Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
2.2.1. Sample Preparation and Saturation Experiments
2.2.2. Characterization of Shale Saturated with Different Fluids
3. Results and Discussion
3.1. Mineralogical and Chemical Characteristics of Shale Treated with Different Fluids
3.2. SEM Measurements
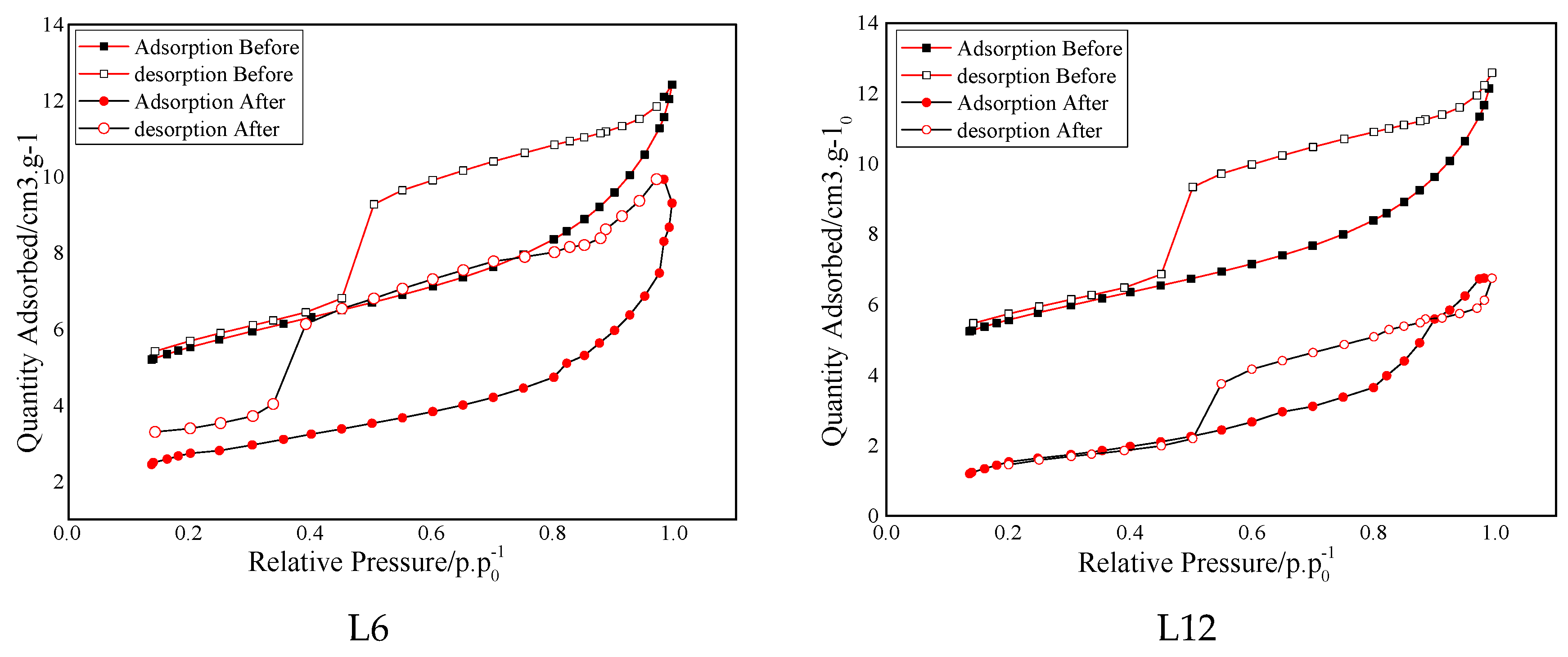
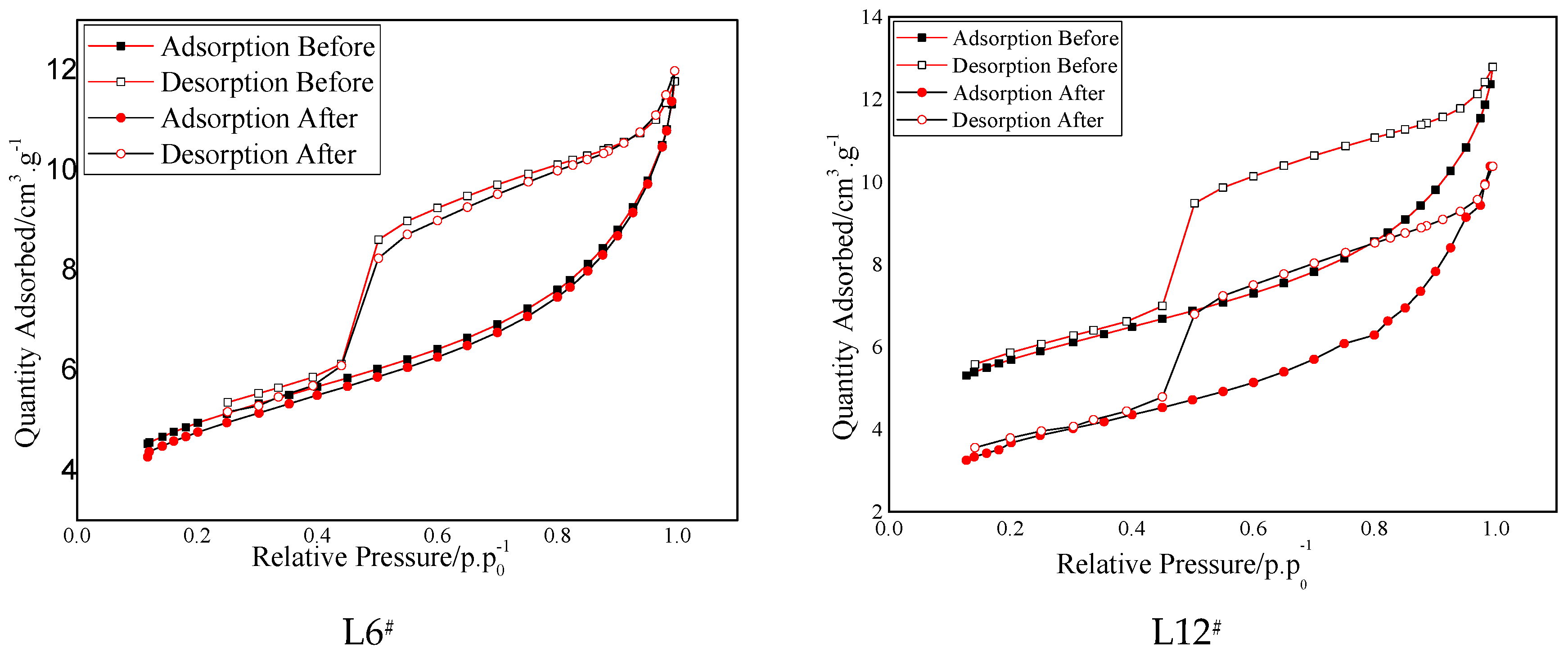
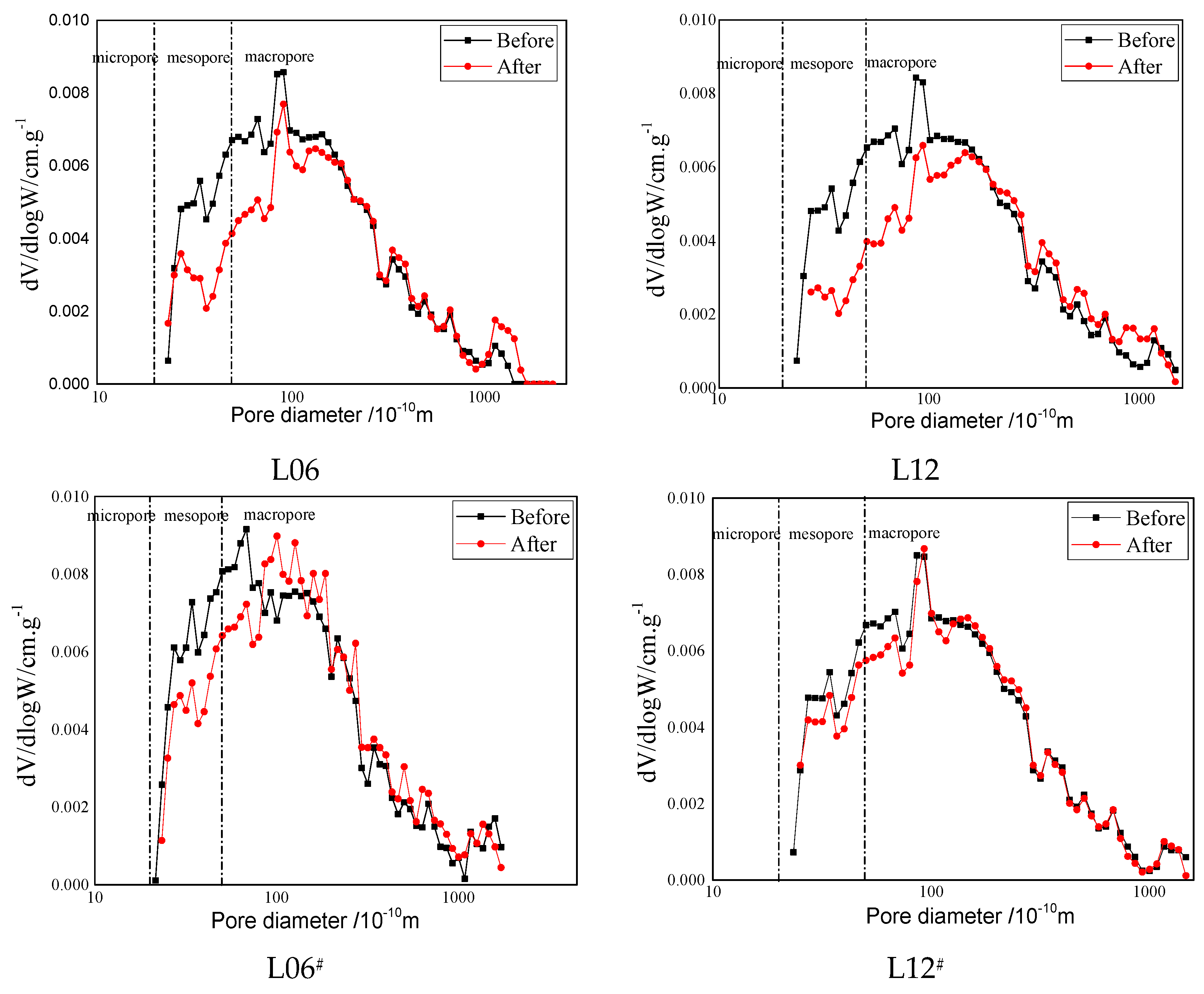
3.3. Low-Pressure Gas Adsorption Test
4. Conclusions
- The XRF analysis revealed that the content of Si increased slightly, while Ca, Al, Fe, and K contents decreased to 10.34%, 3.36%, and 1.37%, respectively, with the increase in CO2 pressure and the addition of brine. XRD results showed that the Longmaxi shale was mainly composed of quartz, dolomite clay, and calcite, with a total content of over 80%, and the content of quartz and dolomite increased, while the content of clay and calcite decreased slightly after saturation.
