1. Introduction
Alkali-rich silica-undersaturated melts commonly contain significant amounts of volatiles that play a key role in the magmatic and postmagmatic evolution of lamprophyres, including kimberlite and ultramafic massifs with carbonatite and their surrounding areas (e.g., [
1,
2,
3]). However, most igneous rocks lack direct evidence of magmatic fluid presence (except microinclusions in minerals) because the fluid that had once been exsolved and physically separated from the parental magma then rapidly left it. Moreover, if exsolved fluids or liquids have not physically separated from the parental magma, they can redissolve, and immiscible textures are not preserved (e.g., [
4]). As volatile-rich lamprophyres decompress as they rapidly rise from lower crustal or upper mantle depths, fluids are oversaturated and exsolved. The quenching of lamprophyres, which typically occur as small bodies, blocks fluid flux and preserves specific textures called ocelli.
The presence of ocelli is a characteristic feature of lamprophyre rocks. Ocelli are small, subrounded, mainly leucocratic structures, commonly filled with carbonates and/or felsic silicates disseminated within a groundmass. Ocelli are interpreted as amygdales—as vesicles filled by late-stage minerals—or as products of silicate–carbonate or silicate–silicate liquid immiscibility ([
5,
6] and reference therein). In some lamprophyres, ocelli have been interpreted as preserved pockets of solidified exsolved immiscible late (residual) magmatic fluid (liquid or gas) [
7,
8]. Ocelli are interpreted as products of silicate melt and carbonate liquid or carbonate-rich fluid immiscibility that occurs in lamprophyre magma (e.g., [
9,
10,
11]).
Ocelli, being droplets of melt or fluid that equilibrate with the host melt, can provide information regarding volatile behaviour in silicate melts during ascent and exsolution, which are complex and not sufficiently understood yet.
This study describes in detail the petrography, mineralogy and carbonate geochemistry of various types of ocelli observed in the lamprophyres from the Chadobets Uplift, southwestern Siberian craton. We show the ocelli’s heterogeneous nature and that they originated because of liquid immiscibility in the magmatic stage and oversaturated fluid exsolution in late magmatic and hydrothermal–magmatic stages.
Rare-earth element (REE) deposits are located in and around carbonatites associated with alkaline rocks. The Chadobets lamprophyres are related to the REE-bearing Chuktukon carbonatites. Our study shows that fluids exsolved in the hydrothermal–magmatic stage of lamprophyre evolution could leach REE from the host lamprophyre and provide REE mobility.
2. Geological Settings and Samples
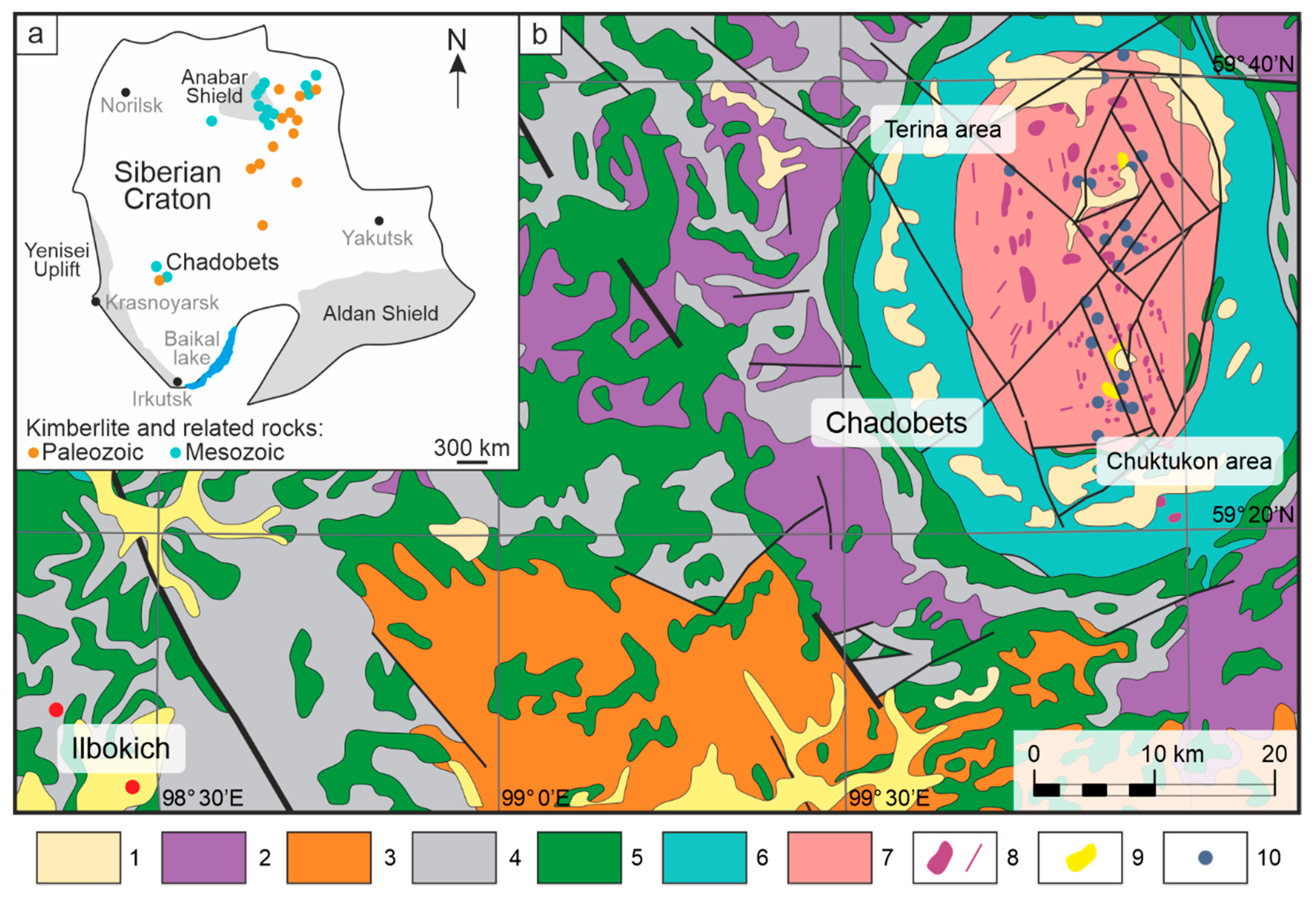
The Chadobets ultramafic lamprophyre (UML) occurrence (also known as the Chadobets massive or Chadobets alkaline complex) is located in the southwestern part of the Siberian Craton within the Irkineeva–Chadobets Trough, the southwestern segment of the Angara–Kotui rift system (
Figure 1). UMLs occur within the Chadobets Uplift that is slightly extended in the north and has 45- and 35-km long axes. Detailed geological descriptions of the Chadobets Uplift and the Chadobets alkaline complex are given in [
12,
13,
14,
15,
16,
17].
