New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods
Abstract
:1. Introduction
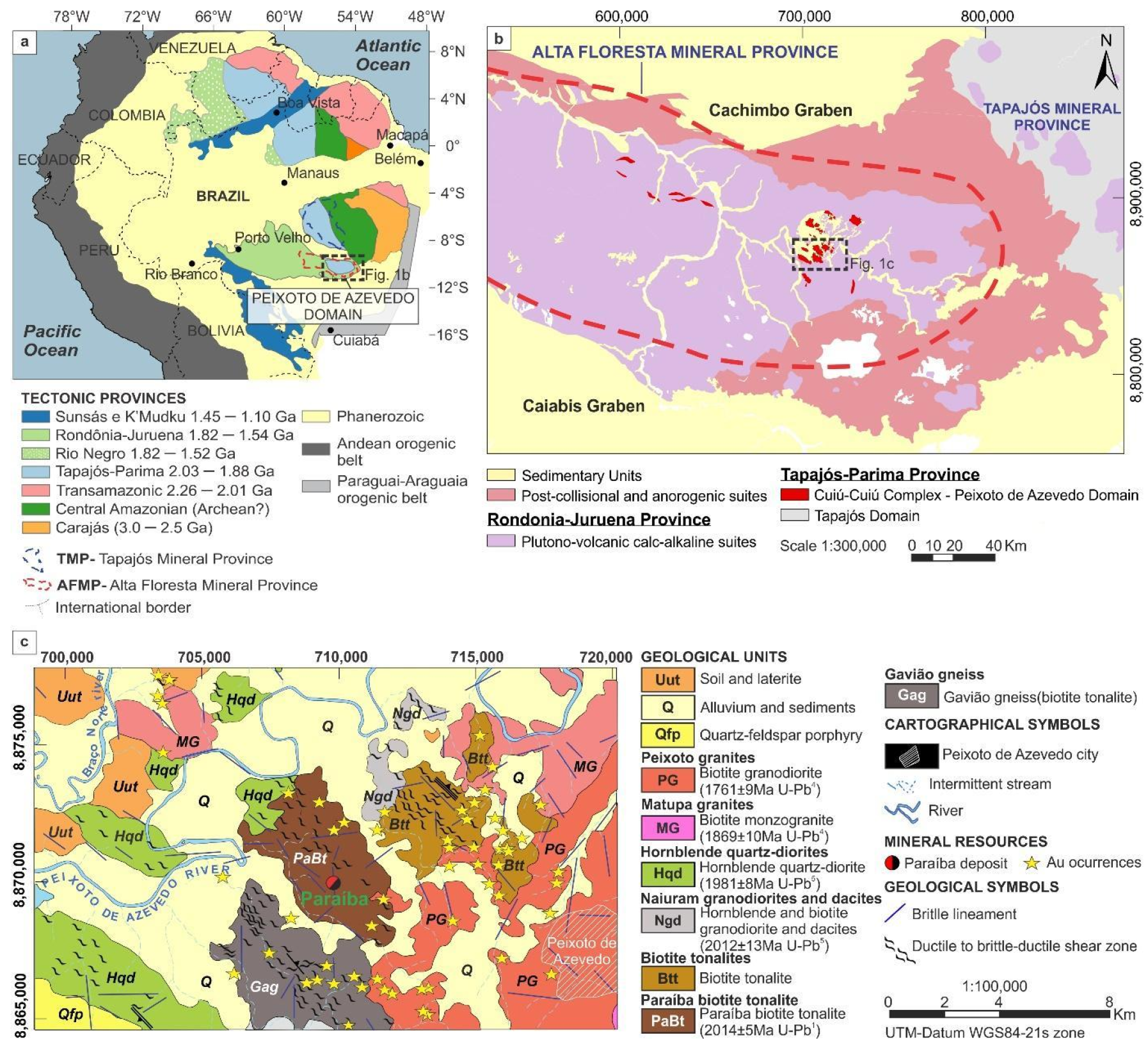
2. Geological Framework
2.1. Eastern Sector Geology of the AFMP
2.2. Local Geology and Characteristics of the Paraíba Deposit
Paraíba Vein
3. Material and Methods
3.1. Field Work
3.2. Reflectance Spectroscopy
3.3. Petrography and Mineral Chemistry
3.4. Imaging Spectroscopy
4. Results
4.1. Distribution and Petrography of Host Rocks
4.2. Hydrothermal Alteration and Mineralization
4.2.1. Group 1 Hydrothermal Alteration Zones
Illitic Zone
Biotite Zone
Chloritic Zone
Gold-Quartz Vein
4.2.2. Group 2 Hydrothermal Alteration Zones
Calcic Zone
Potassic Zone
Phyllic Zone
Propylitic Zone
Epidote Zone
Late Phases and Barren Veinlets
4.3. Mineral Chemistry
4.3.1. White Mica
4.3.2. Biotite-Phlogopite
4.3.3. Chlorite
4.3.4. Epidote
5. Data Integration, Discussion, and Conclusion
5.1. Spectral, Mineral Chemical, and Au-Cu Data Integration
5.2. Interpretation
5.3. Implications for Mineral Exploration
6. Final Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paes de Barros, A.J. Granitos da Região de Peixoto de Azevedo—Novo Mundo e Mineralizações Auríferas Relacionadas—Província Aurífera Alta Floresta (MT). Ph.D. Thesis, Instituto de Geociências, Universidade Estadual de Campinas, Campinas, Brazil, 2007; 154p. [Google Scholar]
- Crusader 2018. Brazilian Gold Clear Path to Production & Committed to Building Value. Available online: https://www.investi.com.au/api/announcements/brv/6fc29655-02d.pdf (accessed on 16 October 2022).
