Industrial Ceramics: From Waste to New Resources for Eco-Sustainable Building Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Parameters
2.3. Analytical Methods
3. Results and Discussion
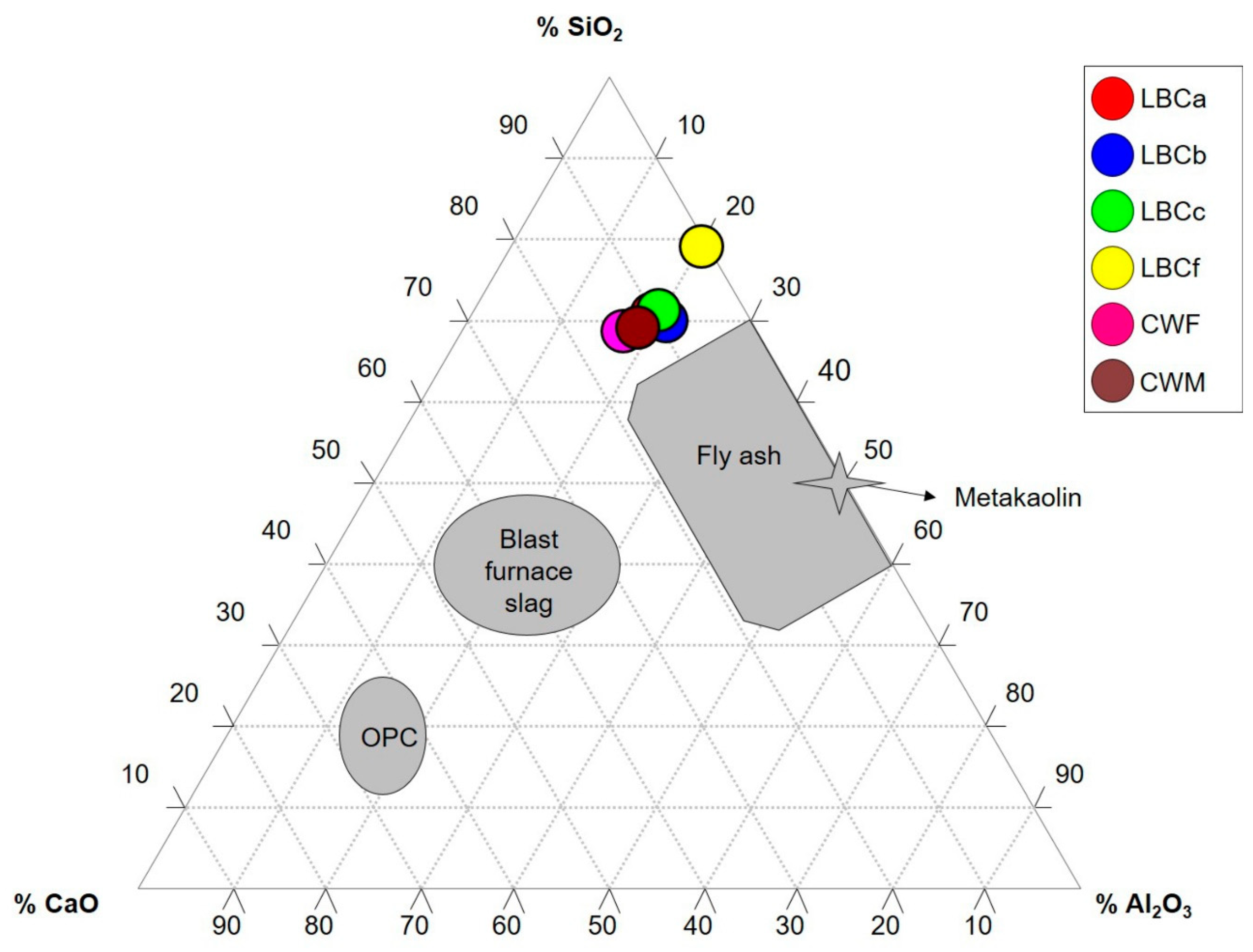
3.1. Chemical Composition of the Ceramic Industrial Wastes
3.2. Mineralogical Composition of the Ceramic Industrial Wastes
3.3. Thermogravimetric Behavior of the Ceramic Industrial Waste
3.4. Reactivity Test in Alkali of Ceramic Industrial Waste
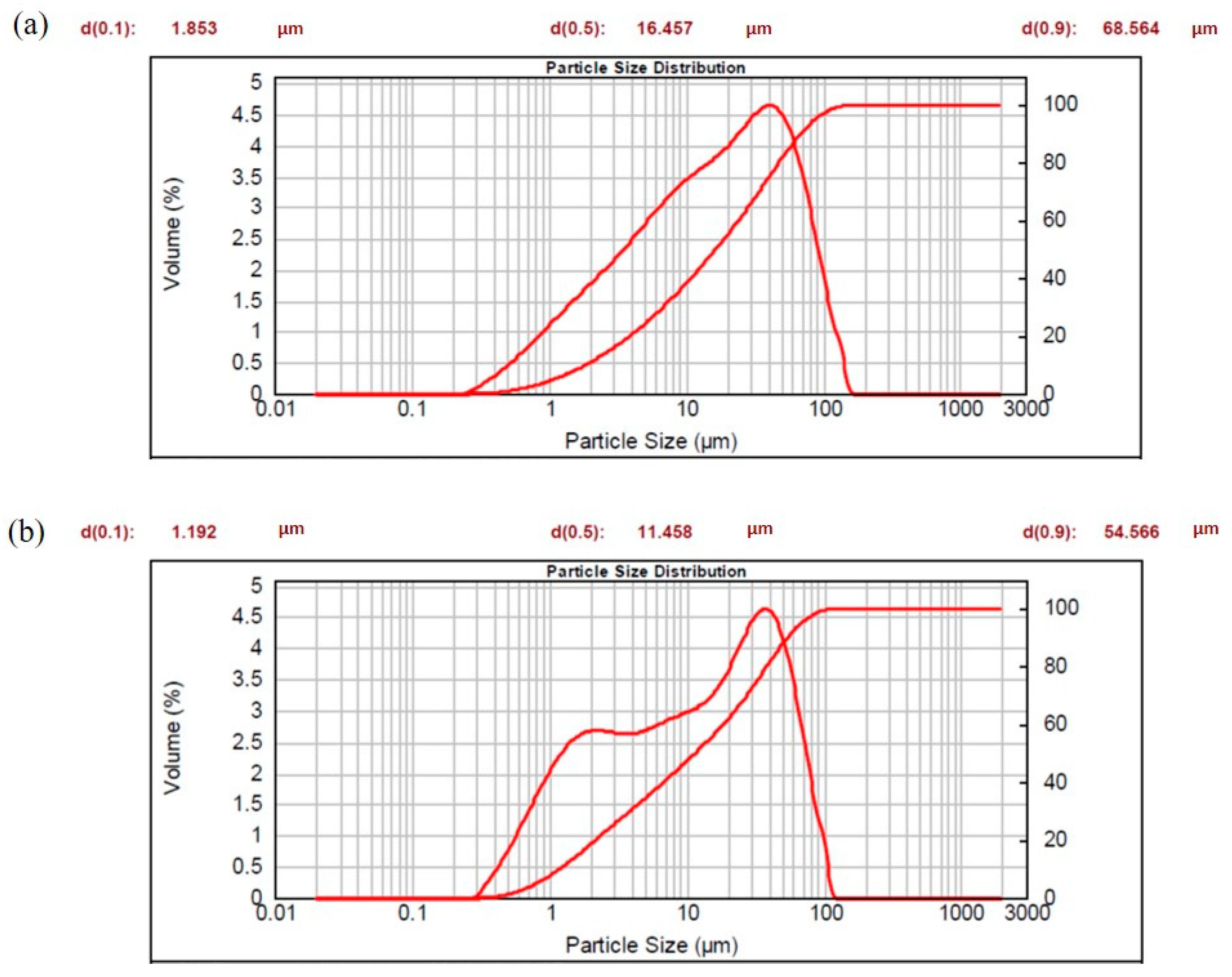
3.5. Granulometric Analysis on Ceramic Waste (Laser Granulometry and BET)
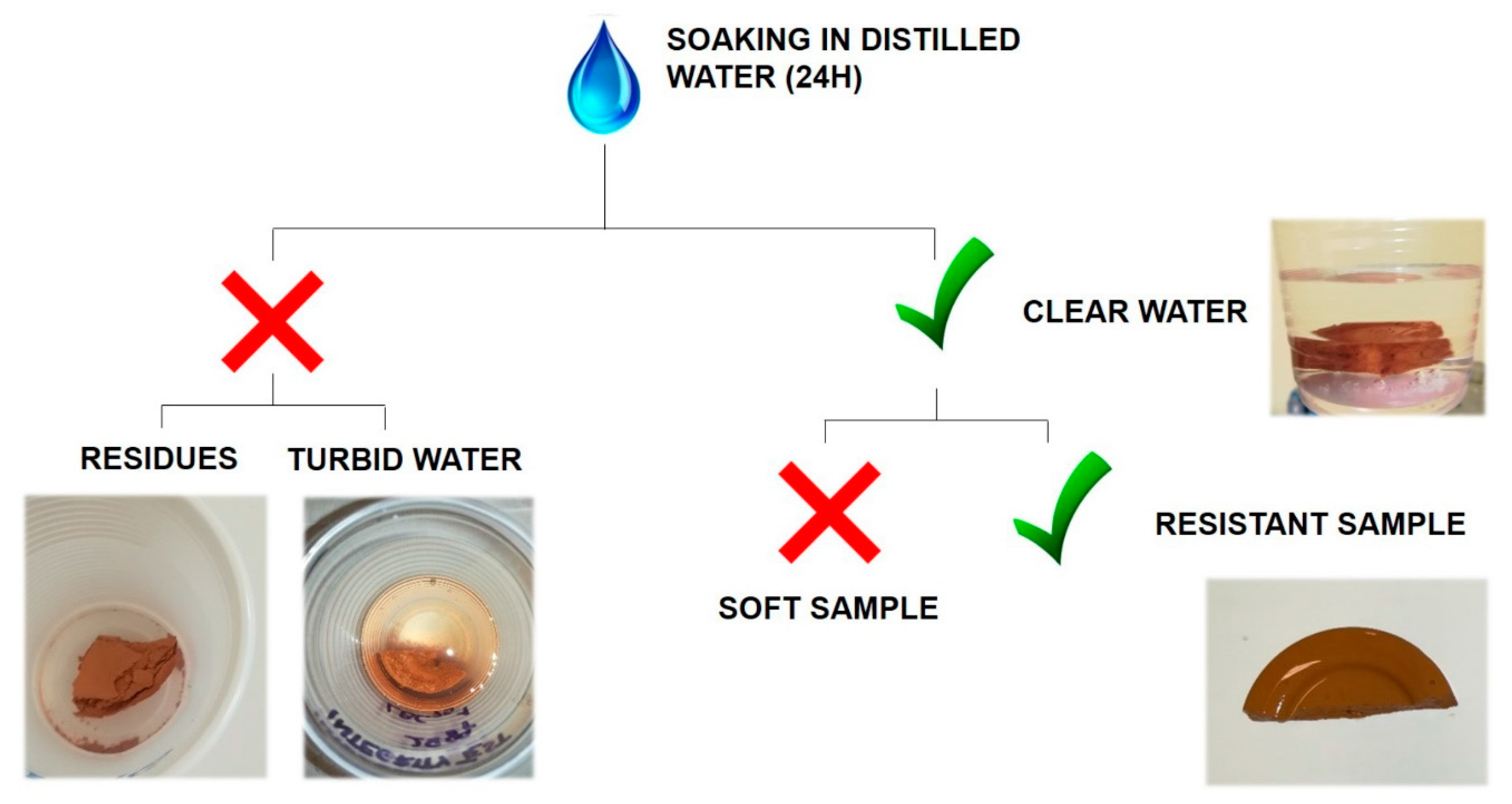
3.6. Visual Observations and Integrity Tests
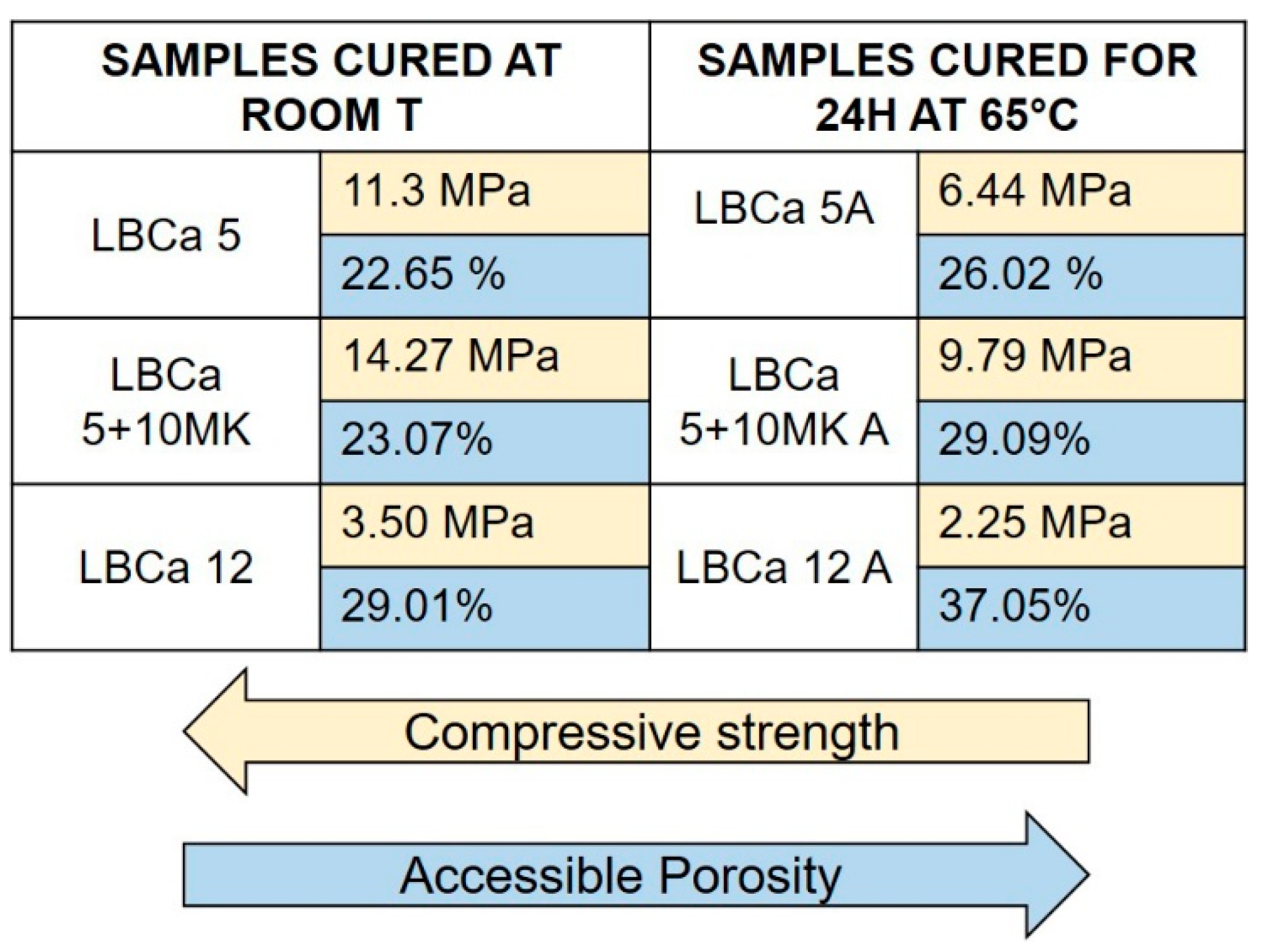
3.7. Mechanical Resistance and Porosity of Alkali-Activated Samples
3.8. Mineralogical Investigation of the Efflorescence
4. Conclusions
- The comparison of performances among the ceramic wastes distinguished two groups related to the presence or absence of calcite. This demonstrates, as we could expect, a different behavior at high temperatures and in alkaline conditions. Nevertheless, by using two ceramics representative of the two individuated groups (LBCa and CWF) for the synthesis, similar results were obtained in terms of workability, curing time, possible efflorescence formations, cracks or shrinkage and water resistance (integrity tests);