The uplift’s core comprises Mesoproterozoic to Cambrian carbonate–terrigenous sediments and is surrounded by dolerite and gabbro–dolerite traps. Triassic magmatic rocks are represented by carbonatites, UMLs such as aillikites, mela-aillikites, clinopyroxene-bearing aillikites and damtjernites with variable abundances of phlogopite, clinopyroxene, monticellite and nepheline [
14,
15,
16]. Alkaline ultramafic rocks are dominant at the northern part of the uplift (Terina occurrence), whereas carbonatites are dominant in the south (Chuktukon occurrence) [
14]. These rocks have been found as dykes, sills, small intrusions and explosion pipes [
12,
17]. Samples of the studied lamprophyres were collected in the northern part of the Chadobets Uplift from sill-shaped outcrop bodies and drill cores from explosion pipes. Rb–Sr isochron and
40Ar/
39Ar dating yielded a Middle Triassic age (243 ± 3 Ma and 241 ± 1 Ma, respectively) for the Chadobets aillikites, indicating post-Siberian Trap emplacement [
15].
In the vicinity of the Chadobets, towards 70 km to the southwest, the Ilbokich UML occurrence is represented by Devonian aillikite and damtjernite dykes. We have described the Ilbokich UMLs in detail earlier [
15,
18].
We studied the composition of ocelli from the Chadobets damtjernite samples. Three samples of this rock with the most abundant and diverse ocelli were selected from our collection for detailed investigation: sample BR-7 with potassium-rich ocelli, sample D-21 with sodium-rich ocelli and sample CHD-30/190 containing ocelli with K and Na minerals. A preliminary description of the Chadobets ocelli was given in [
19].
3. Petrography
The Chadobets alkaline-ultramafic rocks were previously determined as ultramafic lamprophyre (UML) aillikites and damtjernites [
13,
14,
16] based on the classification scheme [
24] that integrated UMLs into the IUGS Classification of Igneous Rocks [
25].
On the basis of the shape, mineralogy, texture and petrographic relation with groundmass, three types of ocelli have been recognised (
Table 1): (i) K–Na–carbonate-silicate ocelli (K-Na-CSO; (ii) Na-carbonate-silicate ocelli (Na-CSO); and (iii) K-rich silicate-carbonate ocelli (K-SCO) and K-rich silicate ocelli (K-SO) (
Figure 2).
Ocelli never contain minerals that crystallised in the early stages of melt evolution, such as olivine and clinopyroxene. Contrarily, they contain minerals that are absent among phenocrysts and groundmass (e.g., sodalite).
The K-Na-CSO are represented in damtjernite CHD-30/190. The rock has porphyritic ocellar textures and contains euhedral phenocrysts of olivine 0.5–1.5 mm in size, irregularly distributed throughout the rock and 15 vol.% of oval ocelli. The groundmass texture is uneven and is represented by small clusters or patches distributed within the mesostasis. Patches of irregular and polygonal shapes sized to 200 µm consist of minerals identical to minerals in ocelli–carbonate, Na-scapolite, K feldspar, albite and apatite. The mesostasis is an aggregate of long laths of phlogopite, apatite euhedral crystals, anhedral crystals of K feldspar, microporous carbonate, minor sulphide and rutile. The carbonate is replaced by small skeletal crystals of clinopyroxene.
The K-Na-CSO show rounded or elongated shapes and sizes up to 600 µm × 800 µm. They are filled with carbonate and massive aggregates of tabular anhedral crystals of sodalite and Na-scapolite (marialite). Potassium feldspar, albite, apatite and minor quartz are present. In contrast with the Na-silicate-bearing carbonate clusters in the groundmass with no clear margins, the ocellus counters are outlined by differences in the texture of the mineral aggregates within the ocelli and the surrounding groundmass. Additionally, tangentially arranged phlogopite flacks often overgrow the outer surface of the ocelli, highlighting its rounded shape (
Figure 3a,b).
The Na-CSO is abundant in damtjernite D-21. The rock has porphyritic ocellar textures. Phenocrysts are represented by euhedral- to subhedral-zoned olivine sized from 0.5 to 1.0 mm. The groundmass is a fine-grained aggregate of euhedral elongated laths of phlogopite, grains of olivine, nepheline, K feldspar, calcite, zeolite, apatite, Ti-magnetite, perovskite and dark needles of a nondiagnostic mineral.
The ocelli are rounded, lens-shaped and dumbbell-shaped and vary between 0.5 and 3.0 mm in size. They often have irregular outlines because of the invasion of groundmass minerals (mainly long prismatic or needle-shaped nepheline and individual crystals of clinopyroxene and phlogopite) inwards. Ocelli consist of carbonates, and laths and tabular crystals or needle-shaped radial aggregates of natrolite and thomsonite dominate. Quartz is interstitial to the Na-silicates. Carbonate–silicate ratios in the ocelli vary significantly from fully carbonate ocelli to ones with 70–80 vol.% occupied by silicate minerals (
Figure 3c,d).
K-rich ocelli are represented in damtjernite BR-7. The rock has porphyritic, sometimes ocellar textures. It hosts phenocrysts of euhedral olivine, fully replaced by an aggregate of secondary minerals that occupied 25% of the rock volume. A fine-grained groundmass consists of olivine, phlogopite, carbonate, potassium feldspar, nepheline, apatite and opaque minerals. Completely altered thin needles, probably amphiboles, are present within the groundmass.
