Tungsten Ores of the Dzhida W-Mo Ore Field (Southwestern Transbaikalia, Russia): Mineral Composition and Physical-Chemical Conditions of Formation
Abstract
:1. Introduction
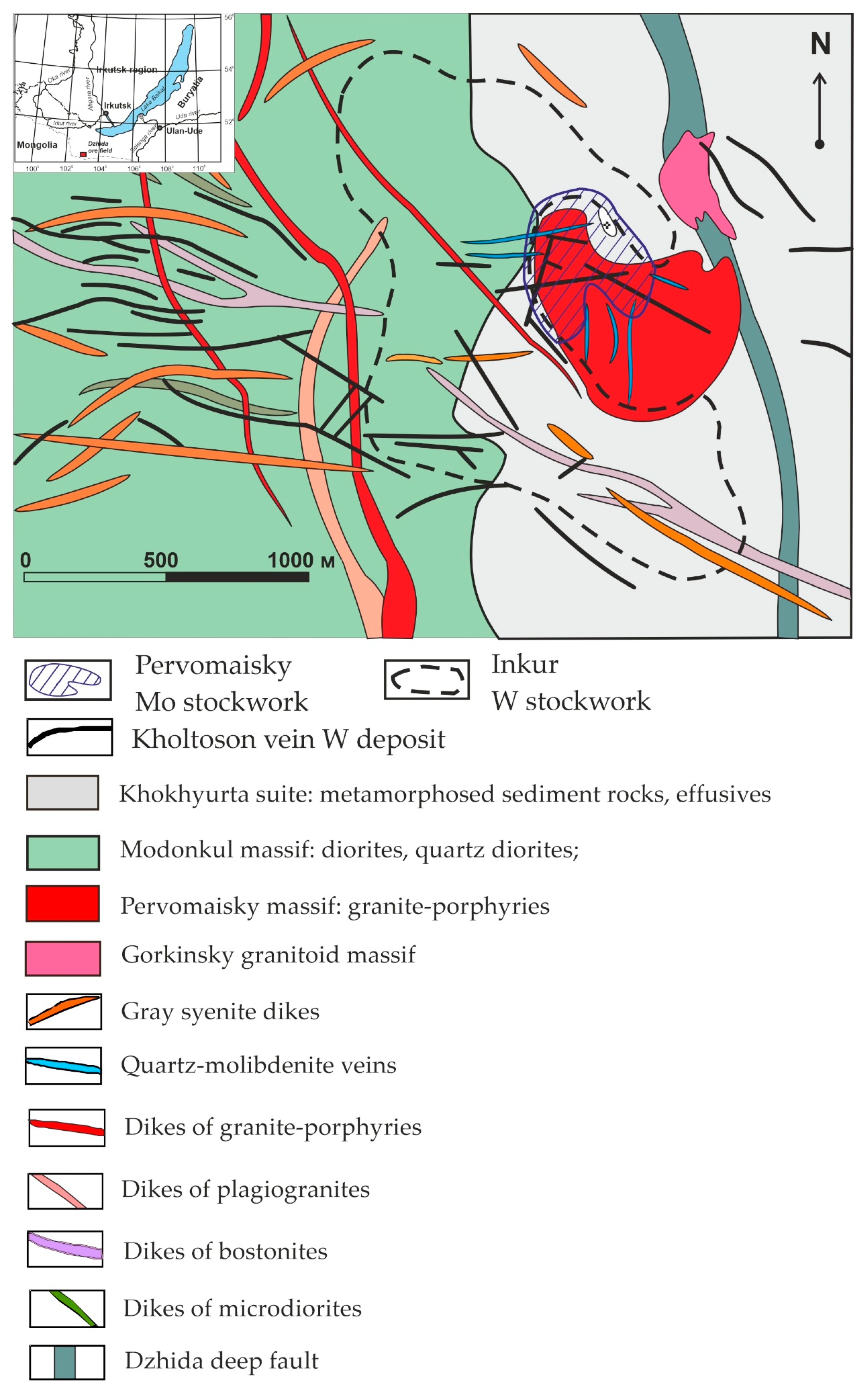
2. Regional Geology
3. Sampling and Analytical Methods
4. Results
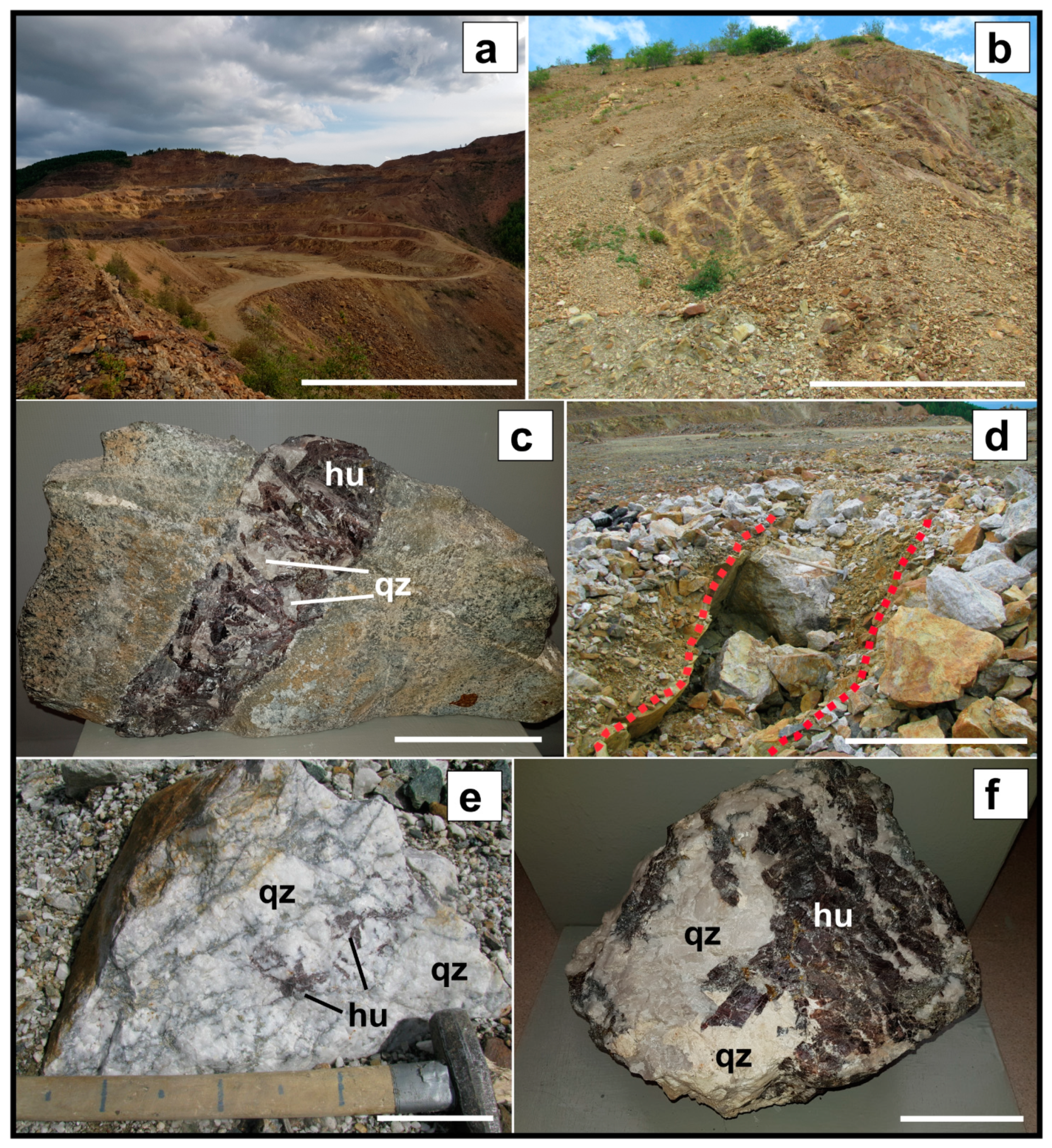
4.1. Deposit Geology
Inkur Deposit
4.2. Kholtoson Deposit
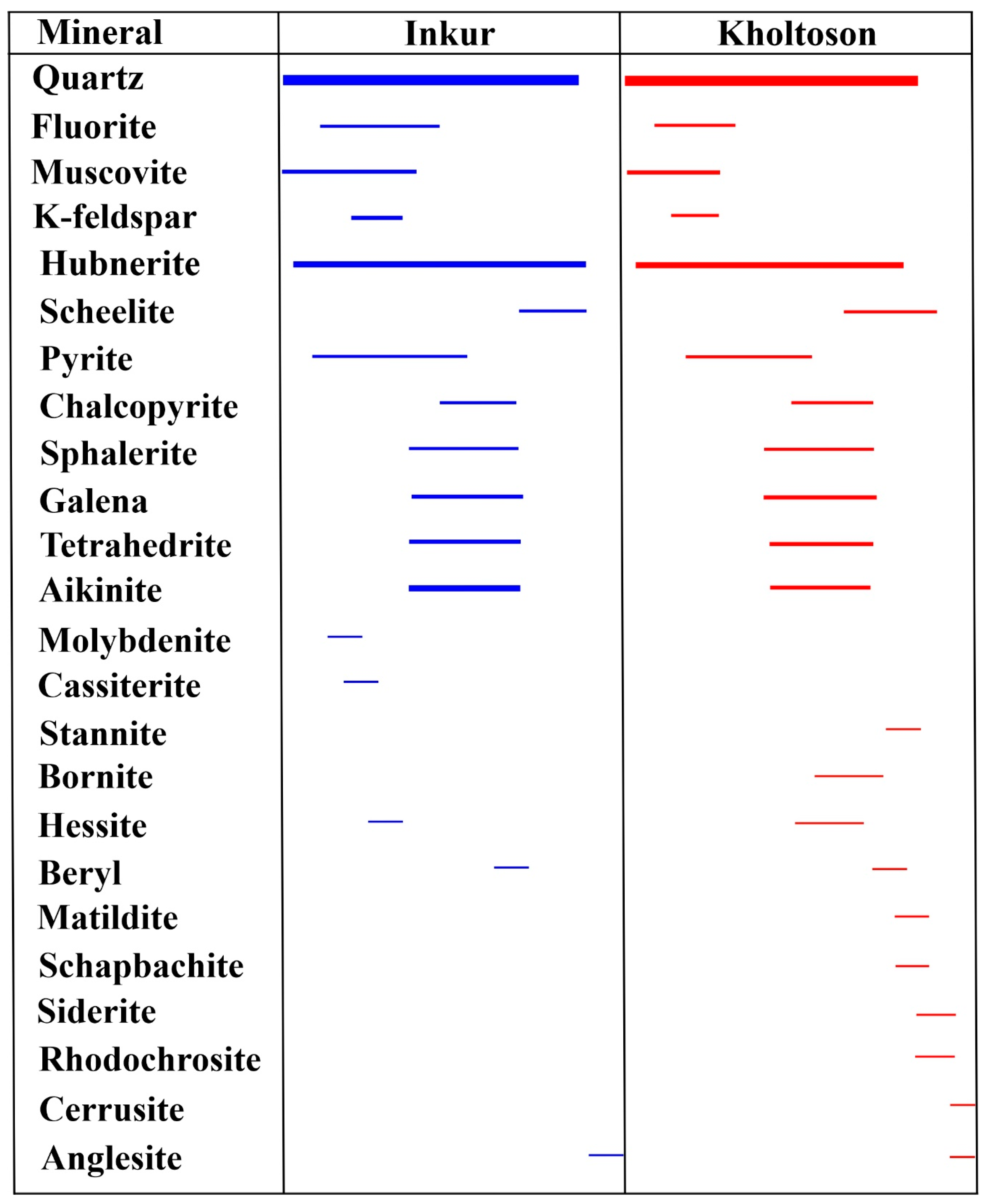
5. Ore Mineralogy
5.1. Inkur Deposit
5.2. Kholtoson Deposit
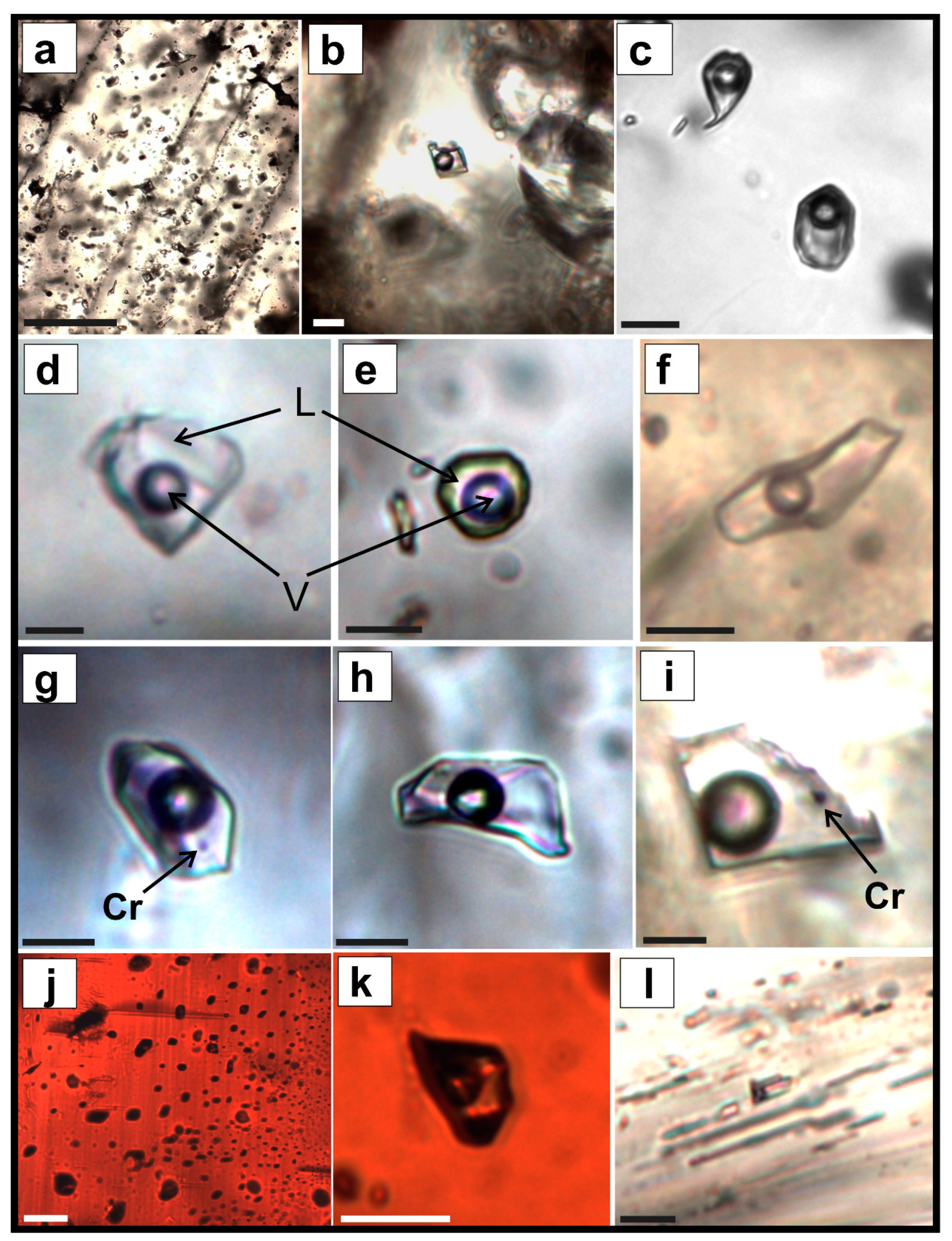
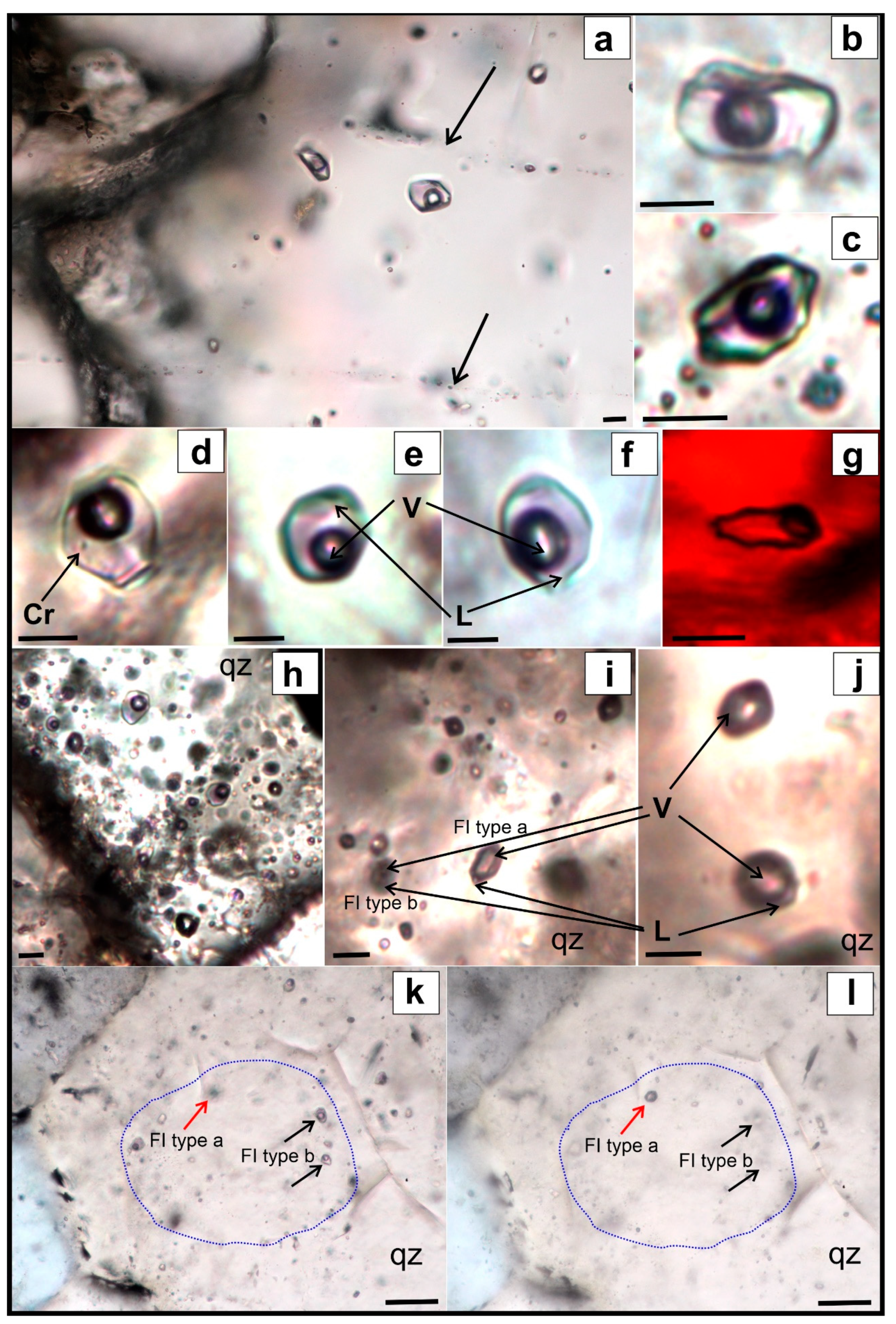
6. Fluid Inclusion Study
6.1. Inkur Deposit
6.2. Kholtoson Deposit
7. Discussion
7.1. Mineralogical Characterization of the Studied Tungsten Deposits
7.2. Physicochemical Parameters of the Ore Formation