- The results obtained reveal that construction industrial wastes of ceramic nature can be activated by using proportioned mixtures of sodium hydroxide (8 M) and sodium silicate (R = 3.3), in accordance with the literature [61], or with only sodium silicate;
- AAMs tend to consolidate between 1 day and 7 days, depending on the formulation and particularly on the amount of additives;
- P and MK, even if added in small amounts (5%–10%), are able to promote the alkaline gel building, and thus the consolidation. Such additives also improve the strength and counteract the formation of efflorescence;
- Where present, the efflorescence seems to be strictly linked to the higher sodium hydroxide or water amount; therefore, as well as with the addition of additives, the formation of efflorescence could be avoided by balancing formulations in a stoichiometric way;
- In contrast to the current literature [4,26,65], this study highlighted that curing at room temperature (around 25 °C) is preferable, whereas thermal curing at 65 °C for 24 h resulted in lower performance in terms of compressive strength and an increase in porosity, and facilitated efflorescence crystallization. Similar results were also found in [39];
- LBCa and CWF proved to be the least reactive ceramics among all the samples studied. Despite that, good results for the synthesis of AAMs were obtained, allowing for better results by using the other, more reactive, ceramic wastes characterized here.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, A.; Kumar, S.; Jha, V.K. Development of Building Material from Geopolymerization of Construction and Demolition Waste (CDW). Trans. Indian Ceram. Soc. 2014, 73, 133–137. [Google Scholar] [CrossRef]
- Allaoui, D.; Nadi, M.; Hattani, F.; Majdoubi, H.; Haddaji, Y.; Mansouri, S.; Oumam, M.; Hannache, H.; Manoun, B. Eco-Friendly Geopolymer Concrete Based on Metakaolin and Ceramics Sanitaryware Wastes. Ceram. Int. 2022, 48, 34793–34802. [Google Scholar] [CrossRef]
- Tan, J.; Cai, J.; Li, J. Recycling of Unseparated Construction and Demolition Waste (UCDW) through Geopolymer Technology. Constr. Build. Mater. 2022, 341, 127771. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Alkaline Activation of Ceramic Waste Materials. Waste Biomass Valoriz. 2013, 4, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Robayo-Salazar, R.; Valencia-Saavedra, W.; de Gutiérrez, R.M. Reuse of Powders and Recycled Aggregates from Mixed Construction and Demolition Waste in Alkali-Activated Materials and Precast Concrete Units. Sustainability 2022, 14, 9685. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yehualaw, M.D.; Vo, D.H.; Huynh, T.P.; Largo, A. Performance Evaluation of Alkali Activated Mortar Containing High Volume of Waste Brick Powder Blended with Ground Granulated Blast Furnace Slag Cured at Ambient Temperature. Constr. Build. Mater. 2019, 223, 657–667. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T.C. Potential Use of Brick Waste as Alternate Concrete-Making Materials: A Review. J. Clean. Prod. 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Panizza, M.; Natali, M.; Garbin, E.; Tamburini, S.; Secco, M. Assessment of Geopolymers with Construction and Demolition Waste (CDW) Aggregates as a Building Material. Constr. Build. Mater. 2018, 181, 119–133. [Google Scholar] [CrossRef]
- Frittelloni, V. Rapporto Rifiuti Speciali Edizione 2022; Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2022; ISBN 9788844811167.