Ocelli occupy 15% of the rock volume. Two subtypes of the K-rich ocelli are presented. The K-SO ocelli have a rounded shape with smooth outlines, and they do not exceed 1–1.5 mm. The ocelli consist of needle-shaped phlogopite, sometimes skeletal with euhedral margins and melt interior crystals, and fine intergrowths of nepheline, potassium feldspar and amphibole needles. These growth textures indicate rapid crystallisation under effective undercooling (
Figure 3e,f).
The K-SCO ocelli are rounded, elongated and dumbbell-shaped to polygonal in shape and vary between 0.1 mm and 4.0 mm in diameter. They are commonly zoned with carbonate in the centre to K feldspar, apatite, phlogopite and Ti-magnetite along the margins. Occasionally, the K-SCO consisted of silicate minerals and altered glass in the centre, and carbonate was situated in the periphery of the ocelli. Thin (1–2 mm) carbonates with minor silicate (Kfs) veinlets interconnected to the K-SCO are common. Silicate minerals are dark mica zoned from phlogopite to biotite, and potassium feldspar formed spherulitic aggregates with the apatite and carbonate inclusions. These spherulitic aggregates mainly grew on the ocelli’s walls and as single spherulites or spherulite clumps in the ocelli’s centres (
Figure 3g,h).
The Chadobets aillikites (samples BR-3 and BR-5) were used as start composition for modelling of melt fractionation using MELTS software (
Section 5.4). Both aillikites are distinguished from other Chadobets aillikites and mela-aillikites with olivine, phlogopite and clinopyroxene phenocrysts by a few olivine phenocrysts, which leads to a weakly porphyritic texture of the rocks. The BR-5 groundmass is composed of olivine, phlogopite, interstitial carbonate, and disseminated apatite, perovskite and Ti-magnetite. The BR-3 groundmass is composed of clinopyroxene elongate prismatic crysts, euhedral phlogopite flakes with abundant inclusions of apatite needles. A mixture of phlogopite and carbonate microlaths with opaque minerals is interstitial between clinopyroxene and phlogopite.
4. Materials and Methods
Textural analyses were conducted using a JEOL scanning electron microscope (JSM-6480LV) at the Laboratory of Local Analytical Methods in the Geology Department at Moscow State University in Russia. Major element compositions of minerals were analysed by the same SEM, using Oxford Instruments X-MaxN energy-dispersive spectrometer with an ultrathin window and a 50-mm2 crystal’s active zone area energy. The accelerating voltage was 20 kV, the beam current was 10 ηA, and the diameter electron beam was focused to a 1–2 μm spot. The instrument was calibrated daily using both natural and synthetic reference material.
Major element compositions of phlogopite were determined by electron microprobe (EMP) analyses using a JEOL JXA-8200 equipped with five wavelength spectrometers at the Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry, Russian Academy of Sciences (IGEM RAS), Moscow, Russia. We used an accelerating beam voltage of 20 kV, a beam current of 20 ηA, a beam diameter of 1–2 μm and a 10 s counting time at peak positions for major elements and 20–40 s for trace elements. The instrument was calibrated daily using both natural and synthetic reference material, and measurements were corrected with the JEOL ZAF correction routine (
Table S3).
Elemental analyses of carbonate were performed on an X-Series II Thermo quadrupole mass spectrometer outfitted with a UP213 New Wave Laser System at the at Laboratory of mineral analysis of IGEM RAS (Moscow, Russia). Data were collected in two analytical sessions within a week. Prior to each analytical session, the instrument was tuned using the NIST-612 glass standard. Ablation was performed by using a laser spot size of 60 μm (circle), a laser fluence of 8–10 J/cm2 and a laser pulse rate of 10 Hz. Helium was used as a carrier gas to transport the laser-generated aerosols from the ablation cell to the ICP-MS. Acquisition time in spot mode was 30 s for the background and 30 s for the mineral analysis. The washout time was 30 s between measuring individual laser spots.
For external calibration, the NIST silicate glass standards (NIST-610 and NIST-612) were used (
Table S2). All standards were run before and after each set of 15 unknowns. Electron Microprobe Ca concentrations in the same spots were used as internal standards for quantification purposes. Data were obtained by reducing the LA-ICP-MS outputs with the Iolite software package [
26]. The measurement errors were 1–3% for REE, U, Th and Pb and 30–50% for Ni, Cu, Zn and Co. The detailed technical description of LA-ICP-MS is provided in [
27]. Time-resolved analysis (TRA) profiles were used to exclude the signal from minor mineral inclusions in carbonates.
Oxygen and carbon isotope analysis was performed by CF-IRMS technique using the Delta V+ isotope mass-spectrometer with the GasBenchII option at the IGEM RAS (Moscow, Russia). Acid decomposition of carbonate samples by H3PO4 was carried out at 70 °C for 2 h. The international standards NBS-18, NBS-19 were used to calibrate measured data vs. V-PDB and V-SMOW. The δ18O and δ13C values were determined with an accuracy of ±0.1‰ and ±0.05‰, respectively.
5. Results
5.1. Mineral Textural Features and Chemistry
5.1.1. Carbonate
Carbonate-rich K-SCO ocelli are characterised by granoblastic calcite aggregates and euhedral calcite crystals with concentric zoning reflecting growth in open spaces. An aggregate of subhedral carbonate grains in the centre of a globule is typical in silicate-rich Na-CSO. Carbonate in the Na-K-CSO presents in granoblastic aggregates. Kidney-shaped calcite aggregates and crystal clusters’ encrusting walls of a globule are observed in silicate-rich ocelli.
In the groundmass, carbonate is anhedral, and often, microporous grains cemented other minerals. Carbonate in a few cases forms elongated spotty zoned grains and fine crystalline aggregates with tiny phlogopite flakes.