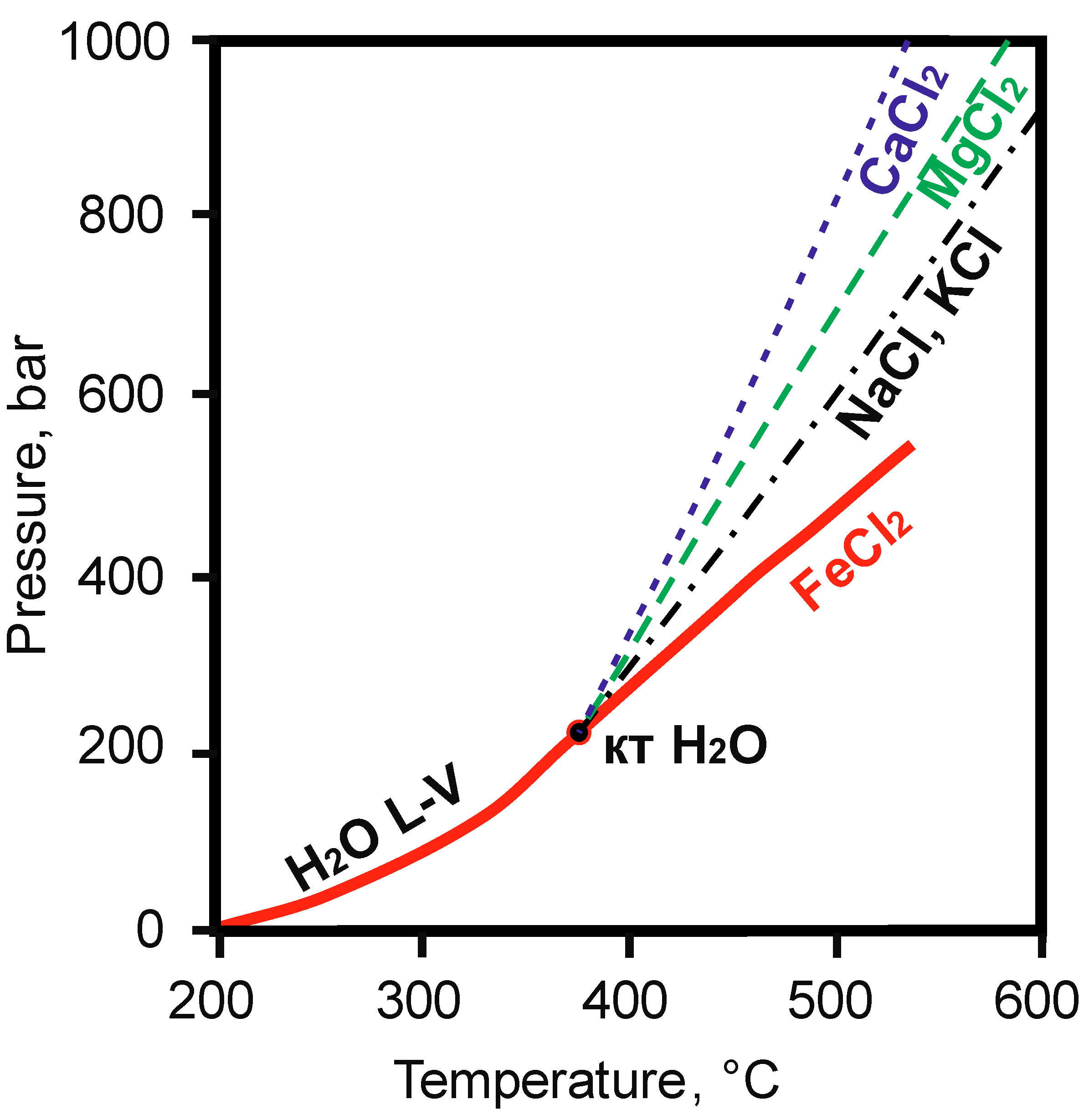
7.3. Pressure Estimation
7.4. Main Factors of Hubnerite and Wolframite Precipitation
8. Conclusions
- Tungsten mineralization of the Dzhida ore field is represented by the Inkur stockwork and the Kholtoson vein deposits, which were formed nearly simultaneously during one stage. Mineral assemblages are similar in both deposits, where more than 20 mineral species have been identified. The main ore mineral is hubnerite, and accompanying minerals are sulfides (pyrite, chalcopyrite, galena, sphalerite, bornite, etc.), sulfosalts (tetrahedrite, aikinite, stannite, etc.), oxides (scheelite, cassiterite), and tellurides (hessite). There were some differences observed only in assemblages of rare and minor ore-forming minerals. For the first time, a rare unusual