- The FESEM analysis results indicate that the surface of the shale sample became rougher, and small bumps and cracks appeared on the shale after saturation with different fluids, revealing mineral dissolution/precipitation, swelling/shrinkage, and development of fractures after long-term exposure to CO2/brine.
- Based on the low-pressure gas adsorption test results, the curves of the isotherms moved down to a certain extent at all the stages of relative pressure. The special surface area decreased sharply after saturation, in particular for the shale saturated with 6 MPa and 12 MPa CO2. In addition, the variation of total pore volume and the pore size showed a similar trend to the special surface areas and reached the maximum variation for the shale saturated with 12 MPa CO2, which indicates that the gas pressure and phase state displayed a remarkable impact on the pore structure of shale.
- The curves of pore size distribution were skewed to larger pore sizes at all diameters, indicating that the number of pores decreased as a result of the reaction, while also illustrating that the effect of saturation with different fluids was mainly concentrated in the micropores and macropores.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansi, M.; Almobarak, M.; Lagat, C.; Xie, Q. Statistical Analysis of Controlling Factors on Enhanced Gas Recovery by CO2 Injection in Shale Gas Reservoirs. Energy Fuels 2023, 37, 965–976. [Google Scholar] [CrossRef]
- Arab, Oil, Gas, and Group, World Energy Outlook 2011 by the International Energy Agency. Arab Oil & Gas. 2011. Available online: https://www.iea.org/reports/world-energy-outlook-2011 (accessed on 24 October 2023).