There are different carbonate in ocelli and lamprophyre groundmass: calcite, dolomite, Fe-dolomite, ankerite and siderite. Carbonate compositions are identical in both ocelli and groundmass for each studied rock. The BR-7 damtjernite with K-SCO contains pure calcite only. The carbonates in the D-21 damtjernite with Na-CSO are calcite, Mg-calcite, Fe-dolomite and dolomite. The CHD-130/90 damtjernite with Na-K-CSO contains calcite, Fe-Mg-calcite, ankerite and siderite (
Figure 4).
Among all carbonates, calcite in the K-SCO has the highest Ba concentration (up to 400 μg/g) and the lowest Sr concentration (331 μg/g on average). Calcite in both Na-CSO and Na-K-CSO is Ba-poor and has no more than 80 μg/g of Ba. Contrary to that in K-SCO, calcite in the Na-CSO and Na-K-CSO is more enriched in Sr and contains 2856 ± 761 μg/g and 1370 ± 1726 μg/g of Sr on average, respectively. Calcite contains no more than 100–150 μg/g of REE in all ocellus types. Fe-dolomite in the Na-CSO has the lowest REE among all carbonate varieties (≈50 μg/g), and Fe-dolomite in the Na-K-CSO contains 170–300 μg/g REE. Ankerite has the highest REE concentration of up to 400 μg/g.
The REE patterns of carbonates in the Na-K-CSO, despite the different levels of REE concentration in calcite, Fe-dolomite and ankerite, are similar (
Figure 5a). They show chondrite-normalised steep negative slopes, with pronounced light REE (LREE) enrichment and heavy REE (HREE) depletion without U- or S-shaped features. The Ce/Er ratio, indicating LREE enrichment relative to HREE, is 15 on average. These patterns have been considered typical of primary carbonate minerals from carbonatites, kimberlites and lamprophyres (e.g., [
11,
28,
29]).
Carbonates from the K-SCO and Na-CSO show diverse REE patterns (
Figure 5b,c). A common feature is pronounced LREE depletion, especially for the carbonate in the Na-K-CSO. Calcite in the K-SCO has a U-shaped REE pattern with Eu-Ho depletion and enrichment relative to chondrite occurring for La–Sm and Er–Yb, and the Ce/Er ratio is 7 on average. Calcite and Fe-dolomite in the Na-CSO show an S-shaped REE pattern with minimum Nd–Dy. The carbonates in the Na-CSO have low Ce/Er ratio values (0.7 on average), indicating elevated HREE/LREE.
LREE depletion and other REE pattern transformations resulting in hump-, U- or S-shaped overall carbonate patterns are increasingly being reported for UML and carbonatites. These geochemical features are interpreted as mainly resulting from the fractionation of LREE-rich minerals preceding the crystallisation of calcite or dolomite or influenced by interactions with hydrothermal and supergene fluids (e.g., [
11,
29,
30,
31] and references therein).
5.1.2. Phlogopite
We studied the phlogopite (including iron-rich varieties previously named biotite) composition of the damtjernite groundmass and ocelli (
Figure 6,
Table S3). The core–rim composition of the zoned phlogopite grains is correlated with those of UML phlogopites worldwide: from the core to the rims, the phlogopite composition is characterised by increasing FeO and decreasing Al
2O
3 and MgO concentrations. The ocellus phlogopites show transitional compositions from the core to the rim in the sample BR-7, indicating that they crystallised during the crystallisation of the groundmass’s minerals. In the ocellus phlogopites in sample D-21, grains with compositions closer to that of the rim are dominant.
5.2. Whole-Rock Chemistry
Detailed descriptions of the Chadobets UML geochemistry have been published previously by us [
15] and Doroshkevich et al. [
13]. Here, we present only the comparative geochemistry of the ocelli-bearing damtjernite samples.
Primitive-mantle-normalised plots of the damtjernite show the general similarity of the trace element patterns for all rocks. These rocks have a lamprophyric geochemical signature: enrichment in large-ion lithophile and high-field-strength elements and high LREE/HREE. The most pronounced differences between the samples can be seen for Zr and Hf: these elements show a slightly negative anomaly in the CHD-30/190 sample with K-Na-CDO, no anomaly in the BR-7 sample with K-SCO and a positive anomaly in the D-21 sample with Na-CSO. Zr shows a positive correlation with TiO
2, FeO and P
2O
5 in the studied damtjernite. The D-21 sample has the highest concentrations of these elements. Additionally, the Sr concentration increases from the CHD-30/190 sample to the D-21 sample, and Ba has an opposite distribution (
Figure 7).
5.3. Oxygen and Carbon Isotope Composition of Carbonate Minerals from Ocelli
Carbonates from the ocelli have δ18O values ranging from +13.5‰ to +21.8‰ and δ
13C values from −2.1‰ to −5.1‰. The K-Na-CSO carbonate (sample 130/90) has the highest δ
18O (+21.6‰ and +21.8‰) and δ
13C (−2.1‰ and −2.4‰) values. The K-SCO carbonate (sample BR-7) shows intermediate δ
18O values and the lowest δ
13C values. By contrast, the Na-CSO carbonate (sample D-21) demonstrated the lowest δ
18O values and intermediate δ
13C values (
Table 3).
A comparison of stable isotopic data for the ocellus carbonate with O and C isotopy from the Chadobets UMLs and carbonatites [
13,
15] shows that the ocellus carbonates are plotted within the Chadobets carbonate field (
Figure 8). Moreover, two trends are described for the carbonates of the Chadobets UMLs [
15]. The first trend towards higher δ
13C and δ
18O values is ascribed to the interaction of primary magmatic carbonates with the surrounding sedimentary carbonates.
The second trend towards low δ13C values reflects the decarbonisation process (carbonate decomposition accompanied by CO2 outgassing). Carbonates from the K-Na-CSO and Na-CSO are plotted in the first trend, and that from the K-SCO with one exception less clearly drawn within the same trend.
5.4. MELTS Modelling of Damtjernite Melt–Fluid Evolution
To constrain the crystallisation and melt evolution conditions of the studied rocks, the Rhyolite-MELTS software package (version 1.2.0, [
34]) was used. The software can model crystallisation in the presence of hydrous-carbonate fluid at 600–1400 °C and 0–3 GPa and was proven to accurately reproduce crystallisation for alkali melts [
35].