mineral of the halogenide class—aluminium hydrofluoride—was found in the ores of the Inkur deposit. Its composition is similar to rosenbergite (AlF[F0.5(H2O)0.5]4·H2O). In the Kholtoson deposit ores, we diagnosed unknown phases—Cu2PbS3 and Cu3Pb3S5—as well as rare sulfosalts matildite (AgBiS2) and schapbachite (Ag0.4Pb0.2Bi0.4S).
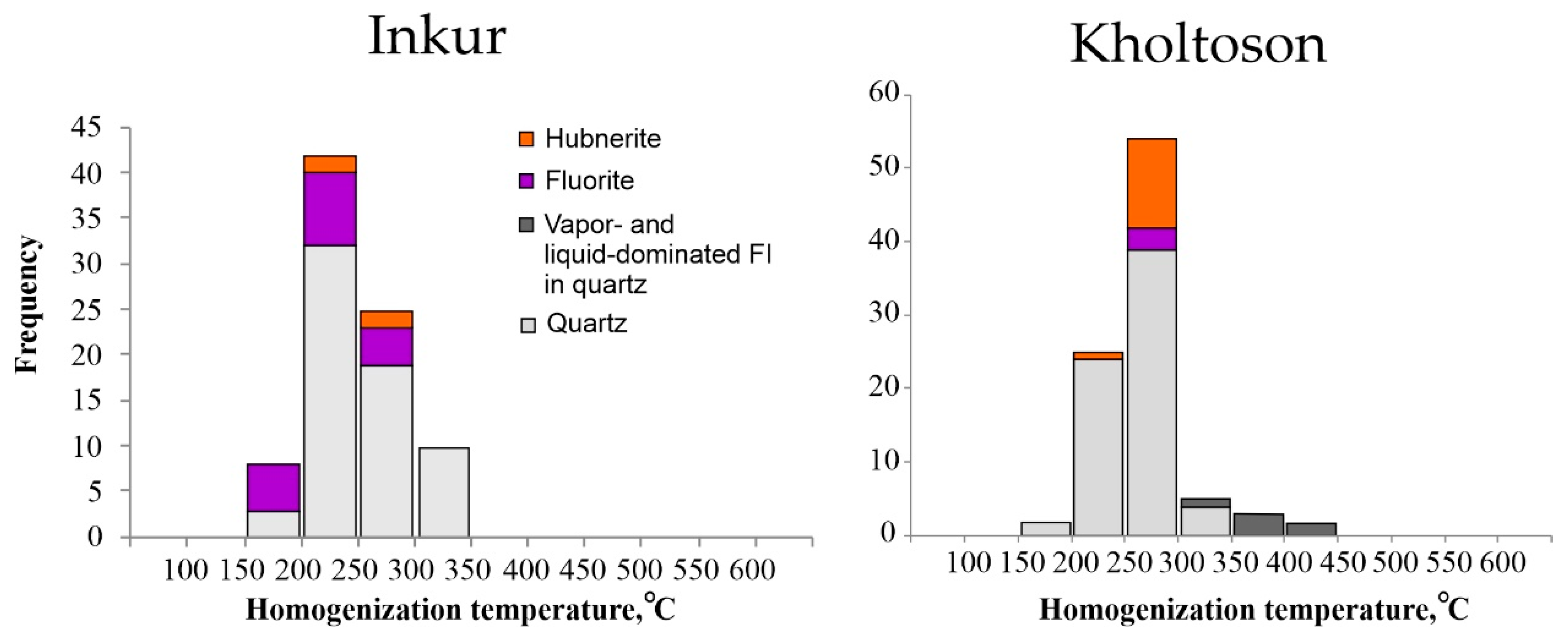
- For both deposits, the fluid inclusion homogenization temperatures of homogeneous trapping FIs overlapped and varied within the range ~195–344 °C, reflecting the minimum conditions for mineral formation. The presence of cogenetic liquid- and vapor-dominated inclusions in the quartz from the ores of the Kholtoson deposit allowed us to estimate the true temperature range of mineral formation as 413–350 °C.