- Paes de Barros, A.J. Contribuição a Geologia e Controle das Mineralizações Auríferas de Peixoto de Azevedo-MT. Master’s Thesis, Instituto de Geociências, Universidade de São Paulo, São Paulo, Brazil, 1994; 145p. [Google Scholar]
- Siqueira, A.J.B. Geologia da Mina de Ouro Filão do Paraíba, Região de Peixoto de Azevedo, Norte de Mato Grosso. Master’s Thesis, Instituto de Geociências, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 1997; 98p. [Google Scholar]
- Mesquita, M.J.; Teixeira, R.; Trevisan, V.; Xavier, R.; Assis, R.; Quispe, P.; Moretti, M.; Agnoletto, E.; Paes de Barros, A.; Miguel, E., Jr. Gold deposits in ductile shear zone of the Paleoproterozoic Alta Floresta Province (Brazil). In Proceedings of the 13th Society for Geology Applied to Mineral Deposits, Nancy, France, 24–27 August 2015. [Google Scholar]
- Pimenta, V.A. Alteração Hidrotermal e Deformação no Depósito do Peteca, Província Aurífera de Alta Floresta (PAAF), Região de Peixoto de Azevedo-MT. Master’s thesis, Institute of Geosciences, UNICAMP, Campinas, Brazil, 2018; 152p. [Google Scholar]
- Santos, J.O.S.; Groves, D.I.; Hartmann, A.; Moura, M.A.; McNaughton, N.J. Gold deposits of the Tapajós and Alta Floresta domains, Tapajós-Parima orogenic belt, Amazon Craton, Brazil. Miner. Depos. 2001, 36, 278–299. [Google Scholar] [CrossRef]
- Moreton, L.C.; Martins, E.G. Geologia e Recursos Minerais de Alta Floresta. Vila Guarita. Escala 1:250.000; Serviço Geológico do Brasil/CPRM: Brasília, Brazil, 2005; 68p. [Google Scholar]
- Silva, M.G.; Abram, M.B. Projeto Metalogenia da Província Aurífera Juruena-Teles Pires, Mato Grosso; Serviço Geológico Brasileiro, CPRM: Oiânia, Brazil, 2008; 212p. [Google Scholar]
- Mesquita, M.J.; Gomes, M.E.B.; Moreira, I.C.; Paes, R.A.S.; Martins, H.E.S.; Matos, J.H.; Ruggiero, A.; Primo, G.M.A.; Ducart, D.F.; Poggi, L.; et al. Paleoproterozoic Gold Deposits at Alta Floresta Mineral Province, Brazil: Two Overprinted Mineralising Events? Geological Society, Special Publications: London, UK, 2022; Volume 516, p. SP516-2021-64. [Google Scholar] [CrossRef]
- Moura, M.A.; Botelho, N.F.; Olívio, G.R.; Kyser, T.K. Granite-related Paleoproterozoic, Serrinha gold deposit, Southern Amazonia, Brazil: Hydrothermal alteration, fluid inclusion and stable isotope constraints on genesis and evolution. Econ. Geol. 2006, 101, 585–605. [Google Scholar] [CrossRef]
- Trevisan, V.G. Estudo Comparativo Entre Mineralizações Filonares de Au ± Cu e Au + Metais de base do Setor Leste da Província de Alta Floresta (MT), Cráton Amazônico. Master’s Thesis, Instituto de Geociências, UNICAMP, Campinas, Brazil, 2015; 129p. [Google Scholar]
- Assis, R.R.; Xavier, R.P.; Creaser, R.A. Linking the Timing of Disseminated Granite-Hosted Gold-Rich Deposits to Paleoproterozoic Felsic Magmatism at Alta Floresta Gold Province, Amazon Craton, Brazil: Insights from Pyrite and Molybdenite Re-Os Geochronology. Econ. Geol. 2017, 112, 1937–1957. [Google Scholar] [CrossRef]
- Assis, R.R. Depósitos Auríferos Associados ao Magmatismo Granítico do Setor Leste da Província de Alta Floresta (MT), Cráton Amazônico: Tipologia das Mineralizações, Modelos Genéticos e Implicações Prospectivas. Master’s Thesis, Institute of Geosciences, UNICAMP, Campinas, Brazil, 2011; p. 474. (In Portuguese). [Google Scholar]
- Assis, R.R. Depósitos Auríferos Associados ao Magmatismo Félsico da Província de Alta Floresta (MT), Cráton Amazônico: Idade das Mineralizações, Geoquímica e Fonte dos Fluidos. Doctorate’s Thesis, Institute of Geociences, UNICAMP, Campinas, Brazil, 2015; 363p. [Google Scholar]
- Moreira, I.C. Petrogênese dos Granitóides do Depósito Paraíba, Domínio Peixoto de Azevedo, Província Aurífera de alta Floresta, Cráton Amazonas. Master’s Thesis, Institute of Geociences, UNICAMP, Campinas, Brazil, 2019; 92p. [Google Scholar]
- Hauff, P.L.; Kruse, F.A.; Madrid, R.J. Gold exploration using illite polytypes defined by X-ray diffraction and reflectance spectroscopy: Gold Forum on Technology and Practices. In Proceedings of the “World Gold 89”, Society for Mining, Metallurgy and Exploration, Littleton, CO, USA, 1989; pp. 76–82. [Google Scholar]