- Gholamic, R.; Raza, A.; Iglauer, S. Leakage risk assessment of a CO2 storage site: A review. Earth-Sci. Rev. 2021, 223, 103849. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wu, K.; Zhang, L.; Hui, G.; Yang, M. Molecular dynamics computations of brine-CO2/CH4-shale contact angles: Implications for CO2 sequestration and enhanced gas recovery. Fuel 2020, 280, 118590. [Google Scholar]
- Fatah, A.; Ben Mahmud, H.; Bennour, Z.; Hossain, M.; Gholami, R. Effect of supercritical CO2 treatment on physical properties and functional groups of shales. Fuel 2021, 303, 121310. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, Z.; Zhang, Y.; Zheng, Z.; Deng, Q. Will the future of shale reservoirs lie in CO2 geological sequestration? Sci. China Technol. Sci. 2020, 63, 10. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, S.; Xian, X.; Zheng, Y.; Yang, K.; Liu, J. Comprehensive Review of Property Alterations Induced by CO2-shale Interaction: Implications for CO2 Sequestration in Shale. Energy Fuels 2022, 36, 8066–8080. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Aman, M.; Espinoza, D.N.; Rodriguez, M.A.; Griego, J.M.; Dewers, T.A.; Feldman, J.D.; Stewart, T.A.; Choens, R.C.; Wilson, J. Shale-brine-CO2 interactions and the long-term stability of carbonate-rich shale caprock. Int. J. Greenh. Gas Control. 2018, 78, 244–253. [Google Scholar] [CrossRef]
- Fatah, A.; Ben Mahmud, H.; Bennour, Z.; Gholamic, R.; Hossain, M. Geochemical and physical alteration of clay-rich shales under supercritical CO2 conditions. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2022, 140, 105291. [Google Scholar] [CrossRef]
- Marcon, V.; Kaszuba, J.P. Carbon dioxide–brine–rock interactions in a carbonate reservoir capped by shale: Experimental insights regarding the evolution of trace metals. Geochim. Et Cosmochim. Acta 2015, 168, 22–42. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.; Huang, Z.; Zhao, J.; Wang, L.; Qiu, R. Changes in microstructure and mechanical properties of shales exposed to supercritical CO2 and brine. Int. J. Rock Mech. Min. Sci. 2022, 160, 105228. [Google Scholar] [CrossRef]
- Harris, R.K.; Kowalewski, J.; Menezes, S.C.D. International Union of Pure and Applied Chemistry Physical Chemistry Division Commission on Molecular Structure and Spectroscopy. Parameters and symbols for use in nuclear magnetic resonance (IUPAC recommendations 1997). Magn. Reson. Chem. 1998, 36, 145–149. [Google Scholar] [CrossRef]
- Tan, J.; Hu, C.; Lyu, Q.; Dick, J.M.; Ranjith, P.G.; Li, L.; Wang, Z. Multi-fractal analysis for the AE energy dissipation of CO2 and CO2+ brine/water treated low-clay shales under uniaxial compressive tests. Fuel 2019, 246, 330–339. [Google Scholar] [CrossRef]
- Dai, X.; Wei, C.; Wang, M.; Ma, R.; Song, Y.; Zhang, J.; Wang, X.; Shi, X.; Vandeginste, V. Interaction mechanism of supercritical CO2 with shales and a new quantitative storage capacity evaluation method. Energy 2023, 264, 126424. [Google Scholar] [CrossRef]