- The obtained pressure value for the Inkur veinlet formation was more than 785 bar, reflecting the minimum FI trapping pressure. At the Kholtoson deposit, the pressure could be estimated at about 300 bar. The drop in pressure during mineral formation of the Kholtoson veins was probably caused by the appearance of large cracks, whereas the Inkur stockwork was formed at a relatively high pressure.
- The deposition of minerals in the studied tungsten deposits proceeded with a decrease in temperature. The main factors of ore precipitation at the deposits of the Dzhida ore field were decreases in temperature. The acidic composition of ore-forming solutions led to the W precipitation as hubnerite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, T.; Pollard, P.J.; Mustard, R.; Mark, G.; Graham, J.L. A comparison of granite-related tin, tungsten, and gold-bismuth deposits: Implications for exploration. SEG Newsl. 2005, 61, 5–17. [Google Scholar]
- Harlaux, M.; Mercadier, J.; Marignac, C.; Peiffert, C.; Cloquet, C.; Cuney, M. Tracing metal sources in peribatholitic hydrothermal W deposits based on the chemical composition of wolframite: The example of the Variscan French Massif Central. Chem. Geol. 2018, 479, 58–85. [Google Scholar] [CrossRef]
- Damdinova, L.B.; Damdinov, B.B.; Huang, X.-W.; Bryansky, N.V.; Khubanov, V.B.; Yudin, D.S. Age, conditions of formation, and fluid composition of the Pervomaiskoe molybdenum deposit (Dzhidinskoe ore field, South-Western Transbaikalia, Russia). Minerals 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Ignatovich, V.I.; YuP, G. Prospects for the expansion of the tungsten mineral resource base. Razvedka i Okhrana Nedr = Prospect Protect. Miner. Resour. 2007, 12, 43–47. (In Russian) [Google Scholar]
- Wei, W.F.; Hu, R.Z.; Bi, X.W.; Peng, J.T.; Su, W.C.; Song, S.Q.; Shi, S.H. Infrared microthermometric and stable isotopic study of fluid inclusions in wolframite at the Xihuashan tungsten deposit, Jiangxi province, China. Miner. Deposita 2012, 47, 1–17. [Google Scholar] [CrossRef]
- Yang, J.-H.; Zhang, Z.; Peng, J.-T.; Liu, L.; Leng, C.-B. Metal source and wolframite precipitation process at the Xihuashan tungsten deposit, South China: Insights from mineralogy, fluid inclusion and stable isotope. Ore Geol. Rev. 2019, 111, 102965. [Google Scholar] [CrossRef]
- Borovikov, A.A.; Goverdovskii, V.A.; Borisenko, A.S.; Bryanskii, N.V.; Shabalin, S.I. Composition and metal contents of ore-forming fluids of the Kalguty Mo-W(Be) deposit (Gorny Altai). Russ. Geol. Geophys. 2016, 57, 647–662. (In Russian) [Google Scholar] [CrossRef]
- Bychkov, A.; Matveeva, S. Thermodynamic model of the formation of ore bodies at the Akchatau wolframite greisenvein deposit. Geochem. Int. 2008, 46, 867–886. [Google Scholar] [CrossRef]
- Besova, M.V. Geology and mineralogy of the Dzhidinsky tungsten deposit. In Mestorozhdeniya Redkikh i Malykh Metallov SSSR = Deposits of Rare and Minor Metals in the USSR; The USSR Academy of Sciences: Moscow/Leningrad, Russia, 1939; Volume 1, pp. 3–88. (In Russian) [Google Scholar]
- Neletov, P.I.; Shalaev, K.A.; Deulya, T.T. Geology of the Dzhidinsky Ore District; Irkutskoe Oblastnoe Izdatel’stvo: Irkutsk, Russia, 1941; p. 282. (In Russian) [Google Scholar]
- Smolyansky, E.N. On the basic regularities in the spatial distribution of molybdenum and tungsten deposits in the Dzhidinsky ore district. Trudy Vostochno-Sibirskogo geologicheskogo instituta SO AN SSSR. Seriya Geologicheskaya 1960, 1, 20–38. (In Russian) [Google Scholar]
- Ignatovich, V.I. Dykes and Molybdenum-Tungsten Mineralization of the Pervomaisk Intrusion of Granite-Porphyry (the Dzhidinsky Deposit); Buryatskoe knizhnoe izdatel’stvo: Ulan-Ude, Russia, 1959. (In Russian) [Google Scholar]
- Ignatovich, V.I. On the structure of the Dzhidinsky ore field. In Materialy po Geologii i po-Leznym Iskopaemym Bu-rASSR = Materials on Geology and Minerals of Buryat ASSR. Iss. 6; Buryatskoe knizhnoe izdatel’stvo: Ulan-Ude, Russia, 1961; pp. 3–22. (In Russian) [Google Scholar]
- Malinovsky, E.P. Defining the spatial location of the ore-forming sources of the Dzhidinsky deposits by the structural analysis data. In Dzhidinskii Rudnyi Raion = Dzhidinsky ore District; Mokhosoev, M., Ed.; Nauka: Novosibirsk, Russia, 1984; pp. 116–126. [Google Scholar]
- Ontoev, D.O. Staged Mineralization and Zoning of Transbaikalia Deposits; Nauka: Moscow, Russia, 1974; p. 244. (In Russian) [Google Scholar]
- Ontoev, D.O. Staged mineralization and zoning of molybdenum-tungsten deposits of the Dzhidinsky ore district. In Dzhidinskii Rudnyi Raion = Dzhidinsky Ore District; Nauka: Novosibirsk, Russia, 1984; pp. 53–76. (In Russian) [Google Scholar]
- Baturina, E.E.; Ripp, G.S. Molybdenum and Tungsten Deposits of Western Transbaikalia (the Main Metallogenic and Geochemical Features); Nauka: Moscow, Russia, 1984; p. 152. (In Russian) [Google Scholar]
- Gordienko, I.V.; Filimonov, A.V.; Minina, O.R.; Gornova, M.A.; Medvedev, A.Y.; Klimuk, V.S.; Elbaev, A.; Tomurtogoo, O. Dzhidinsky island-arc system of the Paleo-Asian Ocean: Structure and main stages of the geodynamic evolution in the Vendian-Paleozoic. Geologiya Geofizika 2007, 48, 120–140. (In Russian) [Google Scholar]
- Distanova, A.N. Late Paleozoic granite intrusions of the western part of the Dzhidinsky zone (Western Trans-baikalia). In Granitoidnye Kompleksy Sibiri = Granitoid Complexes of Siberia. Iss. 440; Kuznetsov, Y.A., Ed.; Nauka: Novosibirsk, Russia, 1979; pp. 3–23. (In Russian) [Google Scholar]
- Khodanovich, P.Y.; Smirnova, O.K. Tungsten-Bearing Beresite Rocks and Local Mineralization Prognosis; Nauka: Novosibirsk, Russia, 1991; p. 208. (In Russian) [Google Scholar]