- Tappert, M.; Rivard, B.; Giles, D.; Tappert, R.; Mauger, A. Automated drill core logging using visible and near-infrared reflectance spectroscopy: A case study from the Olympic Dam IOCG deposit, South Australia. Econ. Geol. 2011, 106, 289–296. [Google Scholar] [CrossRef]
- Laukamp, C.; Cudahy, T.; Cleverly, J.S.; Oliver, N.H.S.; Hewson, R. Airborne hyperspectral imaging of hydrothermal alteration zones in granitoids of the Eastern fold belt, Mount Isa inlier, Australia. Geochem. Explor. Environ. Anal. 2011, 11, 3–24. [Google Scholar] [CrossRef]
- Roache, T.J.; Walshe, J.L.; Huntington, J.F.; Quigley, M.A.; Yang, K.; Bil, B.W.; Blake, K.L.; Hyvärinen, T. Epidote-clinozoisite as hyperspectral tool in exploration for Archean gold. Aust. J. Earth Sci. 2011, 58, 813–822. [Google Scholar] [CrossRef]
- Alva-Jimenez, T.; Tosdal, R.M.; Dilles, J.H.; Dipple, G.; Kent, A.J.R.; Halley, S. Chemical Variations in Hydrothermal White Mica Across the Highland Valley Porphyry Cu-Mo District, British Columbia, Canada. Econ. Geol. 2020, 115, 903–926. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of Rocks and Minerals, and Principles of Spectroscopy. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing, 3rd ed.; Geological Survey Denver: Denver, CO, USA, 1999; Volume 3, Chapter 1. [Google Scholar]
- Hook, S.; Abbott, E.; Grove, C.; Kahle, A.; Palluconi, F. Multispectral thermal infrared in geologic studies. In Manual of Remote Sensing; Rencz, A., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 59–110. [Google Scholar]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Halley, S.; Dilles, J.H.; Tosdal, R.M. Footprints: Hydrothermal alteration and geochemical dispersion around porphyry copper deposits. Soc. Econ. Geol. Newsl. 2015, 100, 1–29. [Google Scholar] [CrossRef]
- Chmielowski, R.M.; Jansson, N.; Fjellerad Persson, M.; Fagerström, P. 3D modelling of hydrothermal alteration associated with VHMS deposits in the Kristineberg area, Skellefte district, northern Sweden. Miner. Depos. 2016, 51, 113–130. [Google Scholar] [CrossRef]
- Neal, L.C.; Wilkinson, J.J.; Mason, P.J.; Chang, Z. Spectral characteristics of propylitic alteration minerals as a vectoring tool for porphyry copper deposits. J. Geochem. Explor. 2018, 184, 179–198. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, F. Analysis of spectral absorption features in hyperspectral imagery. Int. J. Appl. Earth Obs. Geoinf. 2004, 5, 55–68. [Google Scholar] [CrossRef]
- Reyes, A.G. Petrology of Philippine geothermal systems and the application of alteration mineralogy to their assessment. J. Volcanol. Geotherm. Res. 1990, 43, 279–309. [Google Scholar] [CrossRef]
- Mathieu, M.; Roy, R.; Launeau, P.; Cathelineau, M.; Quirt, D. Alteration mapping on drill cores using a HySpex SWIR-320m hyperspectral camera: Application to the exploration of an unconformity-related uranium deposit (Saskatchewan, Canada). J. Geochem. Explor. 2017, 172, 71–88. [Google Scholar] [CrossRef]
- Graham, G.E.; Kokaly, R.F.; Kelley, K.D.; Hoefen, T.M.; Johnson, M.R.; Hubbard, B.E. Application of imaging spectroscopy for mineral exploration in Alaska: A study over porphyry Cu deposits in the Eastern Alaska Range. Econ. Geol. 2018, 113, 489–510. [Google Scholar] [CrossRef]
- Santos, J.O.S.; Hartmann, L.A.; Gaudette, H.E.; Groves, D.I.; McNaughton, N.J.; Fletcher, I.R. A New Understanding of the Provinces of the Amazon Craton Based on Integration of Field Mapping and U-Pb and Sm-Nd Geochronology. Gondwana Res. 2000, 3, 453–488. [Google Scholar] [CrossRef]
- Lacerda Filho, J.V.; Abreu Filho, W.; Valente, C.R.; Oliveira, C.C.; Albuquerque, M.C. Geologia e Recursos Minerais do Estado de Mato Grosso (Escala 1:1.000.000); Mapa; Convênio entre Secretaria de Estado de Indústria, Comércio, Minas e Energia (SICME-MT) e Serviço Geológico do Brasil (CPRM-Goiânia): Goiânia, Brazil, 2004; 200p. [Google Scholar]
- Vasquez, M.L.; Rosa-Costa, L.T. Geologia e Recursos Minerais do Estado do Pará (Escala 1:1.000.000); Mapa; Serviço Geológico do Brasil (CPRM-Belém): Belén, Brazil, 2008; 328p. [Google Scholar]