- Sarkar, M.; Dana, K. Partial Replacement of Metakaolin with Red Ceramic Waste in Geopolymer. Ceram. Int. 2021, 47, 3473–3483. [Google Scholar] [CrossRef]
- Mir, N.; Khan, S.A.; Kul, A.; Sahin, O.; Lachemi, M.; Sahmaran, M.; Koç, M. Life Cycle Assessment of Binary Recycled Ceramic Tile and Recycled Brick Waste-Based Geopolymers. Clean. Mater. 2022, 5, 100116. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Kirchheim, A.P.; Provis, J.L. Management and Valorisation of Wastes through Use in Producing Alkali-Activated Cement Materials. J. Chem. Technol. Biotechnol. 2016, 91, 2365–2388. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential Applications of Geopolymer Concrete in Construction: A Review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Russo, A.; Palmero, P. Alkali-Activation of Recycled Construction and Demolition Waste Aggregate with No Added Binder. Constr. Build. Mater. 2019, 205, 398–413. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Mazzoli, C. Use of Industrial Ceramic Sludge in Brick Production: Effect on Aesthetic Quality and Physical Properties. Constr. Build. Mater. 2016, 124, 219–227. [Google Scholar] [CrossRef]
- Fugazzotto, M.; Cultrone, G.; Mazzoleni, P.; Barone, G. Suitability of Ceramic Industrial Waste Recycling by Alkaline Activation for Use as Construction and Restoration Materials. Ceram. Int. 2023, 49, 9465–9478. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; Vieira, C.M.F.; Ferreira, W.M.; Faria, K.C.P.; Pedroti, L.G.; Mendes, B.C. Potential Use of Ceramic Waste as Precursor in the Geopolymerization Reaction for the Production of Ceramic Roof Tiles. J. Build. Eng. 2020, 29, 101156. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Brice, D.G. Chemical Research and Climate Change as Drivers in the Commercial Adoption of Alkali Activated Materials. Waste Biomass Valoriz. 2010, 1, 145–155. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Pachego-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Cambridge, UK, 2015; ISBN 9781782422884. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Allahverdi, A.; Kani, E.N. Construction Wastes as Raw Materials for Geopolymer Binders. Int. J. Civ. Eng. 2009, 7, 154–160. [Google Scholar]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability Evaluation of Geopolymer and Conventional Concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of Geopolymers Sourced from Construction and Demolition Waste: A Review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Lancellotti, I.; Vezzali, V.; Barbieri, L.; Leonelli, C.; Grillenzoni, A. Construction and Demolition Waste (Cdw) Valorization in Alkali Activated Bricks. In Wastes: Solutions, Treatments and Opportunities III; Taylor and Francis Group: London, UK, 2020; pp. 495–499. ISBN 9780367257774. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of Synthesis Parameters on the Quality of Construction and Demolition Wastes (CDW) Geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.K.; El-Sherbiny, S.A.; El-Magd, A.A.M.A.; Belal, A.; Abadir, M.F. Fabrication of Geopolymer Bricks Using Ceramic Dust Waste. Constr. Build. Mater. 2017, 157, 610–620. [Google Scholar] [CrossRef]
- Leonelli, C.; Romagnoli, M. Geopolimeri: Polimeri Inorganici Attivati Chimicamente, 2nd ed.; ICerS: Bologna, Italy, 2013. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Geraldes, C.F.M.; Lima, A.M.; Delgado-Rodrigues, J.; Mimoso, J.M.; Pereira, S.R.M. Geopolymers as Potential Repair Material in Tiles Conservation. Appl. Phys. A Mater. Sci. Process. 2016, 122, 197. [Google Scholar] [CrossRef]
- Moutinho, S.; Costa, C.; Cerqueira, Â.; Rocha, F.; Velosa, A. Geopolymers and Polymers in the Conservation of Tile Facades. Constr. Build. Mater. 2019, 197, 175–184. [Google Scholar] [CrossRef]
- Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Robayo, R.A.; Mulford, A.; Munera, J.; de Gutiérrez, R.M. Alternative Cements Based on Alkali-Activated Red Clay Brick Waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Rovnaník, P.; Rovnaníková, P.; Vyšvařil, M.; Grzeszczyk, S.; Janowska-Renkas, E. Rheological Properties and Microstructure of Binary Waste Red Brick Powder/Metakaolin Geopolymer. Constr. Build. Mater. 2018, 188, 924–933. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of Alkali Activator Concentration and Curing Condition on Strength and Microstructure of Waste Clay Brick Powder-Based Geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Barone, G.; Caggiani, M.C.; Coccato, A.; Finocchiaro, C.; Fugazzotto, M.; Lanzafame, G.; Occhipinti, R.; Stroscio, A.; Mazzoleni, P. Geopolymer Production for Conservation-Restoration Using Sicilian Raw Materials: Feasibility Studies. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012001. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Tchadjié, L.N.; Tchakoute, H.K.; Kenne, B.B.D.; Elimbi, A.; Njopwouo, D. Synthesis of Geopolymer Composites from a Mixture of Volcanic Scoria and Metakaolin. J. Asian Ceram. Soc. 2014, 2, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.; Lukey, G.C.; van Deventer, J.S.J. The Coexistence of Geopolymeric Gel and Calcium Silicate Hydrate at the Early Stage of Alkaline Activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic Polymers from Alkali Activation of Metakaolin: Effect of Setting and Curing on Structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of Geopolymeric Binder from Blended Waste Concrete Powder and Fly Ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Properties and Microstructure of Alkali-Activated Red Clay Brick Waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay Reactivity: Production of Alkali Activated Cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of Fly Ashes. Potential Reactivity as Alkaline Cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Pulidori, E.; Lluveras-Tenorio, A.; Carosi, R.; Bernazzani, L.; Duce, C.; Pagnotta, S.; Lezzerini, M.; Barone, G.; Mazzoleni, P.; Tiné, M.R. Building Geopolymers for CuHe Part I: Thermal Properties of Raw Materials as Precursors for Geopolymers. J. Therm. Anal. Calorim. 2022, 147, 5323–5335. [Google Scholar] [CrossRef]
- Pelosi, C.; Occhipinti, R.; Finocchiaro, C.; Lanzafame, G.; Pulidori, E.; Lezzerini, M.; Barone, G.; Mazzoleni, P.; Tiné, M.R. Thermal and Morphological Investigations of Alkali Activated Materials Based on Sicilian Volcanic Precursors (Italy). Mater. Lett. 2023, 335, 133773. [Google Scholar] [CrossRef]
- EN 1926 2006; Natural Stone Test Methods—Determination of Uniaxial Compressive Strength. European Commission: Brussels, Belgium, 2006.
- Buchwald, A.; Kaps, C.; Hohmann, M. Alkali-Activated Binders and Pozzolan Cement Binders—Compete Binder Reaction or Two Sides of the Same Story ? In Proceedings of the 11th International Congress on the Chemistry of Cement, Durban, South Africa, 11–16 May 2003; pp. 1238–1247. [Google Scholar]
- Warr, L.N. IMA–CNMNC Approved Mineral Symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali Activation Processes for Incinerator Residues Management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, N.Y.; El-Hemaly, S.A.S.; Al-Wakeel, E.I.; El-Korashy, S.A.; Brown, P.W. Characterization and Evaluation of the Pozzolanic Activity of Egyptian Industrial By-Products: I: Silica Fume and Dealuminated Kaolin. Cem. Concr. Res. 2001, 31, 467–474. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of Aluminosilicate Minerals and By-Products in Alkaline Media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of Specific Surface by the BET Method. Matériaux Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Nazari, A.; Bagheri, A.; Riahi, S. Properties of Geopolymer with Seeded Fly Ash and Rice Husk Bark Ash. Mater. Sci. Eng. A 2011, 528, 7395–7401. [Google Scholar] [CrossRef]
- Azevedo, A.G.D.S.; Strecker, K.; Lombardi, C.T. Produção de Geopolímeros à Base de Metacaulim e Cerâmica Vermelha. Cerâmica 2018, 64, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A Review and Prospects for the Minerals Industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Pecchioni, E.; Fratini, F.; Cantisani, E. Le Malte Antiche e Moderne Tra Tradizione Ed Innovazione; Pàtron Editore: Bologna, Italy, 2008. [Google Scholar]