Fractionation of silica-undersaturated melts can lead from nephelinites to phonolites and carbonatite with or without liquid immiscibility (e.g., [
36,
37,
38] and references therein). The Rhyolite-MELTS with the integrated model of [
35] captures much of the experimentally observed melting behaviour of CO
2-rich mafic melts, including carbonatite melts [
39]. We used the MELTS software for the Chadobets lamprophyres to model a liquid line of descent (LLD) and fluid composition for primitive melt (aillikite) crystallisation and compare the modelled LLDs with those of the studied damtjernites to understand the general conditions and trends of the melt and coexisting fluid evolution.
The initial primitive melt was chosen as the most magnesian with high-Cr and high-Ni aillikites (samples BR-3 and BR-5), representing the products of primitive nonevolved melts [
15]. We do not know the pressure at which the olivine phenocrysts crystallised, and we used indirect data to estimate the pressure. On the basis of experimental data and natural observations for kimberlite and strictly limited data for UMLs indicating 2.0 GPa as the maximum value [
40,
41,
42] and by analogy using the Kola aillikite crystallisation model, which gives 1.5–0.5 as the pressure interval [
38], we take 1.5 GPa as the initial crystallisation pressure of the phenocrysts. We modelled aillikite decompression crystallisation under decreasing pressure from 1.5 to 0.3 GPa to obtain a phenocryst assemblage. Then, we used the residual melt composition after nearly 40% of the initial melt crystallised to model groundmass formation at low pressure (0.1 GPa). The overall fluid content in the melt was assumed to be 7 wt%, with the loss-on-ignition value in the primitive initial aillikite. We suggest the dominance of the carbonate component among volatilities for the initial point of crystallisation (CO
2/H
2O = 5/2) and for the groundmass crystallisation the dominance of the hydrous component (CO
2/H
2O = 2/5).
We chose the FMQ buffer as the crystallisation’s oxygen fugacity (
fO
2) based on fO
2 values estimates obtained by [
14,
16], which ranged from FMQ + 0.1 to FMQ − 0.2 log units for the Chadobets rocks and, by analogy, for the Aillik Bay aillikite crystallisation estimated slightly above the FMQ buffer [
32].
We plotted the LLDs with the composition of the ocelli-bearing damtjernites and other Chadobets rock on bivariate Harker’s diagrams as shown in
Figure 9. The crystallisation of both BR-3 and BR-5 aillikites leads to decreasing SiO
2 and MgO, generally corresponding to the observed trend for the Chadobets UMLs (
Figure 9a). Therefore, the MELTS modelling demonstrated that the residual melt becomes poorer in SiO
2 and MgO, meaning more carbonatitic. One can see on this plot that the studied damtjernites are located on the modelled LLDs.
By contrast, the other rocks are characterised by their higher Mg concentration, which is likely related to higher cumulate proportions. The modelled LLDs satisfactorily match the CaO increase and Al
2O
3 decrease in the Chadobets rocks via melt fractionation (
Figure 9c,d).
A discrepancy remains, however, between the modelled LLDs for Na
2O, K
2O and the observed rock compositions. The model reproduces a moderate increase in Na
2O content for most rocks, but it does not shift to more Na-rich compositions for the damtjernites. Contrary to model predictions, the increase and then decrease in the potassium content, continuously enriched by K
2O, notably in damtjernites, is observed for the Chadobets UMLs (
Figure 9e,f). The significant difference between the model forecasts and the observed alkali content for damtjernites is more likely because the model considers only CO
2 and H
2O in the fluid and not any other component such as alkalis.
At high pressures (1.5–0.95 GPa) and temperatures (1400–1280 °C), the only crystallising phenocryst is high-Mg olivine (0.92 to 0.86 forsterite molecule,
Supplementary Material Data S1), which is well within the petrography of the damtjernites. At low pressure (0.1 GPa) and temperatures (1100–800 °C), the modelled fractionated phases are minor olivine, apatite, sphene, ilmenite, more abundant spinel and dominant phlogopite and clinopyroxene (
Supplementary Material Data S2). The modelled phases resemble the observed groundmass minerals except for the abundance of clinopyroxene and lack of carbonate in the modelled assemblage. They resemble well the mineralogy of the daughter phases of the melt inclusions in olivine, apatite and spinel from the Chadobets aillikite and damtjernite [
14,
43,
44]
All modelled crystallising phases are equilibrated with the exsolved fluid at all temperature ranges. The fluid under high pressure has a significant carbonate composition and evolves to a more hydrous composition via equilibration with melt-fractionated aillikite. The fluid equilibrated with the melt under low pressure evolved to a more carbonate-rich composition during melt crystallisation.
6. Discussion
The origin of ocellar or globular textures in lamprophyres and some other predominantly alkaline rocks (e.g., kimberlite and basanite) has been widely debated in the last century: ocelli have been interpreted as segregations of residual interstitial melt, vesicles filled by late-stage minerals, pseudomorphs after olivine or other minerals, immiscible liquid droplets, nucleation centres for leucocratic minerals or amygdales (e.g., [
5,
6,
7] and references therein). Two main perspectives on ocelli origin presented by Cooper [
5] remain relevant: ocelli are produced either via liquid immiscibility or via the segregation of late-stage liquids/fluids (e.g., [
9,
10,
45]).
We summarised features that indicate globules in our damtjernites as true ocelli. The mineral composition of ocelli, spherulitic and mosaic textures of mineral aggregates within the ocelli and their relation with host rock (invasion of groundmass minerals) indicate that they are not amygdules. The presence of minerals lacking in groundmass (e.g., natrolite, scapolite and sodalite) indicates that they are not segregations of residual interstitial melt. Petrographic features and mineral composition evidence that the ocelli are not vesicles filled by late-stage minerals. They are also not pseudomorphs after olivine.