- Quispe, P.E. Geologia, Geoquímica e Geocronologia dos Granitoides Foliados e Rochas Subvulcânicas da Região de Peixoto de Azevedo, Setor Leste da AFGP, MT. Master’s Thesis, Institute of Geosciences, UNICAMP, Campinas, Brazil, 2016. [Google Scholar]
- Silva, F.R. Geoquímica e Geocronologia U-Pb (SHRIMP) de Granitos da Região de Peixoto de Azevedo—Província Aurífera de Alta Floresta–MT. Master’s Thesis, Universidade Federal de Mato Grosso, Cuiabá, Brasil, 2014; 82p. [Google Scholar]
- Tassinari, C.C.G.; Macambira, M.J.B. Geochronological Provinces of the Amazonian Craton. Episodes 1999, 22, 174–182. [Google Scholar] [CrossRef]
- Santos, J.O.S. Os Terrenos Paleoproterozóicos da Província do Tapajós e as Mineralizações de Ouro Associadas. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2000; 208p. [Google Scholar]
- Alves, C.L.; Rizzotto, G.J.; Rios, F.S.; Sant’Ana, M.A.B. The Orosirian Cuiú-Cuiú arc in Peixoto de Azevedo domain, Southern of Amazonian craton. J. South Am. Earth Sci. 2020, 102, 102648. [Google Scholar] [CrossRef]
- Miguel, E., Jr. Mineralizações auríferas do Lineamento Peru-Trairão, Província Aurífera de Alta Floresta-MT: Controle Estrutural e Idade U-Pb das Rochas Hospedeiras. Master’s Thesis, Institute of Geosciences, UNICAMP, Campinas, Brazil, 2011; p. 81. (In Portuguese). [Google Scholar]
- Dezula, S.E.M.; Barros, M.A.S.A.; Pierosan, R.; Santos, J.O.S.; Assis, R.R. Granito Aragão—Suíte intrusiva Nhandú—Um granito oxidado, tipo A2, de 1967 a 1964 Ma na Província Aurífera Alta Floresta—Cráton Amazônico. Geologia USP. Série Científica 2018, 18, 3–20. [Google Scholar] [CrossRef]
- Barros, M.A.S.; Barros, A.J.P.; Santos, J.O.S.; Rocha, M.L.B.P. Extension of the Tapajós domains to the Alta Floresta gold province: Evidence from U-Pb SHRIMP ages of the Nhandu intrusive suite at 1962 and 1967 Ma; 14◦ Simpósio de Geologia da Amazônia, Belém: Pará, Brasil, 2015. (In Portuguese) [Google Scholar]
- Moura, M.A. Omaciço Granítico Matupá no Depósito de ouro Serrinha (MT): Petrologia, Alteração Hidrometal e Metalogenia. Ph.D. Thesis, Institute of Geosciences, Universidade de Brasília, Brasília, Brazil, 1998; 238p. [Google Scholar]
- Rocha, M.L.B.P.; Junior, F.C.; Santos, J.O.S.; Sant’Ana, M.A.B.; Pinho, F.E.C.; Neal, J.M.; da Costa, P.C.; Roberts, C. U-Th-Pb Shrimp dating of hydrothermal monazite from the Trairão Gold Deposit—Alta Floresta Gold Province (Amazon Craton). Braz. J. Geol. 2020, 50. [Google Scholar] [CrossRef]
- Pinho, M.A.S.B.; Chemale, F., Jr.; Van Schumus, W.R.; Pinho, F.E.C. U-Pb and Sm-Nd evidence for 1.76–1.77 Ga magmatism in the Moriru region, Mato Grosso, Brazil: Implications for province boundaries in the SW Amazon Craton. Precambrian Res. 2003, 126, 1–25. [Google Scholar] [CrossRef]
- Duarte, T.B.; Rodrigues, J.B.; Ribeiro, P.S.E.; Scandolara, J.E. Tectonic evolution of the Juruena magmatic arc between the Aripuanã and Juruena rivers: Northwest Mato Grosso State, Brazil. Braz. J. Geol. 2012, 42, 824–840. [Google Scholar] [CrossRef]
- Scandolara, J.E.; Correa, R.T.; Fuck, R.A.; Souza, V.S.; Rodrigues, J.B.; Ribeiro, P.S.E.; Frasca, A.A.S.; Saboia, A.M.; Lacerda Filho, J.V. Paleo-Mesoproterozoic arc accretion along the southwestern margin of the Amazonian craton: The Juruena accretionary orogen and possible implications for Columbia supercontinent. J. South Am. Earth Sci. 2017, 73, 223–247. [Google Scholar] [CrossRef]
- Rizzotto, G.J.; Alves, C.L.; Rios, F.S.; Barros, M.A.D.S.A. The Nova Monte Verde metamorphic core complex: Tectonic implications for the southern Amazonian craton. J. South Am. Earth Sci. 2019, 91, 154–172. [Google Scholar] [CrossRef]
- Rizzotto, G.J.; Alves, C.L.; Rios, F.S.; Barros, M.A.D.S.A. The Western Amazonia Igneous Belt. J. South Am. Earth Sci. 2019, 96, 102326. [Google Scholar] [CrossRef]
- Alves, C.L.; Rizzotto, G.J.; Rios, F.S.; Gonçalves, G.F. Áreas de Relevante Interesse Mineral: Projeto Evolução Crustal e Metalogenia da Província Mineral Juruena-Teles-Pires; CPRM Serviço Geológico do Brasil: Brasília, Brazil, 2019; p. 231. (In Portuguese) [Google Scholar]