- Zhang, S.; Xian, X.; Zhou, J.; Zhang, L. Mechanical behavior of Longmaxi black shale saturated with different fluids: An experimental study. RSC Adv. 2017, 7, 42946–42955. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Zuo, S.; Chen, S.; Liu, Z.; Yin, H.; Li, Y. Influence of supercritical CO2 exposure on water wettability of shale: Implications for CO2 sequestration and shale gas recovery. Energy 2022, 242, 122551. [Google Scholar] [CrossRef]
- Yekeen, N.; Khan, J.A.; Ali, M.; Elraies, K.A.; Okunade, O.A.; Ridha, S.; Al-Yaseri, A. Impact of nanoparticles–surfactant solutions on carbon dioxide and methane wettabilities of organic-rich shale and CO2/brine interfacial tension: Implication for carbon geosequestration. Energy Rep. 2022, 8, 15669–15685. [Google Scholar] [CrossRef]
- Sanguinito, S.; Goodman, A.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawat, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying dry supercritical CO2-induced changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Tang, J.; Li, H.; Zhou, L.; Han, S.; Ge, Z.; Xia, B.; Shen, H.; Zhang, J. Relationship between pore structure and mechanical properties of shale on supercritical carbon dioxide saturation. Energy 2019, 172, 270–285. [Google Scholar] [CrossRef]
- Li, N.; Jin, Z.; Zhang, S.; Wang, H.; Yang, P.; Zou, Y.; Zhou, T. Micro-mechanical properties of shale due to water/supercritical carbon dioxide-rock interaction. Pet. Explor. Dev. 2023, 50, 1001–1012. [Google Scholar] [CrossRef]
- Alafnan, S. Utilization of supercritical carbon dioxide for mechanical degradation of organic matters contained in shales. Fuel 2022, 316, 123427. [Google Scholar] [CrossRef]
- Hazarika, S.; Boruah, A.; Kumar, H. Study of pore structure of shale formation for CO2 storage. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, J.; Xian, X.; Zhang, C.; Gan, Q.; Dong, Z. Effect of supercritical CO2-water-shale interaction on mechanical properties of shale and its implication for carbon sequestration. Gas Sci. Eng. 2023, 111, 204930. [Google Scholar] [CrossRef]
- Ozotta, O.; Liu, K.; Gentzis, T.; Carvajal-Ortiz, H.; Ostadhassan, M. Pore Structure Alteration of Organic-Rich Shale with Sc-CO2 Exposure: The Bakken Formation. Energy Fuels 2021, 35, 5074–5089. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Wang, X.; Hu, Q.; Hu, D.; Yi, J. Paleo-ocean redox environments of the Upper Ordovician Wufeng and the first member in lower Silurian Longmaxi formations in the Jiaoshiba area, Sichuan Basin. Can. J. Earth Sci. 2016, 53, 426–440. [Google Scholar] [CrossRef]
- Liu, S.; Ma, W.; Jansa, L.; Huang, W.; Zeng, X.; Zhang, C. Characteristics of the shale gas reservoir rocks in the Lower Silurian Longmaxi Formation, East Sichuan Basin, China. Energy Explor. Exploit. 2013, 31, 187–219. [Google Scholar] [CrossRef]