- Khodanovich, P.Y. Molybdenum-tungsten deposits of the Dzhidinsky ore field. In Mestorozhdeniya Zabaikal’ya = Deposits of Transbaikalia. Vol. 1. Book 1; Geoinformmark: Chita/Moscow, Russia, 1995; pp. 149–163. (In Russian) [Google Scholar]
- Chernyshev, I.V.; Gol’tsman, Y.V.; Bairova, E.D.; Ivanova, G.F. Rb-Sr-geochronometry of the processes of granites sequential formation, greisenization and hydrothermal mineralization: Dzhidinsky W-Mo deposit, Western Transbaikalia. Doklady Akademii Nauk 1998, 360, 537–540. (In Russian) [Google Scholar]
- Reyf, F.G.; Bazheev, E.D. Magmatic Process and Tungsten Mineralization; Nauka: Novosibirsk, Russia, 1982; p. 158. (In Russian) [Google Scholar]
- Reyf, F.G. Conditions and Mechanisms of Formation of Granite Ore-Magmatic Systems (by the Data of Fluid Inclusions Studies); Institute of Mineralogy, Geochemistry, and Crystal Chemistry of Rare Elements: Moscow, Russia, 2009; p. 498. (In Russian) [Google Scholar]
- Povilaitis, M.M. The Main Mineralogical Features of the Dzhidinsky Molybdenum-Tungsten Deposit; The USSR Academy of Sciences: Moscow, Russia, 1960; p. 165. (In Russian) [Google Scholar]
- Povilaitis, M.M.; Mozgova, N.N.; Senderova, V.M. Bismuth minerals in the Dgidinsky molybdenum-tungsten deposit (West Transbaikal). Zapiski Vsesoyuznogo Mineral-Ogicheskogo Obshchestva 1969, 98, 655–664. (In Russian) [Google Scholar]
- Stel’machonok, K.Z. On the synchronization of the formation of the ore-bearing fractures and the molybdenum mineralization at the Pervomaisk stockwork deposit (Transbaikalia), and the causes of the fractures formation. Doklady Akademii Nauk 1994, 337, 382–385. (In Russian) [Google Scholar]
- Stel’machonok, K.Z. On the near-simultaneous formation of the single-system veins in the molybdenite stockwork ore body (the Dzhidinsky deposit, Transbaikalia). Doklady Akademii Nauk 1995, 341, 399–402. (In Russian) [Google Scholar]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; De Vivo, B., Frezzotti, M.L., Eds.; Verginia Tech: Blacksburg, VA, USA, 1994; pp. 117–130. [Google Scholar]
- Borisenko, A.S. Study of the salt composition of gas-liquid inclusions in minerals by the method of cryometry. Geologiya i Geofizika 1977, 8, 16–27. (In Russian) [Google Scholar]
- Shadlun, T.N.; Ontoev, D.O.; Basova, G.V.; Vjalsov, L.N.; Muravjeva, I.V. Copper and silver sulfobismuthite from the Djidinsky deposit. Zapiski Vsesoyuznogo Mineralog-Icheskogo Obshchestva. 1969, 98, 452–463. (In Russian) [Google Scholar]
- Goldstein, R.H.; Reynolds, T.J. Systematics of fluid inclusions in diagenetic minerals. SEPM Short C 1994, 31, 1–199. [Google Scholar]
- Lüders, V.; Romer, R.L.; Gilg, H.A.; Bodnar, R.J.; Pettke, T.; Misantoni, D. A geochemical study of the Sweet Home Mine, Colorado Mineral Belt, USA: Hydrothermal fluid evolution above a hypothesized granite cupola. Miner. Depos. 2009, 44, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Roedder, E. Fluid Inclusions. Rev. Mineral. 1984, 12, 644. [Google Scholar]
- Redder, E. Fluid Inclusions in Minerals; Mir: Moscow, Russia, 1987; pp. 1–2. (In Russian) [Google Scholar]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H. Fluid inclusion evidence for magmatic-hydrothermal fluid evolution in the porphyry copper-molybdenum deposit at Butte, Montana. Econ. Geol. 2008, 103, 307–334. [Google Scholar] [CrossRef]
- Tarantola, A.; Diamond, L.W.; Stünitz, H.; Thust, A.; Pec, M. Modification of fluid inclusions in quartz by deviatoric stress. III: Influence of principal stresses on inclusion density and orientation. Contrib. Miner. Pet. 2012, 164, 537–550. [Google Scholar] [CrossRef]
- Diamond, L.W.; Tarantola, A. Interpretation of fluid inclusions in quartz deformed by weak ductile shearing: Reconstruction of differential stress magnitudes and pre-deformation fluid properties. Earth Planet. Sci. Lett. 2015, 417, 107–119. [Google Scholar] [CrossRef]
- Naumov, V.B.; Dorofeev, V.A.; Mironova, O.F. Physical-chemical parameters of the hydrothermal deposits for-mation according to the studies of fluid inclusions. 1. Deposits of tin and tungsten. Geokhimiya 2011, 10, 1063–1082. (In Russian) [Google Scholar]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. HokieFlincs_H2O-NaCl: A Microsoft excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O—NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Bakker, R. AqSo_NaCl: Computer program to calculate p-T-V-x properties in the H2O-NaCl fluid system applied to fluid inclusion research and pore fluid calculation. Comput. Geosci. 2018, 115, 122–133. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. Synthetic fluid inclusions XX. Critical PTx properties of H2O–FeCl2 fluids. Geochim. Cosmochim. Acta 2015, 148, 50–61. [Google Scholar] [CrossRef]
- Polya, D.A. Pressure-dependence of wolframite solubility for hydrothermal vein formation. Trans. Inst. Min. Metall. Sect. B Appl. Earth Sci. 1990, 99, B120–B124. [Google Scholar]
- Heinrich, C.A. The chemistry of hydrothermal tin(−tungsten) ore deposition. Econ. Geol. 1990, 85, 457–481. [Google Scholar] [CrossRef]
- Samson, I.M. Fluid evolution and mineralization in a subvolcanic granite stock: The Mount Pleasant W-Mo-Sn deposits, New Brunswick, Canada. Econ. Geol. 1990, 85, 145–163. [Google Scholar] [CrossRef]
- Xi, B.B.; Zhang, D.H.; Zhou, L.M.; Zhang, W.H.; Wang, C. Characteristics of ore-forming fluid evolution in Dajishan tungsten deposit, Quannan county, Jiangxi (in Chinese with English abstract). Acta Geol. Sin. 2008, 82, 956–966. [Google Scholar]
- Gong, Q.J.; Yu, C.W.; Zhang, R.H. Physical chemistry study on the ore-forming process of Shizhuyuan tungsten-polymetallic deposit (in Chinese with English abstract). Earth Sci. Front. 2004, 11, 617–625. [Google Scholar]