- Souza, J.P.; Frasca, A.A.S.; Oliveira, C.C. Geologia e Recursos Minerais da Província Mineral de Alta Floresta; Relatório Integrado; Serviço Geológico Brasileiro, CPRM: Brasília, Brazil, 2005; 164p. [Google Scholar]
- Leite, J.A.D.; Saes, G.S. Geocronologia Pb/Pb de zircões detríticos e análise estratigráfica das coberturas sedimentares proterozóicas do Sudoeste do Cráton Amazônico. Geol. USP Sér. Cien. 2003, 3, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, V.G.; Xavier, R.; Assis, R.R.; Bartolomeu, J.O.; Matos, J.H.S.N.; Mesquita, M.J. Paleoproterozoic porphyry-related Au-(Cu, Mo) systems in the Alta Floresta Gold Province, southern Amazonian Craton, Brazil: The case of the X1 and Paraíba deposits. In Proceedings of the 14th Biennial Meeting 2017, Quebec City, QC, Canada, 20–23 August 2017. [Google Scholar]
- Trevisan, V.G.; Xavier, R.P.; Hagemann, S.; Kemp, A.; Loucks, R.; Gao, J.-F. Geology setting and metallogenesis of the Alta Floresta Province, southern Amazon Craton (Brazil). In Proceedings of the 15th Biennial SGA Meeting, Glasgow, UK, 27–30 August 2019; Volume 3, pp. 1109–1112. [Google Scholar]
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.M.; Lowers, H.A.; Driscoll, R.L.; et al. USGS Spectral Library Version 7: US Geological Survey Data Series; USGS: Denver, CO, USA, 2017; Volume 1035, 61p. [Google Scholar] [CrossRef] [Green Version]
- Haest, M.; Cudahy, T.; Laukamp, C.; Gregory, S. Quantitative mineralogy from infrared spectroscopic data. I. Validation of mineral abundance and composition scripts at the Rocklea channel iron deposit in Western Australia. Econ. Geol. 2012, 107, 209–228. [Google Scholar] [CrossRef]
- Prado, E.M.G.; Silva, A.M.; Ducart, D.F.; Toledo, C.L.B.; de Assis, L.M. Reflectance spectroradiometry applied to a semi-quantitative analysis of the mineralogy of the N4ws deposit, Carajás Mineral Province, Pará, Brazil. Ore Geol. Rev. 2016, 78, 101–119. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Sways, G.A. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef] [Green Version]
- Duke, E.F. Near infrared spectra of muscovite, Tschermak substitution, and metamorphic reaction progress: Implications for remote sensing. Geology 1994, 22, 621–624. [Google Scholar] [CrossRef]
- Guidotti, C.V. Micas in metamorphic rocks. In Micas, Reviews in Mineralogy; Bailey, S.W., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 13, pp. 357–467. [Google Scholar]
- Miyashiro, A.; Shido, F. Tschermak Substitution in Low- and Middle-grade Pelitic Schists. J. Petrol. 1985, 26, 449–487. [Google Scholar] [CrossRef]
- Herrmann, W.; Blake, M.D.; Doyle, M.G.; Huston, D.L.; Kamprad, J.; Merry, N.; Pontual, S. Short wavelength infrared (SWIR) spectral analysis of hydrothermal alteration zones associated with base metal sulfide deposits at Rosebery and Western Tharsis, Tasmania, and Highway-Reward, Queensland. Econ. Geol. 2001, 96, 939–955. [Google Scholar] [CrossRef]
- Guggenheim, S.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Eberl, D.D.; Formoso, M.L.L.; Galan, E.; Merriman, R.J.; Peacor, D.R.; et al. Order, disorder and crystallinity in phyllosilicates and the use of the “Crystallinity Index”. Report of the association Internationale pour l’Etude des Argiles (AIPEA). Clay Miner. 2002, 37, 389–393. [Google Scholar] [CrossRef]
- Doublier, M.P.; Roache, T.; Potel, S. Short-wavelength infrared spectroscopy: A new petrological tool in low-grade to very low-grade pelites. Geology 2010, 38, 1031–1034. [Google Scholar] [CrossRef]
- Kamps, O.M.; Ruitenbeek, F.J.A.; Mason, P.R.D.; Van der Meer, F.D. Near-Infrared Spectroscopy of Hydrothermal versus Low-Grade Metamorphic Chlorites. Minerals 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Ye, M.; Han, H.; Ren, G.; Han, L.; Zhang, Z. Near-infrared spectroscopic study of chlorite minerals. J. Spectrosc. 2018, 2018, 6958260. [Google Scholar] [CrossRef]