- Pacheco-Torgal, F.; Tam, V.W.Y.; Labrincha, J.A.; Ding, Y.; de Brito, J. Handbook of Recycled Concrete and Demolition Waste; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Rowles, M.R.; O’Connor, B.H. Chemical and Structural Microanalysis of Aluminosilicate Geopolymers Synthesized by Sodium Silicate Activation of Metakaolinite. J. Am. Ceram. Soc. 2009, 92, 2354–2361. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of Geopolymer Concrete for a Cleaner and Sustainable Environment e A Review of Mechanical Properties and Microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of Waste Brick Powder in Alkali Activated Aluminosilicates: Functional and Environmental Aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A.; Provis, J.L. Efflorescence Control in Geopolymer Binders Based on Natural Pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and Subflorescence Induced Microstructural and Mechanical Evolution in Fly Ash-Based Geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
- Leonelli, C. Definizione, Preparazione, Proprietà Ed Applicazioni. In Geopolimeri: Polimeri Inorganici Attivati Chimicamente; Leonelli, C., Romagnoli, M., Eds.; ICerS: Bologna, Italy, 2013; pp. 1–22. [Google Scholar]
- Sun, Z.; Cui, H.; An, H.; Tao, D.; Xu, Y.; Zhai, J.; Li, Q. Synthesis and Thermal Behavior of Geopolymer-Type Material from Waste Ceramic. Constr. Build. Mater. 2013, 49, 281–287. [Google Scholar] [CrossRef]



| Major Oxides (wt%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | LOI | Tot. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MK | 58.56 | 34.03 | 2.57 | - | 2.08 | - | 0.69 | 1.88 | 0.2 | - | nd | 100 |
| P | 18.09 | 7.24 | 3.2 | 3.84 | 53.07 | 0.28 | 1.16 | 1.13 | 0.35 | 3.24 | 9.28 | 100 |
| Major Oxides (wt%) | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | Others | LOI | Tot. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBCa | 60.67 | 16.30 | 5.69 | 0.08 | 2.31 | 8.73 | 1.35 | 3.27 | 0.69 | 0.14 | 0.30 | 0.47 | 100 |
| LBCb | 57.91 | 17.33 | 7.53 | 0.11 | 2.58 | 7.41 | 2.35 | 2.30 | 1.13 | 0.35 | 0.53 | 0.47 | 100 |
| LBCc | 61.17 | 17.03 | 5.63 | 0.04 | 2.08 | 7.81 | 0.49 | 4.02 | 0.76 | 0.17 | 0.29 | 0.51 | 100 |
| LBCf | 72.37 | 18.37 | 0.99 | 0.01 | 0.54 | 0.80 | 3.22 | 2.37 | 0.68 | 0.12 | 0.18 | 0.35 | 100 |
| CWF | 57.33 | 14.32 | 5.51 | 0.08 | 2.43 | 11.99 | 1.16 | 2.49 | 0.73 | 0.17 | 0.33 | 3.46 | 100 |
| CWM | 56.83 | 15.20 | 5.63 | 0.07 | 2.52 | 10.15 | 1.39 | 2.80 | 0.70 | 0.18 | 0.21 | 4.32 | 100 |
| Sample | Qz | Fsp | Hem | Di | Gh | Wo | Mnt | Cal | Ms | Mul |
|---|---|---|---|---|---|---|---|---|---|---|
| LBCa | X | X | X | X | X | X | / | / | / | / |
| LBCb | X | X | X | X | / | / | X | / | / | / |
| LBCc | X | X | X | X | X | / | / | / | / | / |
| LBCf | X | X | / | / | / | / | / | / | / | X |
| CWF | X | X | X | X | X | / | / | X | X | / |
| CWM | X | X | X | / | / | / | / | X | X | / |
| Sample | 0–80 °C | 80–130 °C | 130–200 °C | 200–500 °C | 500–600 °C | 600–900 °C | Residue (%) |
|---|---|---|---|---|---|---|---|
| LBCa | 0.15 | 0.05 | 0.09 | 99.7 | |||
| LBCb | 0.14 | 0.19 | 99.7 | ||||
| LBCc | 0.4 | 99.6 | |||||
| LBCf | 0.12 | 0.4 | 99.5 | ||||
| CWF * | 0.36 | 2.7 | 97 | ||||
| CWM * | 0.78 | 0.24 | 3.2 | 95.8 |
| Sample | Solid Residue (wt%) | Soluble Fraction (wt%) | Si (mg/L) | Al (mg/L) | Si + Al (mg/L) | (Si + Al)/Soluble Fraction (wt%) |
|---|---|---|---|---|---|---|
| LBCa | 90 | 10 | 9.5 | 3.1 | 12.63 | 12 |
| LBCb | 84 | 16 | 8.1 | 3 | 11.07 | 7 |
| LBCf | 83 | 17 | 15.7 | 3.8 | 19.48 | 11 |
| CWF | 94 | 6 | 10 | 3.6 | 13.61 | 22 |
| CWM | 70 | 30 | 22.1 | 14.6 | 36.7 | 12 |
| Sample | SiO2% s Tot | Al2O3% s Tot | SiO2% s Silica Tot | Al2O3% s Alumina Tot | [SiO2]/[Al2O3]reactive |
|---|---|---|---|---|---|
| LBCa | 0.002 | 0.001 | 0.328 | 0.359 | 3.397 |
| LBCb | 0.002 | 0.001 | 0.299 | 0.327 | 3.057 |
| LBCf | 0.003 | 0.001 | 0.464 | 0.391 | 4.678 |
| CWF | 0.002 | 0.001 | 0.373 | 0.475 | 3.145 |