As shown above, the textures of carbonate and other minerals in the ocelli—euhedral grain shapes, spherulitic and mosaic textures of mineral aggregates and oscillatory zoning of carbonate—indicate that they grew into spaces filled by fluid or melt. Studies on carbonate-ocelli-bearing lamprophyre dykes from various occurrences (e.g., Yunnan Province [
9], Italy [
10] and New Zealand [
11]) suggest that ocelli are the product of silicate melt and carbonate liquid or carbonate-rich fluid immiscibility during the magmatic evolution of lamprophyres.
To test the liquid immiscibility or other factors that led to the origin of the ocelli in the Chadobets damtjernites, we calculated the trace element and REE patterns for the carbonates in the ocelli and normalised trace element concentrations in carbonate to those in the host lamprophyre (
Figure 10). We used this approach despite the host lamprophyre compositions being bulk lamprophyre and ocelli and even though the ocelli contain silicate phases in addition to carbonate: the ocelli only make up a maximum of 15% of the host rock volume, and carbonates mainly predominate in the ocelli. Various ocellus types are characterised by different trace elements and REE patterns of carbonates normalised to host lamprophyres.
6.1. K-Na-CSO and Liquid Immiscibility
The MELTS model shows that the CHD-30/190 damtjernite sample is the most evolved among all the studied damtjernites. This rock has the lowest SiO
2 and MgO contents and plots on the lower temperature part of the LLDs relative to the other damtjernites (
Figure 9). The evolved nature of the CHD-30/190 damtjernite is favourable to the accumulation of carbonate- and alkali-rich residual melt.
The K-Na-CSO carbonate has a Sr concentration higher than that of the host lamprophyre but is depleted of other trace elements. All carbonate varieties (calcite, dolomite and ankerite) from the K-Na-CSO have REE patterns similar to those of the host damtjernite with a negative REE slope typical of igneous carbonate minerals (e.g., [
29]). The REE patterns normalised to the host rock shows that the K-Na-CSO carbonate has an REE concentration nearly equal to that of the host lamprophyre except for the slightly depleted LREE (
Figure 10a).
The CHD-30/190 damtjernite sample and the K-Na-CSO contain carbonate–silicate irregular patches much smaller than the ocelli and consist of the same minerals as those of the ocelli: alkaline feldspars, Na-scapolite, apatite and carbonate. This texture resembles typical features of spinodal decomposition: a quick initial separation of two phases, which can produce fractal ramified structures and much slower, subsequently coarsening structures transformed into droplets to minimise the interfacial energy (e.g., [
46,
47] and references therein).
The identical mineralogy and carbonate composition of the ocelli and the carbonate–silicate patches in the groundmass suggest that the ocelli are droplets of an exsolved carbonate-rich melt. The patches are likely proto-ocelli: initial small segregations of exsolved melt that may accumulate and transform into isolated droplets of melt.
Apart from the textural evidence, the geochemical signature of the K-Na-CSO carbonate is consistent with the origin by immiscibility of silicate melt and residual carbonate-rich melt. To verify this suggestion, we compared the trace element patterns normalised to the host lamprophyre for the K-Na-CSO carbonate with partition coefficients for carbonatite–silicic melt immiscibility to the anhydrous potassium system at 1 GPa by [
48]. One can see in
Figure 10a,b that the normalised REE patterns are consistent with the experimental partition coefficients and Sr, Y and Zr, with the distribution between the carbonates from K-Na ocelli and the host lamprophyre matching well with the liquid immiscibility process.
LREE depletion in carbonates more probably relates to the preceding crystallisation of LREE-accommodating minerals (e.g., apatite). The relatively low P2O5 content in other Chadobets rocks and modelled LLDs indicate the loss of some apatite crystals. Discrepancies in barium content may have resulted from phlogopite crystallisation, and Rb depletion is consistent with this suggestion.
Therefore, we suppose that the textural and mineralogical features of the K-Na-CSO and their host rock can be interpreted as droplets of Na-K-carbonate-rich melt exsolved from evolved silicate–carbonate damtjernite melt probably via spinodal decomposition.
6.2. Na-CSO: Late Magmatic Fluid Exsolution and Readsorption
The damtjernite from the D-21 sample, which contains the Na-CSO, is characterised by notable differences in composition relative to other damtjernites and aillikites: it is enriched with Na, Ti, Fe and P and has a pronounced positive Zr–Hf anomaly. These geochemical features may have appeared because of the loss of the carbonate component from the preceding carbonate–silicate melt. The silicate melt from which a carbonate melt was separated should be characterised by high Ti, Fe, Zr and Hf, as evidenced by partition coefficients for carbonatite–silicate melt immiscibility [
48]. This immiscibility leads to the slight Na enrichment of the carbonatitic melt relative to the silicate one [
48].
However, the D-21 damtjernite has an elevated Na content, significantly higher than in the other Chadobets lamprophyres and modelled LLDs (
Figure 9), which is contradictory to a silicate residual after carbonate melt separation. Nevertheless, the widespread occurrence of fenitised rocks surrounding the carbonatitic massifs, highly alkali-rich fluid and melt inclusions in minerals from carbonatites and fenites suggests that an alkali-rich, often Na-rich carbonate fluid co-exists with and then separates from the carbonate and carbonate–silicate melts (e.g., [
2] and references therein; [
49,
50]).
The REE patterns for the Na-CSO carbonates normalised to the host D-21 damtjernite show strong LREE depletion and moderate to nondepleted HREE from Tb to Yb. Sr concentrations in the carbonates are a factor of two higher than those in the host rock (
Figure 10). Alkali-rich fluids exsolved from carbonatites are characterised by elevated HREE/LREE ratios, and potassic fluids preferentially transport HREE relative to sodic fluids [
2]. The moderate HREE/LREE ratios observed in the Na-CSO carbonates with HREE content not higher than that in the host damtjernite are consistent with sodic fluids separated from the ocelli.
Phlogopite flakes in the Na-CSO have compositions matching those of the rims of phlogopite crystals within the damtjernite groundmass. This is evident in the late magmatic stages of melt evolution when the Na-rich fluid has exsolved and formed the ocelli. The invasion of groundmass minerals into the Na-CSO indicates that the melt was still present when they were formed.