- Lypaczewski, P.; Rivard, B. Estimating the Mg# and AlVI content of biotite and chlorite from shortwave infrared reflectance spectroscopy: Predictive equations and recommendations for their use. Appl. Earth Obs. Geoinf. 2018, 68, 116–126. [Google Scholar]
- Boardman, J.W.; Kruse, F.A.; Green, R.O. Mapping target signatures via partial unmixing of AVIRIS data. In Summaries of the Fifth Annual JPL Airborne Earth Science Workshop. 1: AVIRIS Workshop; JPL: Pasadena, CA, USA, 1995; pp. 23–26. [Google Scholar]
- Guidotti, C.V. Compositional variation of muscovite in medium- to high-grade metapelites of northwestern Maine. Am. Mineral. 1987, 63, 878–884. [Google Scholar]
- Tappert, M.C.; Rivard, B.; Giles, D.; Tappert, R.; Mauger, A.J. The mineral chemistry, near-infrared, and mid-infrared reflectance spectroscopy of phengite from the Olympic Dam IOCG deposit, South Australia. Ore Geol. Rev. 2013, 53, 26–38. [Google Scholar] [CrossRef]
- Foster, M.D. Interpretation of the Composition and a Classification of the Chlorites; US Geological Survey Professional Paper 414-A; US Geological Survey: Washington, DC, USA, 1962; 33p. [Google Scholar]
- Bourdelle, F.; Cathelineau, M. Low-temperature chlorite geothermometry: A graphical representation based on a T–R2+ –Si diagram. Eur. J. Mineral. 2015, 27, 617–626. [Google Scholar] [CrossRef]
- Wiewióra, A.; Weiss, Z. Crystallochemical classifications of phyllosilicates based on the unified system of projection of chemical composition: II The chlorite group. Clay Miner. 1990, 25, 8392. [Google Scholar] [CrossRef]
- Kartashov, P. Clasification diagram for REE-bearing members of the epidote group based on crystallochemical data. In Workshop on Accessory Minerals; Univ Warsaw: Warsaw, Poland, 2014; pp. 19–21. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to Rock-Forming Minerals, 2nd ed.; Longman Scientific & Technical: Essex, UK, 1992; 696p. [Google Scholar]
- Bourdelle, F.; Parra, T.; Chopin, C.; Beyssac, O. A new chlorite geothermometer for diagenetic to low-grade meta- morphic conditions. Contrib. Mineral. Petrol. 2013, 165, 723–735. [Google Scholar] [CrossRef]
- Jefferies, S.P.; Holdsworth, R.E.; Wibberley, C.A.J.; Shimamoto, T.; Spiers, C.J.; Niemeijer, A.R.; Lloyd, G.E. The nature and importance of phyllonite development in crustal-scale fault cores: An example from the Median Tectonic Line, Japan. J. Struct. Geol. 2006, 28, 220–235. [Google Scholar] [CrossRef]
- Sibson, R.H.; Robert, F.; Poulsen, K.H. High-angle reverse faults, fluid-pressure cycling, and mesothermal gold-quartz deposits. Geology 1988, 16, 551–555. [Google Scholar] [CrossRef]
- Groves, D.; Goldfarb, R.; Gebre-Mariam, M.; Hagemann, S.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Robert, F.; Hart, C.J.R. Gold deposits in metamorphic belts: Overview of current understanding, outstanding problems, future research, and exploration significance. Econ. Geol. 2003, 98, 1–29. [Google Scholar]
- Hagemann, S.; Cassidy, K.F. Archean orogenic lode gold deposits. In Gold in 2000 (Reviews in Economic Geology); Hagemann, S.G., Brown, P.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Volume 13, pp. 9–68. [Google Scholar] [CrossRef]
- Robert, F.; Poulsen, K.H. Vein formation and dformation in greenstone gold deposits. Rev. Econ. Geol. 2001, 14, 111–155. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Groves, D.I.; Gardoll, S. Orogenic gold and geologic time: A global synthesis. Ore Geol. Rev. 2001, 18, 1–75. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Deng, J.; Yang, L.-Q.; Li, R.-H.; Groves, D.I.; Santosh, M.; Wang, Z.-L.; Sai, S.-X.; Wang, S.-R. Regional structural control on the distribution of world-class gold deposits: An overview from the Giant Jiaodong Gold Province, China. Geol. J. 2018, 54, 378–391. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Goldfarb, R.J.; Zhang, L. Structural geometry of orogenic gold deposits: Implications for exploration of world-class and giant deposits. Geosci. Front. 2018, 9, 1163–1177. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Deng, J.; Wang, Q.; Yang, L.; Zhang, L. A holistic model for the origin of orogenic gold deposits and its implications for exploration. Miner. Depos. 2020, 55, 275–292. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Zhang, L. A scale-integrated exploration model for orogenic gold deposits based on a mineral system approach. Geosci. Front. 2020, 11, 719–738. [Google Scholar] [CrossRef]