| CWM | 0.005 | 0.003 | 0.832 | 1.815 | 1.714 |
| Sample | D50 | Surface Area (m2/g) |
|---|---|---|
| LBCa | 1–2 mm | 0.5272 |
| ~15 µm | 1.0155 | |
| CWF | >125 μm | 47.8880 |
| ~12 µm | 43.1090 |
| Sample | St (min) | Ct (Days) | Homogeneity | Shrinkage | Cracks | Salts | Integrity Tests | |
|---|---|---|---|---|---|---|---|---|
| Stability in Water | Resistance to Pincer | |||||||
| LBCa75 1 | / | 28 | no | yes | no | no | no | no |
| LBCa75 2 | / | 28 | no | yes | no | no | no | no |
| LBCa75 3+50P | / | 28 | yes | yes | no | no | yes | no |
| LBCa75 4 | / | 28 | no | yes | no | no | no | no |
| LBCa10 1 | 5 | 28 | yes | yes | no | no | no | no |
| LBCa10 2 | 5 | 28 | yes | yes | no | no | no | no |
| LBCa10 3+50P | 5 | 1 | yes | yes | no | no | yes | no |
| LBCa10 4 | 5 | 28 | no | yes | no | no | no | no |
| LBCa 5 | 5 | 1 | no | yes | no | no | no | no |
| LBCa 5A | 5 | 1 | no | yes | no | yes | yes | no |
| LBCa 5+10MK | 5 | 1 | yes | yes | no | no | yes | no |
| LBCa 5+10MK_A | 5 | 1 | no | yes | no | yes | yes | no |
| LBCa 5+20MK | 5 | 1 | yes | yes | no | no | yes | no |
| LBCa 6 | 5 | 1 | yes | yes | no | no | no | no |
| LBCa 6+10MK | 5 | 1 | no | yes | no | no | no | no |
| LBCa 6+20MK | 5 | 1 | yes | yes | no | no | no | no |
| LBCa 7 | 5 | 1 | yes | yes | no | no | no | no |
| LBCa 8 | 5 | 1 | yes | yes | no | no | no | no |
| LBCa 9 | 5 | 7 | yes | yes | no | no | no | no |
| LBCa 10 | 5 | 7 | yes | yes | no | no | no | no |
| LBCa 11 | 5 | 1 | no | yes | no | no | yes | no |
| LBCa 12 | 5 | 1 | yes | yes | no | yes | yes | no |
| LBCa 12A | 5 | 1 | yes | yes | no | yes | yes | no |
| LBCa 13 | 5 | 7 | yes | yes | no | no | yes | yes |
| LBCa 13+10MK | 5 | 1 | yes | yes | no | yes | yes | yes |
| LBCa 13+20MK | 5 | 1 | yes | no | no | no | yes | yes |
| LBCa 14 | 5 | 7 | yes | yes | no | no | yes | yes |
| LBCa 14+10MK | 5 | 1 | yes | yes | no | no | yes | yes |
| LBCa 14+20MK | 5 | 1 | yes | no | no | no | yes | yes |
| LBCa 14+5P | 5 | <1 | yes | yes | no | no | yes | yes |
| LBCa 14+10P | 2 | <1 | yes | yes | no | no | yes | yes |
| LBCa 14+20P | 2 | <1 | yes | yes | no | no | yes | yes |
| LBCa 15 | 5 | 7 | yes | yes | no | yes | yes | no |
| LBCa 15+10MK | 5 | 1 | yes | yes | no | yes | yes | yes |
| LBCa 15+20MK | 5 | 1 | yes | no | no | yes | yes | yes |
| CWF 13 | 5 | 7 | yes | yes | no | no | yes | yes |
| CWF 13+10MK | 5 | 1 | yes | yes | no | no | yes | yes |
| CWF 13+20MK | 5 | 1 | no | no | no | no | yes | yes |
| CWF 14 | 5 | 7 | yes | yes | no | no | yes | yes |
| CWF 14+10MK | 5 | 1 | yes | yes | no | no | yes | yes |
| CWF 14+20 MK | 5 | 1 | yes | no | no | no | yes | yes |
| CWF 15 | 5 | 7 | yes | yes | no | yes | no | yes |
| CWF 15+10MK | 5 | 1 | yes | yes | no | yes | no | yes |
| CWF 15+20MK | 5 | 1 | yes | no | no | yes | no | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fugazzotto, M.; Mazzoleni, P.; Lancellotti, I.; Camerini, R.; Ferrari, P.; Tiné, M.R.; Centauro, I.; Salvatici, T.; Barone, G. Industrial Ceramics: From Waste to New Resources for Eco-Sustainable Building Materials. Minerals 2023, 13, 815. https://doi.org/10.3390/min13060815
Fugazzotto M, Mazzoleni P, Lancellotti I, Camerini R, Ferrari P, Tiné MR, Centauro I, Salvatici T, Barone G. Industrial Ceramics: From Waste to New Resources for Eco-Sustainable Building Materials. Minerals. 2023; 13(6):815. https://doi.org/10.3390/min13060815
Chicago/Turabian StyleFugazzotto, Maura, Paolo Mazzoleni, Isabella Lancellotti, Rachel Camerini, Pamela Ferrari, Maria Rosaria Tiné, Irene Centauro, Teresa Salvatici, and Germana Barone. 2023. "Industrial Ceramics: From Waste to New Resources for Eco-Sustainable Building Materials" Minerals 13, no. 6: 815. https://doi.org/10.3390/min13060815
APA StyleFugazzotto, M., Mazzoleni, P., Lancellotti, I., Camerini, R., Ferrari, P., Tiné, M. R., Centauro, I., Salvatici, T., & Barone, G. (2023). Industrial Ceramics: From Waste to New Resources for Eco-Sustainable Building Materials. Minerals, 13(6), 815. https://doi.org/10.3390/min13060815