- Fatah, A.; Mahmud, H.; Bennour, Z.; Gholamic, R.; Hossain, M. Geochemical modelling of CO2 interactions with shale: Kinetics of mineral dissolution and precipitation on geological time scales. Chem. Geol. 2022, 592, 120742. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Abu-Mahfouz, I.S.; Yekeen, N.; Wolff-Boenisch, D. Organic-rich source rock/H2/brine interactions: Implications for underground hydrogen storage and methane production. J. Energy Storage 2023, 63, 106986. [Google Scholar] [CrossRef]
- Jung, H.B.; Um, W.; Cantrell, K.J. Effect of oxygen co-injected with carbon dioxide on Gothic shale caprock–CO2–brine interaction during geologic carbon sequestration. Chem. Geol. 2013, 354, 1–14. [Google Scholar] [CrossRef]
- Sakthivel, S.; Yekeen, N.; Theravalappil, R.; Al-Yaseri, A. Influence of carbon nanodots on the Carbonate/CO2/Brine wettability and CO2-Brine interfacial Tension: Implications for CO2 geo-storage. Fuel 2024, 355, 129404. [Google Scholar] [CrossRef]
- Fatah, A.; Mahmud, H.B.; Bennour, Z.; Gholamic, R.; Hossain, M. The impact of supercritical CO2 on the pore structure and storage capacity of shales. J. Nat. Gas Sci. Eng. 2022, 98, 104394. [Google Scholar] [CrossRef]
- Lyu, Q.; Wang, K.; Hu, C.; Shi, J.; Tan, J.; Zhang, G.; Chen, S.; Ranjith, P.G. Effects of supercritical CO2/water imbibition under dynamic pressures on shale mechanics and acoustic emission characteristics. Fuel 2022, 321, 124087. [Google Scholar] [CrossRef]
- Ozotta, O.; Kolawole, O.; Malki, M.L.; Ore, T.; Gentzis, T.; Fowler, H.; Liu, K.; Ostadhassan, M. Nano- to macro-scale structural, mineralogical, and mechanical alterations in a shale reservoir induced by exposure to supercritical CO2. Appl. Energy 2022, 326, 120051. [Google Scholar] [CrossRef]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Tan, J.; Hu, C.; Lyu, Q.; Feng, G.; Chen, S. Experimental investigation on the effects of different fracturing fluids on shale surface morphology. J. Pet. Sci. Eng. 2022, 212, 110356. [Google Scholar] [CrossRef]
- Lyu, Q.; Shi, J.; Tan, J.; Dick, J.M.; Kang, X. Effects of shale swelling and water-blocking on shale permeability. J. Pet. Sci. Eng. 2022, 212, 110276. [Google Scholar] [CrossRef]
- Currenti, G.; Cantucci, B.; Montegrossi, G.; Napoli, R.; Misnan, M.S.; Rashidi, M.R.; Bakar, Z.A.A.; Harith, Z.Z.; Bahri, N.H.; Hashim, N. CO2 Leakage Scenarios in Shale Overburden. Minerals 2023, 13, 1016. [Google Scholar] [CrossRef]
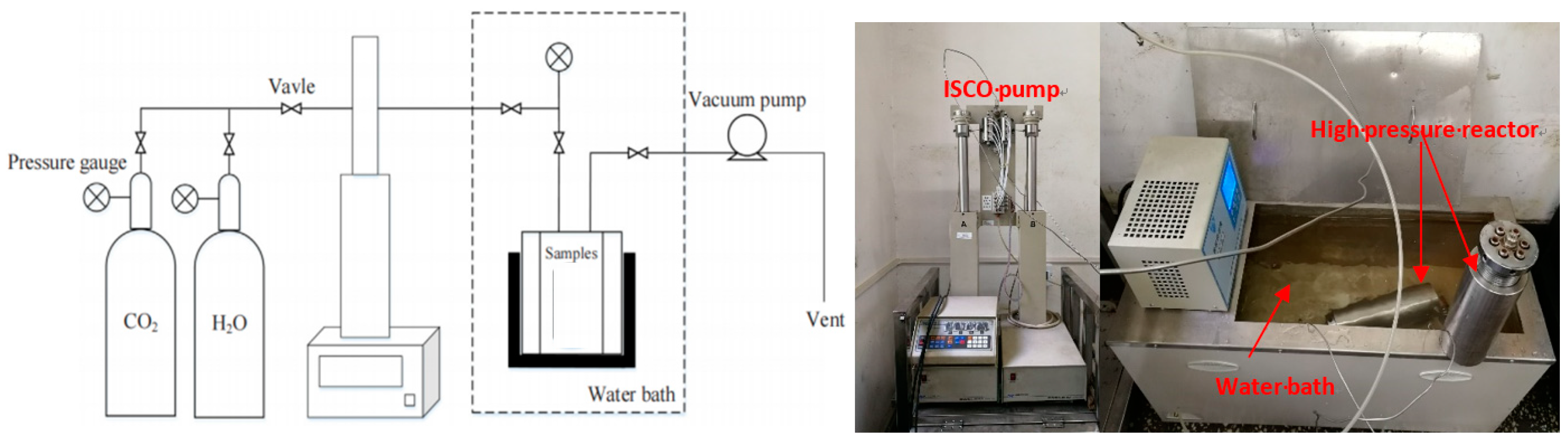


| Saturation Fluids | Carbon Dioxide Pressure | Temperature | Volume of Brine | Exposure Time | Label |
|---|---|---|---|---|---|
| CO2 saturation | 6 MPa | 45 °C | - | 100 days | L6 |
| CO2 saturation | 12 MPa | 45 °C | - | 100 days | L12 |
| 10%NaCl (brine) + CO2 saturation | 6 MPa | 45 °C | 300 mL | 100 days | L6# |
| 10%NaCl (brine) + CO2 saturation | 12 MPa | 45 °C | 300 mL | 100 days | L12# |
| Shale Samples | Main Elements (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Si | Ca | Al | Fe | K | S | Mg | Na | P | |