The invasion of nepheline, clinopyroxene and phlogopite crystals from the groundmass to the ocelli breaks their contours and makes them disappear into the groundmass (
Figure 3). We suggest that this texture demonstrates an active chemical reworking and can be interpreted by ocellus dissolution into the late-stage melt. When the pressure decreases and CO
2 escapes from a melt, the solubility of the carbonate melt or fluid could increase, and the carbonate liquid or fluid that has been exsolved and not physically separated from the parental magma should redissolve ([
4,
7]).
Therefore, we ascribe the observed texture, mineralogy and geochemistry of the Na-CSO carbonates to the late magmatic separation and subsequent partial dissolution of the Na-rich fluid.
6.3. K-Silicate–Carbonate Ocelli: Postmagmatic Carbothermal Fluid Separation
Damtjernite from the BR-7 sample has high MgO and SiO
2 contents and is the least evolved melt among all the studied ocelli-bearing rocks as the lamprophyric liquid evolved towards a silica-poor and low-Mg carbonate-rich composition, as the MELTS model has shown (
Figure 9). This rock has a low (Na + K)/Al (molar) ratio and enrichment in K
2O and Ba.
The K-SCO carbonate is moderately depleted of all elements relative to the host damtjernite. Compared with the carbonates from other ocelli, it has higher Rb, Zr and Ba concentrations and is depleted of Sr. The REE patterns normalised to the host damtjernite show strong MREE depletion, slight LREE depletion and no depletion and even enrichment of HREE (
Figure 10). The geochemical features of the K-SCO calcite do not correspond to an igneous carbonate in lamprophyres (e.g., [
45]). A significant difference between the REEs of the whole rock and the carbonate it hosts has been observed for hydrothermally altered lamprophyres from the Southern Alps, New Zealand [
51].
The calcite geochemistry, most notably its REE patterns (
Figure 5), is similar to that of the hydrothermal carbonate observed at the Weishan REE carbonatite deposit [
30]. The mobility of REE in hydrothermal and carbothermal fluids is complex. Many factors, such as fluid composition, temperature, pH, salinity and
XCO
2, play a significant role in REE variations observed in natural carbonates (e.g., [
52] and references therein; [
53] and references therein). Anenburg et al. [
2] have shown recently that alkaline potassium-rich carbonate fluids can transport HREE dominantly. HREE enrichment in carbonates under these concentrations in the host lamprophyre is observed for late-stage hydrothermal carbonates in the lamprophyres from the Southern Alps, New Zealand [
51].
The REE patterns of the K-SCOcalcite have two components: an LREE–MREE interval with a negative slope such as that in the igneous calcite from the CHD-30/190 damtjernite and an HREE interval with strong enrichment typical for hydrothermal carbonates (
Figure 9). We suggest that the REEs in the fluid from which the calcite precipitated were partly inputted directly from the host damtjernite and partly were transported by hydrothermal fluids as REE complexes. Experimental studies and numerical simulations of the stability of REE complexes in hydrothermal fluids (e.g., [
52,
53]) show that different ligands (halogenides and bicarbonate/carbonate) can control the REE mobility.
The presence of only pure calcite and the lack of any dolomite and ankerite can indirectly indicate low-temperature conditions for the K-SCOformation relative to those of the other ocelli containing Mg- and Fe-carbonate minerals (e.g., [
51] and references therein). The network of veinlets interconnecting the K-SCO indicates that the host rock was mechanically rigid but not completely crystallised. The percolation theory predicts that spherical vesicles will form a connected network at this point, allowing relatively easy and rapid gas transport [
54].
The isotopic composition of C and O in the K-SCO carbonate plots a decarbonisation trend towards low δ
13C values, reflecting carbonate decomposition accompanied by CO
2 outgassing (
Figure 8).
On the basis of the textural, mineralogical and geochemical features of the K-CDO, we suggest that the ocelli of this type are a product of a hydro(carbo)thermal K-rich fluid that interacted with almost crystallised damtjernite in a magmatic–hydrothermal stage.
6.4. Fluid–Melt System Evolution and Significance for the Chuktukon Carbonatites
The diversity of the ocelli from the Chadobets damtjernites exhibits a sequence of multistage processes of fluid–melt system evolution under lamprophyre crystallisation and rapid cooling.
The structural difference between silicate and carbonate melts can result in the distinct chemical separation of these two phases (e.g., review in [
55]). The rapid ascent of the volatile (hydrous and carbonate)-rich, silica-undersaturated, alkali-rich lamprophyre melt from lower crustal or upper mantle depths results in the oversaturation of hydrous fluid-phase and carbonate–melt components (e.g., [
7]). Pressure decrease leads to decreased water and carbonate solubility in a silicate melt, and the melt exsolves volatiles (e.g., [
56]). The evolution of the melt–fluid alkaline silica-undersaturated system is of critical importance to REE deposits in carbonatites (e.g., [
2,
57]). Considering that the Chadobets lamprophyres are temporally, spatially and originally related to the Chuktukon carbonatitic massif, which bears an REE mineralisation ([
13] and references therein), understanding what magmatic and hydrothermal processes during lamprophyre evolution lead to carbonatite formation and are responsible for REE enrichment is important.
Recently, Doroshkevich et al. [
13] suggested that the Chuktukon carbonatites originated via the silicate–carbonate liquid immiscibility mechanism because the trace element distributions between the Chuktukon carbonatites and melilitites compared with the experimentally obtained coefficients are consistent with this finding. However, investigations found no evidence of liquid immiscibility through melt inclusions. Our study demonstrated that the Chadobets lamprophyres are capable of producing carbonatitic melts through liquid immiscibility. The K-Na-CDO, with the most evolved damtjernite, originated when alkali- and carbonate-rich residual melts accumulated. On the basis of the compositions of the ocellus minerals (i.e., sodalite, scapolite and natrolite), we suggest that the ocelli formed from a carbonate-rich melt containing high amounts of Na, Cl and S and a significant fraction of silicate components such as Al and Si. We conclude that this melt was exsolved from the evolved silicate–carbonate damtjernite melt through liquid immiscibility on the basis of the igneous geochemistry of the carbonate in the ocelli and textural evidence.