- Powell, R.; Will, T.M.; Phillips, G.N. Metamorphism in Archean greenstone belts: Calculated fluid compositions and implications for gold mineralization. J. Metamorph. Geol. 1991, 9, 141–150. [Google Scholar] [CrossRef]
- Cassidy, K.F.; Groves, D.; Mcnaughton, N. Late-Archaean granitoid-hosted lode-gold deposits, Yilgarn Craton, Western Australia: Deposit characteristics, crustal architecture and implications for ore genesis. Ore Geol. Rev. 1998, 13, 65–102. [Google Scholar] [CrossRef]
- Santos, A.C.; Geraldes, M.C.; Santos, W.H.; Ferreira, L.O. Paraíba Ore and the Alta Floresta Gold Province (SW Amazon Craton): Petrography, Pb and S Isotopes in Pyrite from Mineralized Vein. Anu. Inst. Geociênc. UFRJ 2018, 41, 690–701. [Google Scholar] [CrossRef]
- Lowell, J.D.; Guilbert, J.M. Lateral and vertical alteration-mineralization zoning in porphyry ore deposits. Econ. Geol. 1970, 65, 373–408. [Google Scholar] [CrossRef]
- Gustafson, L.B.; Hunt, J.P. The Porphyry Copper Deposit at El Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Cao, K.; Yang, Z.M.; Mavrogenes, J.; White, N.C.; Ji, F.-X.; Li, Y.; Li, W.-K. Geology and Genesis of the Giant Pulang Porphyry Cu-Au District, Yunnan, Southwest China. Soc. Econ. Geol. 2019, 114, 275–301. [Google Scholar] [CrossRef]













| White Mica Groups | ||||||
|---|---|---|---|---|---|---|
| Method | Characteristics | Wm–1 | Wm–2 | Wm–3 | Wm–4 | Wm–5 |
| Mineral description, petrography | Alteration group | 1 | 1 | 2 | 2 | 2 |
| Alteration zone | illitic | chloritic | phyllic | epidote | late phases | |
| Alteration type | pervasive/selective | pervasive | pervasive | pervasive | fissural | |
| Structurally controlled | no | phyllonitic foliation | no | breccia | veinlet | |
| Mineral assemblage | Wm–1 + Qz | Chl–2 + Wm–2 + Cb + Qz + Ttn + Py + Ccp ± native Au | Wm–3 +Rt + Ilm + Qz | Wm–4 + Ep–2 + Qz ± Cal ± Py ± Ccp ± Mol | Qz + Wm–5 | |
| Habit | fine grain-size lamellar | lamellar fish | lamellar to fibro-radial | tabular | fibro-radial | |
| Grain size (μm) | 5 and 80 | 50–200 | 200–1000 | 50–150 | 20–100 | |
| Mineralization | barren | Au | barren | Au, Cu, Mo | barren | |
| Reflectance spectroscopy | Al-OH absorption wavelength range (nm) | 2198–2207 | 2210–2225 | 2207–2215 | 2213–2221 | 2212–2215 |
| Al-OH absorption wavelength composition | Ms | Ms/Phg to Phg | Ms/Phg to Phg | Ms/Phg to Phg | Ms/Phg to Phg | |
| Crystallinity | 1–2.3 (Ill/Sm to Ill) | 1–15 (Ill/Sm to Ms) | 4–8 (Ms) | 4–15 (Ms) | 4–8 (Ms) | |
| EPMA data | Si (apfu) | 3.16 to 3.20 | 3.15 to 3.31 | 3.09 to 3.20 | 3.17 to 3.24 | 3.11 to 3.17 |
| Al (t) (apfu) | 2.44 to 2.47 | 2.23 to 2.44 | 2.32 to 2.47 | 2.22 to 2.46 | 2.36 to 2.40 | |
| Fe (t) (apfu) | 0.18 to 0.20 | 0.11 to 0.22 | 0.21 to 0.26 | 0.12 to 0.20 | 0.20 to 0.27 | |
| Mg (apfu) | 0.17 to 0.18 | 0.23 to 0.47 | 0.18 to 0.26 | 0.24 to 0.46 | 0.26 to 0.28 | |
| Biotite Groups | |||||
|---|---|---|---|---|---|
| Method | Characteristics | Bt–1 | Bt–2 | Bt–3 | Bt–4 |
| Mineral description, petrography | Alteration group | 1 | 1 | 1 | 2 |
| Alteration zone | biotite | biotite | biotite | potassic | |
| Alteration type | pervasive | pervasive | pervasive | pervasive | |
| Structurally controlled | phyllonitic foliation | phyllonitic foliation | phyllonitic foliation | no | |
| Mineral assemblage | Bt + Cal + Py + Ccp | Bt + Cal + Py + Ccp | Phl + Cal + Py + Ccp | Bt + Mc + Qz + Anh + Mag-Hem | |
| Habit/natural color pleocroism | lamellar fish yellowish to brown | lamelar fish yellowish to brown | lamelar fish light brown to dark greenish | lamellar light to dark brown | |
| Grain size (μm) | 25 and 100 | 25 and 500 | 1000 to 3000 | 1000 to 3000 | |
| Mineralization | traces of Au | traces of Au | traces of Au | no | |