- So, C.S.; Yun, S.T. Origin and evolution of W-Mo-producing fluids in a granitic hydrothermal system: Geochemical studies of quartz vein deposits around the Susan Granite, Hwanggangri District, Republic of Korea. Econ. Geol. 1994, 89, 246–267. [Google Scholar] [CrossRef]
- Graupner, T.; Kempe, U.; Dombon, E.; Pätzold, O.; Leeder, O.; Spooner, E.T.C. Fluid regime and ore formation in the tungsten (−yttrium) deposits of Kyzyltau (Mongolian Altai): Evidence for fluid variability in tungsten-tin ore systems. Chem. Geol. 1999, 154, 21–58. [Google Scholar] [CrossRef]
- Yokart, B.; Barr, S.M.; Williams-Jones, A.E.; Macdonald, A.S. Latestage alteration and tin-tungsten mineralization in the Khuntan Batholith, northern Thailand. J. Asian Earth Sci. 2003, 21, 999–1018. [Google Scholar] [CrossRef]
- Beuchat, S.; Moritz, R.; Pettke, T. Fluid evolution in the W-Cu-Zn-Pb San Cristobal vein, Peru: Fluid inclusion and stable isotope evidence. Chem. Geol. 2004, 210, 201–224. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhao, Z.; Chou, I.M. Roles of carbonate/CO2 in the formation of quartz-vein wolframite deposits: Insight from the crystallization experiments of hubnerite in alkali-carbonate aqueous solutions in a hydrothermal diamond-anvil cell. Ore Geol. Rev. 2018, 95, 40–48. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, C. Wolframite solubility and precipitation in hydrothermal fluids: Insight from thermodynamic modeling. Ore Geol. Rev. 2020, 117, 103289. [Google Scholar] [CrossRef]

| Assemblage | Inkur Deposit | Kholtoson Deposit | |
|---|---|---|---|
| Minerals | |||
| Gangue minerals | Major | Quartz SiO2 | Quartz SiO2 |
| Minor | Fluorite CaF2 | Fluorite CaF2 | |
| K-feldspar KAlSi3O8 | K-feldspar KAlSi3O8 | ||
| Muscovite KAl2[AlSi3O10](OH)2 | Muscovite KAl2[AlSi3O10](OH)2 | ||
| Ore minerals | Major | Hubnerite MnWO4 | Hubnerite MnWO4 |
| Pyrite FeS2 | Pyrite FeS2 | ||
| Chalcopyrite CuFeS2 | Chalcopyrite CuFeS2 | ||
| Minor | Sphalerite ZnS | Sphalerite ZnS | |
| Galena PbS | Galena PbS | ||
| Tetrahedrite Cu3SbS3 | Tetrahedrite Cu3SbS3 | ||
| Aikinite PbCuBiS3 | Aikinite PbCuBiS3 | ||
| Molybdenite MoS2 | Scheelite—CaWO4 | ||
| Scheelite CaWO4 | Hessite Ag2Te | ||
| - | Cassiterite SnO2 | Stannite Cu2FeSnS4 | |
| - | - | Siderite FeCO3 Rhodochrosite—MnCO3 | |
| Rare | Hessite Ag2Te | Schapbachite Ag0.4Bi0.4S | |
| Bornite Cu5FeS4 | Bornite Cu5FeS4 | ||
| Beryl Al2[Be3(Si6O18)] | Matildite—AgBiS2 | ||
| - | - | Unknown phases Cu2PbS3, Cu2Pb3S5 | |
| Secondary minerals | Anglesite PbSO4 | Anglesite PbSO4 | |
| Rosnbergite(?)AlF[F0.5(H2O)0.5]4 · H2O | Cerussite PbCO3 | ||
| № | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | Na2O | K2O | F | H2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inkur Deposit | |||||||||||
| 1. | 47.22 | - | 28.57 | 0.42 | 0.61 | 2.44 | - | 12.08 | 3.06 | 4.5 | 99.11 |
| 2. | 47.48 | 0.33 | 28.52 | 0.46 | 0.53 | 2.72 | - | 12.36 | 2.43 | 4.5 | 99.42 |
| 3. | 47.99 | - | 28.84 | 0.33 | 0.68 | 2.27 | - | 12.30 | 2.35 | 4.5 | 99.48 |
| 4. | 48.74 | - | 26.90 | - | 0.50 | 3.55 | - | 12.55 | 3.16 | 4.5 | 100.13 |
| 5. | 48.50 | - | 29.44 | - | 0.64 | 2.46 | - | 12.01 | 3.00 | 4.5 | 100.78 |
| 6. | 49.05 | - | 27.35 | - | 0.94 | 2.93 | - | 12.23 | 3.13 | 4.5 | 100.22 |
| 7. | 47.78 | - | 28.14 | 1.31 | 1.02 | 2.34 | - | 12.33 | 2.14 | 4.5 | 99.92 |
| 8. | 48.27 | - | 29.00 | - | 0.73 | 2.56 | - | 12.27 | 2.43 | 4.5 | 100.12 |
| 9. | 47.87 | - | 28.19 | 0.96 | 0.37 | 2.38 | - | 12.67 | 2.80 | 4.5 | 99.97 |
| 10. | 47.30 | - | 31.69 | 0.93 | 0.51 | 1.48 | 0.32 | 12.29 | 1.74 | 4.5 | 100.99 |
| 11. | 47.96 | - | 30.20 | - | 0.46 | 2.15 | 0.32 | 12.31 | 2.60 | 4.5 | 100.59 |
| Kholtoson Deposit | |||||||||||
| 1. | 47.96 | - | 29.93 | 0.44 | 0.72 | 2.26 | - | 11.56 | 1.96 | 4.5 | 99.33 |
| 2. | 46.38 | - | 29.97 | 0.68 | 0.59 | 1.89 | 0.38 | 11.84 | 2.48 | 4.5 | 98.71 |
| 3. | 48.63 | 0.44 | 29.91 | 0.00 | 0.54 | 2.64 | 0.32 | 11.68 | 1.74 | 4.5 | 100.08 |
| 4. | 48.14 | 0.35 | 29.46 | 0.48 | 0.4 | 2.5 | - | 11.58 | 2.69 | 4.5 | 100.1 |
| 5. | 48.18 | - | 27.4 | 1.18 | 0.92 | 2.39 | 0.39 | 11.3 | 1.73 | 4.5 | 97.99 |
| 6. | 48.8 | - | 29.93 | 0.44 | 1.38 | 2.16 | - | 11.58 | 2.47 | 4.5 | 101.26 |
| 7. | 46.81 | - | 31.5 | 0.37 | 0.67 | 1.66 | - | 12.02 | 2.52 | 4.5 | 100.05 |
| 8. | 47.41 | - | 29.19 | 0 | 1.15 | 2.17 | - | 12.00 | 3.21 | 4.5 | 99.63 |
| 9. | 50.83 | - | 25.74 | 0.00 | 0.63 | 3.73 | - | 11.22 | 2.50 | 4.5 | 98.84 |
| № | Fe | Mn | Ca | W | O | Total |
|---|---|---|---|---|---|---|
| Inkur Deposit | ||||||
| 1. | - | 18.69 | - | 63.44 | 16.94 | 99.08 |
| 2. | - | 18.06 | - | 63.84 | 17.61 | 99.50 |
| 3. | 0.61 | 17.76 | - | 62.82 | 18.05 | 99.25 |
| 4. | 0.59 | 17.78 | - | 63.13 | 18.40 | 99.88 |
| 5. | - | 18.78 | - | 63.03 | 17.84 | 99.64 |
| 6. | - | 18.31 | - | 63.97 | 17.43 | 99.72 |
| Kholtoson Deposit | ||||||
| 7. | 18.66 | - | 63.01 | 18.56 | 100.23 | |
| 8. | 0.49 | 19.34 | - | 62.48 | 17.83 | 100.14 |
| 9. | 0.53 | 18.24 | - | 63.37 | 17.64 | 99.78 |
| 10. | 0.46 | 18.70 | - | 62.97 | 17.73 | 99.86 |
| 11. | 0.98 | 17.86 | - | 62.86 | 18.33 | 100.03 |
| 12. | 0.87 | 18.15 | - | 62.96 | 17.67 | 99.64 |
| 13. | - | 18.41 | - | 64.21 | 16.53 | 99.16 |
| 14. | 0.64 | 18.42 | - | 62.73 | 18.04 | 99.82 |
| 15. | - | 18.52 | - | 64.16 | 17.10 | 99.78 |
| 16. | - | - | 14.18 | 66.00 | 20.54 | 100.72 |
| 17. | - | - | 14.11 | 67.30 | 19.20 | 100.61 |
| 18. | - | - | 14.24 | 66.99 | 18.83 | 100.06 |
| 19. | - | - | 14.37 | 66.69 | 18.56 | 99.62 |
| 20. | 0.93 | - | 13.68 | 65.09 | 19.34 | 99.04 |
| 21. | 1.25 | - | 14.15 | 62.81 | 22.27 | 100.47 |