Moreover, our study has shown that lamprophyre-derived carbonate-rich fluids of late magmatic and magmatic–hydrothermal stages can contribute to REE mineralisation. The D-21 damtjernite demonstrates a fluid regime in the late magmatic stage for the silicate fraction of the silicate–carbonate melt after the carbonate fraction has been separated. On the basis of the Na-CSO composition, we suggest that this melt effectively separates alkali (Na)-rich carbonate fluids. If the lamprophyre melt lost some CO
2, then fluid solubility in the melt must rise [
7], and the ocelli can partially be redissolved. The separation of alkali (K)-rich silicate–carbonate fluid during the magmatic–hydrothermal stage of lamprophyre melt evolution is evidenced by the K-CDO. The Na-CSO and K-SCO are enriched in different alkali metals. An experimental study [
58] demonstrated that K-rich silicate melts could accommodate more CO
2 in their structure than those that are Na-rich, which is consistent with the differences in the composition of the Na-CSO and K-CDO.
The geochemical signature of the carbonates from these ocelli shows that the fluid could leach REEs from the host lamprophyre and provide for REE mobility. Both late magmatic and hydro(carbo)thermal fluids show HREE enrichment (
Figure 5 and
Figure 10).
The silicate–carbonate liquid immiscibility within alkaline-ultramafic melts could occur at the late stages of magma evolutions and could reach a complete separation of carbonate-bearing fluid or carbonatitic melts in alkaline-ultramafic magmas due to the reaction of decarbonation [
59]. Monticellite-containing alkaline-ultramafic rocks including kimberlites [
59] and damtjernites [
60] could be considered as natural examples of the separation of carbonate-bearing fluid phases or carbonatitic melts in alkaline-ultramafic magmas. For example, the study of melt inclusions within groundmass minerals from some damtjernite from the Anabar province [
60] show that alkaline-ultramafic magmas completely lost the carbonate component at the groundmass crystallisation stage. The latter is consistent with the presence of carbonate-bearing varieties of alkaline-ultramafic rocks, including carbonatites in the Anabar region [
61].
6.5. Comparison with Kimberlite Fluid
A comparison of the chemistry and formation sequence of the ocelli in the Chadobets lamprophyre with those of recently studied primary melt and secondary fluid inclusions in olivine rims at the Bultfontein kimberlite [
62] demonstrates a similarity in fluid composition and dynamics during the late stages of rock emplacement. Primary inclusions in olivine rims trapped alkali-Si-bearing Ca–Mg-rich carbonate melts, representing either a fractionated product of the kimberlite magma or a fluid exsolved after the crystallisation of olivine and some other minerals. Secondary fluid inclusions in olivine rims are dominated by Na ± K-rich C–O–H–Cl compositions, representing residual fluids during groundmass crystallisation [
62]. The alkali-Si-bearing Ca–Mg-rich carbonate melts/fluids of the primary inclusions compared with the K-Na-CSO and residual Na ± K-rich C–O–H–Cl fluids of the secondary inclusions have a similarity with the Na-CSO and K-SCO.
Na and K-rich C–O–H–Cl ± Ba, Sr, and REE residual fluids were also investigated in melt inclusions within kimberlite groundmass minerals such as chromite and perovskite apatite, monticellite from different kimberlite occurrences [
63,
64,
65].
Nevertheless, the differences between the two cases should be marked. The fluid exsolved earlier before the groundmass was fully crystallised, represented by the K-SCO in the Chadobets lamprophyre, was more silicate-rich and carbonate-depleted than the fluid from the primary inclusion in the olivine rims at the Bultfontein kimberlite.
7. Conclusions
The Chadobets lamprophyres (aillikites and damtjernites) contain elliptical to polyhedral silicate–carbonate globules (ocelli) filled with carbonate and/or felsic silicate minerals. A detailed study of the ocelli, disseminated in UMLs, shows that they vary by origin. On the basis of their morphology, mineralogy and relation with the surrounding groundmass, we distinguish three types of ocelli: carbonate-dominant, containing carbonate, scapolite and sodalite ocelli (K-Na-CSO); carbonate–silicate ocelli, containing natrolite and sodalite (Na-CSO); and carbonate-dominant, containing potassium feldspar and phlogopite (K-SCO).
We suppose that the textural, mineralogical and geochemical features of the ocelli and their host rock can be interpreted as follows: (i) the K-Na-CSO are droplets of an alkali–carbonate melt that separated from residual alkali and carbonate-rich melt in highly evolved damtjernite; (ii) the Na-CSO are droplets of late magmatic fluid—dense fluids once exsolved from a melt and then began to dissolve; (iii) the K-SCO are bubbles of K-P-CO2 fluid liberated from an almost crystallised magma during the magmatic–hydrothermal stage.
Thus, we suggest that the fractional crystallisation of UML magma can cause residual carbonatite melt separation and rapid decompression during the ascent of volatile-rich aillikite and especially damtjernite magma, leading to fluid oversaturation and exsolution. Differences between the Na-CSO and the K-SCO morphologies (polyhedral for the K-SCO versus elliptical for the Na-CSO) and interaction styles with the host groundmass (active chemical reworking for the Na-CSO and slight mechanical deformations for the K-CSO) indicate the earlier formation of the Na-CSO and the late formation of the K-CSO.
The K-Na-CSO presented the most evolved damtjernite and originated when the alkali- and carbonate-rich residual melt accumulated. Na-CSO was formed by Na-rich fluid separation during the late magmatic stage, and the K-SCO corresponds to the magmatic–hydrothermal stage.
The geochemical signature of the K-SCO carbonate shows that the late fluid could leach REE from the host lamprophyre and provide for REE mobility. Both late magmatic and hydro(carbo)thermal fluids show HREE enrichment.
A study of the ocelli origin in the Chadobets lamprophyre indicates the complexity of the melt–fluid system in the late stages of alkalic magmatism.