| Before reaction | 41.40 | 34.62 | 13.00 | 3.65 | 2.52 | 1.56 | 1.03 | 1.06 | 0.89 | 0.06 |
| L06 | 41.60 | 34.57 | 12.95 | 3.63 | 2.50 | 1.54 | 1.04 | 1.05 | 0.89 | 0.06 |
| L12 | 42.25 | 35.62 | 11.56 | 3.48 | 2.43 | 1.42 | 1.03 | 1.10 | 0.86 | 0.05 |
| L06# | 43.41 | 35.16 | 10.83 | 3.42 | 1.86 | 1.47 | 1.17 | 1.07 | 1.19 | 0.06 |
| L12# | 44.60 | 36.54 | 10.34 | 3.36 | 2.09 | 1.37 | 0.90 | 0.96 | 1.29 | 0.06 |
| Mineralogical Analysis | Before Saturation (%, wt) | L6 (%, wt) | L12 (%, wt) | L6# (%, wt) | L12# (%, wt) |
|---|---|---|---|---|---|
| Quartz | 44.8 | 44.9 | 47.1 | 46.8 | 49.2 |
| Calcite | 19.8 | 19.7 | 19.3 | 18.4 | 17.6 |
| barite | 1.0 | 1.0 | 1.1 | 1.2 | 1.3 |
| plagioclase | 3.5 | 3.4 | 3.1 | 3.3 | 3.0 |
| Dolomite | 13.9 | 14.0 | 13.4 | 14.2 | 15.1 |
| marcasite | 1.5 | 1.5 | 1.2 | 1.3 | 0.9 |
| K-feldspar | 1.4 | 1.4 | 1.1 | 1.2 | 1.0 |
| pyrite | 5.1 | 5.0 | 4.8 | 4.94 | 4.7 |
| analcite | 0.9 | 0.9 | 1.0 | 0.9 | 1.1 |
| clay | 8.1 | 8.2 | 7.9 | 7.76 | 6.1 |
| Sample | Special Surface Area (m2·g−1) (Before/After) | Variation (%) | Pore Volume (10−2 cm3·g−1) (Before/After) | Variation (%) | Pore Size (nm) (Before/After) | Variation (%) |
|---|---|---|---|---|---|---|
| L6 | 18.77/10.09 | −46.24 | 151/144 | −4.6 | 6.25/7.91 | 26.6 |
| L12 | 18.87/5.55 | −70.60 | 153/107 | −30.1 | 6.34/9.20 | 45.1 |
| L6# | 17.04/16.51 | −3.10 | 147/143 | −2.7 | 6.57/6.78 | 3.2 |
| L12# | 19.32/12.33 | −36.20 | 155/146 | −5.8 | 6.34/6.86 | 8.2 |
| Sample | State | Pore Volume (10−2 cm3·g−1) | Percentage (%) | ||||
|---|---|---|---|---|---|---|---|
| Micropore | Mesopore | Macorpore | Micropore | Mesopore | Macorpore | ||
| L6 | Before | 36.53 | 47.32 | 67.47 | 24.14 | 31.27 | 44.58 |
| After | 6.38 | 32.79 | 104.99 | 4.43 | 22.75 | 72.83 | |
| L12 | Before | 37.19 | 47.28 | 68.68 | 24.28 | 30.87 | 44.84 |
| After | 4.21 | 21.56 | 81.46 | 3.93 | 20.10 | 75.97 | |
| L6# | Before | 29.63 | 43.48 | 73.83 | 20.16 | 29.59 | 50.24 |
| After | 26.73 | 44.10 | 82.72 | 17.41 | 28.72 | 53.87 | |
| L12# | Before | 38.10 | 47.96 | 68.84 | 24.60 | 30.96 | 44.44 |
| After | 12.76 | 40.63 | 92.36 | 8.75 | 27.88 | 63.37 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Shen, Z.; He, Y.; Zhu, Z.; Ren, Q.; Zhang, L. Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China. Minerals 2023, 13, 1387. https://doi.org/10.3390/min13111387
Zhang S, Shen Z, He Y, Zhu Z, Ren Q, Zhang L. Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China. Minerals. 2023; 13(11):1387. https://doi.org/10.3390/min13111387
Chicago/Turabian StyleZhang, Shuwen, Ziyi Shen, Yan He, Zhonghua Zhu, Qingguo Ren, and Liang Zhang. 2023. "Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China" Minerals 13, no. 11: 1387. https://doi.org/10.3390/min13111387
APA StyleZhang, S., Shen, Z., He, Y., Zhu, Z., Ren, Q., & Zhang, L. (2023). Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China. Minerals, 13(11), 1387. https://doi.org/10.3390/min13111387