| № | Fe | Co | Cu | Zn | Ag | Cd | Sn | Mo | Pb | Bi | Sb | Te | As | S | O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | - | - | 11.09 | - | - | - | - | - | 40.94 | 32.46 | - | - | - | 15.77 | - | 100.27 |
| 2. | - | - | 10.71 | - | - | - | - | - | 41.51 | 31.83 | - | - | - | 15.52 | - | 99.58 |
| 3. | - | - | 10.60 | - | - | - | - | - | 42.72 | 30.84 | - | - | - | 15.01 | - | 99.16 |
| 4. | - | - | 10.63 | - | - | - | - | - | 41.84 | 31.36 | - | - | - | 15.59 | - | 99.41 |
| 5. | - | - | - | - | - | - | 71.88 | - | - | - | - | - | - | - | 27.54 | 99.41 |
| 6. | 30.19 | - | 34.81 | - | - | - | - | - | - | - | - | - | - | 34.99 | - | 99.99 |
| 7. | 30.01 | - | 35.05 | - | - | - | - | - | - | - | - | - | - | 34.06 | - | 99.13 |
| 8. | 30.19 | - | 34.63 | - | - | - | - | - | - | - | - | - | - | 34.13 | - | 98.95 |
| 9. | - | - | - | - | 3.77 | - | - | - | 77.72 | 5.31 | - | - | - | 12.52 | - | 99.31 |
| 10. | - | - | - | - | 6.69 | - | - | - | 76.35 | 3.32 | - | - | - | 12.33 | - | 98.69 |
| 11. | - | - | - | - | 3.5 | - | - | - | 77.97 | 6.69 | - | - | - | 12.28 | - | 100.44 |
| 12. | - | - | - | - | 2.61 | - | - | - | 80.83 | 4.15 | - | - | - | 12.76 | - | 100.36 |
| 13. | - | - | - | - | 2.58 | - | - | - | 81.01 | 4.01 | - | - | - | 11.92 | - | 99.52 |
| 14. | - | - | - | - | 3.66 | - | - | - | 78.52 | 5.23 | - | - | - | 12.36 | - | 99.77 |
| 15. | - | - | - | - | 2.88 | - | - | - | 80.25 | 5.4 | - | - | - | 12.49 | - | 101.02 |
| 16. | - | - | - | - | 7.24 | - | - | - | 76.75 | 4.09 | - | - | - | 12.14 | - | 100.22 |
| 17. | - | - | - | - | - | - | - | - | 87.23 | - | - | - | - | 12.26 | - | 99.49 |
| 18. | - | - | - | - | - | - | - | - | 86.99 | - | - | - | - | 12.23 | - | 99.22 |
| 19. | - | - | - | - | 63.69 | - | - | - | - | - | - | 36.85 | - | - | - | 100.54 |
| 20. | - | - | - | - | 61.35 | - | - | - | - | - | - | 37.02 | - | - | - | 98.37 |
| 21. | - | - | - | - | 58.87 | - | - | - | - | - | - | 35.23 | - | - | - | 94.1 |
| 22. | - | - | - | - | 58.68 | - | - | - | - | - | - | 39.72 | - | - | - | 98.4 |
| 23. | - | - | - | - | 57.81 | - | - | - | - | - | - | 40.29 | - | - | - | 98.1 |
| 24. | - | - | - | - | - | - | - | 55.82 | - | - | - | - | - | 43.95 | - | 99.76 |
| 25. | 46.64 | - | - | - | - | - | - | - | - | - | - | - | - | 52.51 | - | 99.14 |
| 26. | 46.88 | - | - | - | - | - | - | - | - | - | - | - | - | 52.7 | - | 99.58 |
| 27. | 47.5 | - | - | - | - | - | - | - | - | - | - | - | - | 53.44 | - | 100.94 |
| 28. | 46.6 | - | - | - | - | - | - | - | - | - | - | - | - | 52.78 | - | 99.38 |
| 29. | 46.49 | 0.48 | - | - | - | - | - | - | - | - | - | - | - | 52.59 | - | 99.56 |
| 30. | 46.34 | 0.56 | - | - | - | - | - | - | - | - | - | - | - | 52.24 | - | 99.14 |
| 31. | - | - | - | 66.66 | - | 0.58 | - | - | - | - | - | - | - | 33.06 | - | 100.3 |
| 32. | - | - | - | 67.42 | - | 0.66 | - | - | - | - | - | - | - | 32.78 | - | 100.85 |
| 33. | - | - | - | 65.8 | - | 0.53 | - | - | - | - | - | - | - | 32.81 | - | 99.14 |
| 34. | - | - | - | 66.45 | - | 0.69 | - | - | - | - | - | - | - | 32.73 | - | 99.86 |
| 35. | - | - | - | 66.15 | - | 0.62 | - | - | - | - | - | - | - | 32.2 | - | 98.97 |
| 36. | 1.21 | - | - | 64.29 | - | 0.67 | - | - | - | - | - | - | - | 32.57 | - | 98.74 |
| 37. | - | - | - | 65.57 | - | 0.96 | - | - | - | - | - | - | - | 32.59 | - | 99.12 |
| 38. | - | - | - | 64.97 | - | 0.94 | - | - | - | - | - | - | - | 32.45 | - | 98.36 |
| 39. | 1.48 | - | 38.21 | 6.53 | 0.85 | - | - | - | - | 1.59 | 18.23 | - | 6.63 | 24.85 | - | 98.37 |
| 40. | 0.45 | - | 39.98 | 7.26 | - | - | - | - | - | - | 22.14 | - | 4.50 | 25.93 | - | 100.26 |
| 41. | - | - | 39.07 | 7.26 | 0.85 | - | - | - | - | 1.52 | 21.83 | - | 5.27 | 25.84 | - | 101.64 |
| 42. | - | - | 38.79 | 6.83 | 0.95 | - | - | - | - | - | 22.07 | - | 4.95 | 25.67 | - | 99.25 |
| 43. | - | - | 39.37 | 7.44 | 0.8 | - | - | - | - | - | 21.04 | - | 5.04 | 25.73 | - | 99.43 |
| 44. | 0.52 | - | 39.36 | 7.84 | 0.84 | - | - | - | - | - | 22.20 | - | 5.08 | 25.35 | - | 101.2 |
| 45. | 0.52 | - | 38.45 | 7.62 | - | - | - | - | - | - | 19.76 | - | 6.57 | 25.96 | - | 98.87 |
| 46. | - | - | 38.07 | 7.58 | 1.08 | - | - | - | - | - | 21.93 | - | 4.80 | 25.31 | - | 98.77 |
| 47. | 0.38 | - | 38.84 | 9.09 | - | - | - | - | - | - | 18.59 | - | 7.07 | 25.53 | - | 99.51 |
| 48. | 0.46 | - | 39.22 | 7.07 | 0.65 | - | - | - | - | - | 17.21 | - | 8.80 | 25.71 | - | 99.12 |
| 49. | - | - | 37.89 | 7.09 | 1.21 | - | - | - | - | - | 22.07 | - | 4.95 | 25.48 | - | 98.69 |
| № | Fe | Co | Cu | Zn | Ag | Cd | Sn | Pb | Bi | Sb | Te | As | S | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | - | - | 11.19 | - | - | - | - | 41.67 | 31.90 | - | - | - | 15.15 | 99.91 |
| 2. | - | - | 11.60 | - | - | - | - | 42.87 | 30.86 | - | - | - | 14.92 | 100.25 |
| 3. | - | - | 11.67 | - | - | - | - | 42.00 | 31.29 | - | - | - | 15.15 | 100.13 |
| 4. | - | - | 10.82 | - | - | - | - | 42.24 | 31.64 | - | - | - | 15.26 | 99.96 |
| 5. | - | - | 30.82 | - | - | - | - | 48.52 | - | - | - | - | 23.12 | 102.46 |
| 6. | - | - | 30.69 | - | - | - | - | 47.94 | - | - | - | - | 22.75 | 101.38 |
| 7. | - | - | 13.89 | - | - | - | - | 69.38 | - | - | - | - | 16.49 | 99.75 |
| 8. | - | - | 13.47 | - | - | - | - | 69.23 | - | - | - | - | 16.32 | 99.01 |
| 9. | 11.94 | - | 59.43 | - | - | - | - | - | - | - | - | - | 28.03 | 99.39 |
| 10. | 11.43 | - | 59.09 | - | - | - | - | - | - | - | - | - | 28.71 | 99.24 |
| 11. | 30.81 | - | 34.86 | - | - | - | - | - | - | - | - | - | 34.99 | 100.65 |
| 12. | 30.21 | - | 33.83 | - | - | - | - | - | - | - | - | - | 35.34 | 99.38 |
| 13. | 30.57 | - | 33.51 | - | - | - | - | - | - | - | - | - | 35.21 | 99.30 |
| 14. | - | - | - | - | 1.44 | - | - | 82.62 | 3.15 | - | - | - | 12.32 | 99.54 |
| 15. | - | - | - | - | 0.83 | - | - | 85.46 | 1.95 | - | - | - | 11.93 | 100.16 |