| Reflectance spectroscopy | Fe-OH absorption wavelength range (nm) | ~2253 | ~2250 | ~2246 | ~2250 |
| Mg-OH absorption wavelength composition (nm) | ~2354 | ~2352 | ~2346 and ~2380 | ~2352 | |
| EPMA data | Si (apfu) | 2.88 to 2.99 | 2.78 to 2.89 | 2.94 to 3.27 | 2.83 |
| Al (t) (apfu) | 1.09 to 1.25 | 1.31 to 1.44 | 0.81 to 1.19 | 1.41 | |
| Fe (t)/(Fe (t) + Mg) | 0.23 to 0.30 | 0.29 to 0.35 | 0.11 to 0.18 | 0.41 | |
| Mg/Fe | 2.32 to 3.34 | 1.89 to 2.41 | 4.46 to 8.41 | 1.44 | |
| Mn (apfu) | 0.01 to 0.02 | 0.05 to 0.06 | 0.02 to 0.03 | 0.02 | |
| Chlorite Groups | ||||
|---|---|---|---|---|
| Method | Characteristic | Chl–1 | Chl–2 | Chl–3 |
| Mineral description, petrography | Alteration group | 1 | 1 | 2 |
| Alteration zone | chloritic | chloritic | propylitic | |
| Alteration type | pervasive | pervasive | pervasive/fissural | |
| Structurally controlled | phyllonitic foliation | phyllonitic foliation | no | |
| Mineral assemblage | Chl–1 + Cal+ Tnt + Qz + Py + Ccp | Chl–2 + Wm–2 + Cal+ Qz + Ttn + Py + Ccp ± native Au | Chl–3 + Ep–1 + Cb + Py ± Ccp | |
| Habit | lamellar fish | lamellar | massive | |
| Grain size (μm) | 120-200 | 200 to 1000 | 200 and 750 | |
| Mineralization | barren | traces of Au | barren | |
| Reflectance spectroscopy | Mg-OH absorption wavelength range (nm) | 2337–2340 | 2331–2339 | 2340–2344 |
| Al-OH absorption wavelength composition | Mg-Fe chlorite | Mg-Fe chlorite | Fe-Mg chlorite | |
| EPMA data | Si (apfu) | 2.74 to 2.79 | 2.84 to 3.00 | 2.72 to 2.98 |
| Al (t) (apfu) | 2.47 to 2.45 | 2.08 to 2.39 | 2.17 to 2.59 | |
| Fe (t) (apfu) | 1.36 to 1.48 | 1.33 to 1.59 | 1.72 to 1.82 | |
| Mg (apfu) | 3.08 to 3.25 | 3.10 to 3.49 | 2.76 to 2.99 | |
| Mn (apfu) | 0.05 to 0.12 | 0.04 to 1.12 | 0.04 to 0.05 | |
| Crystallization temperature (°C) | 350 | 300 | 220 to 350 | |
| Epidote Groups | ||||
|---|---|---|---|---|
| Method | Characteristic | Ep–1 | Ep–2 | Ep–3 |
| Mineral description, petrography | Alteration group | 2 | 2 | 2 |
| Alteration zone | propylitic | epidote | epidote | |
| Alteration type | pervasive | cement | cement | |
| Structurally controlled | no | breccia | overprint the phyllonite rocks | |
| Mineral assemblage | Ep–1 + Chl–3 + Cal + Py ± Qz | Ep–2 + Cal + Wm–4 + Py + Ccp + Mol + ± native Au | Ep–2 + Cal + Wm–4 + Py + Ccp + Mol + ± native Au | |
| Habit | sub-idiomorphic prismatic | large idiomorphic crystals | large idiomorphic crystals to massive | |
| Grain size (μm) | 1 to 50 | 100 to 500 | 100 to 500 | |
| Mineralization | no | Au, Cu, Mo | Au, Cu, Mo | |
| Reflectance spectroscopy | OH-related absorption wavelength (nm) | 1542 to 1545 | 1542 to 1545 | 1542 to 1559 |
| Al-OH absorption wavelength composition | epidote endmember | epidote endmember | Al-rich clinozoisite endmember | |
| EPMA data | Si (apfu) | 3.0 | 3.0 | 3.0 |
| Al (t) (apfu) | 2.15 to 2.2 | 2.1 to 2.3 | 2.1 to 2.4 | |
| Fe (t) (apfu) | 0.81 to 0.89 | 0.9 to 0.8 | 0.7 to 1.0 | |
| Ca (apfu) | 1.92 to 1.95 | 1.8 to 2.0 | 1.9 to 2.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggi, L.; Ducart, D.F.; Mesquita, M.J.; Moreira, I.C.; Gomes, M.E.B.; de Souza Filho, C.R. New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods. Minerals 2022, 12, 1327. https://doi.org/10.3390/min12101327
Poggi L, Ducart DF, Mesquita MJ, Moreira IC, Gomes MEB, de Souza Filho CR. New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods. Minerals. 2022; 12(10):1327. https://doi.org/10.3390/min12101327
Chicago/Turabian StylePoggi, Luciano, Diego Fernando Ducart, Maria José Mesquita, Igor Camargo Moreira, Márcia Elisa Boscato Gomes, and Carlos Roberto de Souza Filho. 2022. "New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods" Minerals 12, no. 10: 1327. https://doi.org/10.3390/min12101327
APA StylePoggi, L., Ducart, D. F., Mesquita, M. J., Moreira, I. C., Gomes, M. E. B., & de Souza Filho, C. R. (2022). New Insights into the Evolution and Footprints of the Paraíba Au-Cu-Mo Deposit, Alta Floresta Mineral Province (Brazil), through Integration of Spectral and Conventional Methods. Minerals, 12(10), 1327. https://doi.org/10.3390/min12101327