| 16. | - | - | - | - | - | - | - | 87.7 | - | - | - | - | 12.81 | 100.51 |
| 17. | - | - | - | - | 1.34 | - | - | 83.85 | 3.08 | - | - | - | 12.70 | 100.97 |
| 18. | - | - | - | - | 1.99 | - | - | 83.61 | 2.88 | - | - | - | 12.49 | 100.97 |
| 19. | - | - | - | - | 1.26 | - | - | 84.09 | 2.29 | - | - | - | 12.63 | 100.27 |
| 20. | - | - | - | - | 63.79 | - | - | - | - | - | 37.19 | - | - | 100.98 |
| 21. | - | - | - | - | 63.18 | - | - | - | - | - | 37.19 | - | - | 100.37 |
| 22. | 46.63 | 0.54 | - | - | - | - | - | - | - | - | - | - | 52.34 | 99.51 |
| 23. | 47.46 | - | - | - | - | - | - | - | - | - | - | - | 53.08 | 100.54 |
| 24. | 47.11 | - | - | - | - | - | - | - | - | - | - | - | 53.01 | 100.13 |
| 25. | 46.52 | 0.52 | - | - | - | - | - | - | - | - | - | - | 52.85 | 99.89 |
| 26. | - | - | - | - | 27.43 | - | - | 10.05 | 46.12 | - | - | - | 15.98 | 99.58 |
| 27. | - | - | - | - | 27.73 | - | - | 5.05 | 50.86 | - | - | - | 15.91 | 99.55 |
| 28. | - | - | - | - | 28.77 | - | - | 6.06 | 50.59 | - | - | - | 16.49 | 101.92 |
| 29. | - | - | - | 65.91 | - | 1.34 | - | - | - | - | - | - | 31.99 | 99.23 |
| 30. | - | - | - | 66.38 | - | 1.15 | - | - | - | - | - | - | 32.15 | 99.68 |
| 31. | 0.41 | - | - | 65.46 | - | 0.95 | - | - | - | - | - | - | 32.2 | 99.02 |
| 32. | - | - | - | 67.11 | - | 1.13 | - | - | - | - | - | - | 32.11 | 100.34 |
| 33. | 0.34 | - | - | 65.36 | - | 1.1 | - | - | - | - | - | - | 32.66 | 99.46 |
| 34. | - | - | - | 66.26 | - | 1.18 | - | - | - | - | - | - | 32.83 | 100.28 |
| 35. | 0.3 | - | - | 66.42 | - | 0.99 | - | - | - | - | - | - | 32.31 | 100.03 |
| 36. | 0.55 | - | - | 65.59 | - | 0.64 | - | - | - | - | - | - | 32.87 | 99.64 |
| 37. | - | - | - | 66.61 | - | 0.8 | - | - | - | - | - | - | 33.01 | 100.41 |
| 38. | 8.52 | - | 37.46 | 7.16 | - | - | 17.79 | - | - | - | - | - | 29.07 | 100.01 |
| 39. | 8.95 | - | 37.57 | 5.13 | - | - | 19.12 | - | - | - | - | - | 29.6 | 100.37 |
| 40. | 9.71 | - | 38.19 | 4.79 | - | - | 18.41 | - | - | - | - | - | 29 | 100.09 |
| 41. | 9.76 | - | 37.63 | 4.53 | - | - | 18.49 | - | - | - | - | - | 29.39 | 99.8 |
| 42. | 8.74 | - | 38.01 | 6.46 | - | - | 17.83 | - | - | - | - | - | 28.76 | 99.81 |
| 43. | 9.28 | - | 37.65 | 5.21 | - | - | 18.36 | - | - | - | - | - | 28.93 | 99.43 |
| 44. | 10.42 | - | 37.1 | 5.14 | - | - | 18.83 | - | - | - | - | - | 29.34 | 100.83 |
| 45. | - | - | 40.11 | 7.63 | - | - | - | - | - | 20.19 | - | 6.13 | 25.79 | 99.85 |
| 46. | - | - | 38.78 | 7.03 | - | - | - | 0.00 | 2.26 | 20.03 | - | 5.49 | 25.81 | 99.41 |
| 47. | - | - | 38.83 | 7.47 | 0.49 | - | - | 2.18 | - | 21.09 | - | 4.77 | 25.66 | 100.50 |
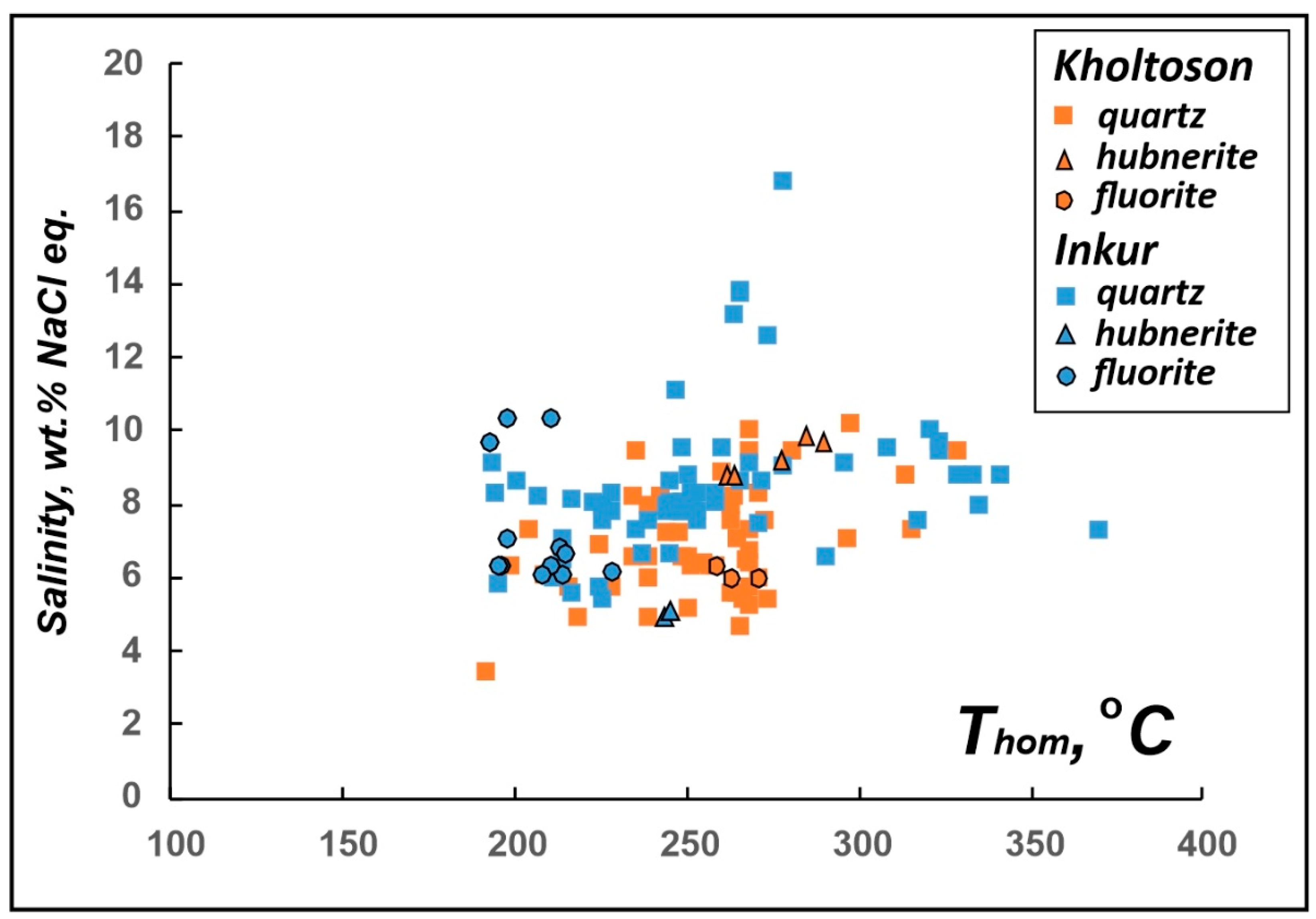
| Deposit | Host Mineral | FI Type | Th | Tice | Teut | Tsdph | Salinity Equivalent NaCl wt.% [3] | Salt System Type [4] | |
|---|---|---|---|---|---|---|---|---|---|
| °C | |||||||||
| Inkur | Quartz | - | ≥343…195 | −4.2…−10.6 | −52…−50…−49.2 (−23.4…−23—accelerated ice melting) | - | 6.7–14.6 | CaCl2-MgCl2-H2O CaCl2-KCl-H2O CaCl-H2O NaCl-KCl-H2O | |
| Fluorite | - | ≥265…195 | −7…−3.8 | -55 … -49 (−24…−23.2—accelerated ice melting) | 184…187 | 6.2–10.5 | CaCl2-NaCl-H2O CaCl-H2O NaCl-KCl-H2O | ||
| Hubnerite | - | ≥278…245 | −3.2…−3.1 | - | - | 5.1–5.3 | - | ||
| Muscovite | - | ≥202…167 | −3.5 | - | - | 5.7 | - | ||
| Kholtoson | Quartz | Homogenious | ≥344…210 | −7.2…−2.9 | −38…−36 −50…−49 −55 | - | 4.8–10.7 | MgCl-KCl-H2O NaCl-FeCl2-H2O FeCl3-H2O CaCl2-KCl-H2O CaCl-H2O CaCl2-NaCl-H2O | |
| Heterogenious | a—vapor-dominated | ≥413…350 (homogenization in vapor) | - | - | - | - | - | ||
| b—liquid-dominated | ≥400…370 | ~−4.4 | −48…47? | - | ~7 | CaCl-H2O | |||
| Fluorite | - | - | ≥272…260 | −3.9…−3.7 | −49…−48 | - | 6-6.3 | CaCl-H2O | |
| Hubnerite | - | - | ≥290…250 | −6.5…−5.7 | −55…54 | - | 8.8–9.9 | CaCl2-NaCl-H2O | |
| № | Sample № | Specimen № | The Average Value of Characteristics Determined from Two Extractions, wt.% | |||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ca | Mg | Na | K | Ca/Na | |||
| 1 | 25−1 | Ink-11 | 0.06 | 5.43 | 0.12 | 0.84 | 0.99 | 7.45 |
| 2 | 26−1 | Ink-16 | 0.02 | 3.58 | 0.08 | 1.92 | 0.73 | 2.139 |
| 3 | 27−1 | Ink-26 | 0.03 | 2.38 | 0.05 | 2.34 | 0.63 | 1.164 |
| 4 | 28−1 | Ink-28 | 0 | 1.90 | 0.07 | 1.98 | 0.62 | 1.101 |
| 5 | 36−1 | Khol-14 | 0.02 | 5.15 | 0.19 | 0.78 | 1.37 | 7.574 |
| 6 | 37−1 | Khol-20 | 0.04 | 2.10 | 0.13 | 1.08 | 1.29 | 2.225 |
| 7 | 38−1 | Khol-23 | 0.02 | 2.28 | 0.11 | 1.25 | 0.88 | 2.092 |
| 8 | 39−1 | Khol-25 | 0.01 | 0.84 | 0.03 | 1.24 | 0.3 | 0.776 |
| 9 | 40−1 | Khol-25 | 0.34 | 0.99 | 0.16 | 1.68 | 2.16 | 0.675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damdinova, L.B.; Damdinov, B.B. Tungsten Ores of the Dzhida W-Mo Ore Field (Southwestern Transbaikalia, Russia): Mineral Composition and Physical-Chemical Conditions of Formation. Minerals 2021, 11, 725. https://doi.org/10.3390/min11070725
Damdinova LB, Damdinov BB. Tungsten Ores of the Dzhida W-Mo Ore Field (Southwestern Transbaikalia, Russia): Mineral Composition and Physical-Chemical Conditions of Formation. Minerals. 2021; 11(7):725. https://doi.org/10.3390/min11070725
Chicago/Turabian StyleDamdinova, Ludmila B., and Bulat B. Damdinov. 2021. "Tungsten Ores of the Dzhida W-Mo Ore Field (Southwestern Transbaikalia, Russia): Mineral Composition and Physical-Chemical Conditions of Formation" Minerals 11, no. 7: 725. https://doi.org/10.3390/min11070725
APA StyleDamdinova, L. B., & Damdinov, B. B. (2021). Tungsten Ores of the Dzhida W-Mo Ore Field (Southwestern Transbaikalia, Russia): Mineral Composition and Physical-Chemical Conditions of Formation. Minerals, 11(7), 725. https://doi.org/10.3390/min11070725





