The Influence of the Physicochemical Characteristics of Ores on the Efficiency of Underground Well Leaching of Uranium Deposits in Kazakhstan
Abstract
:1. Introduction
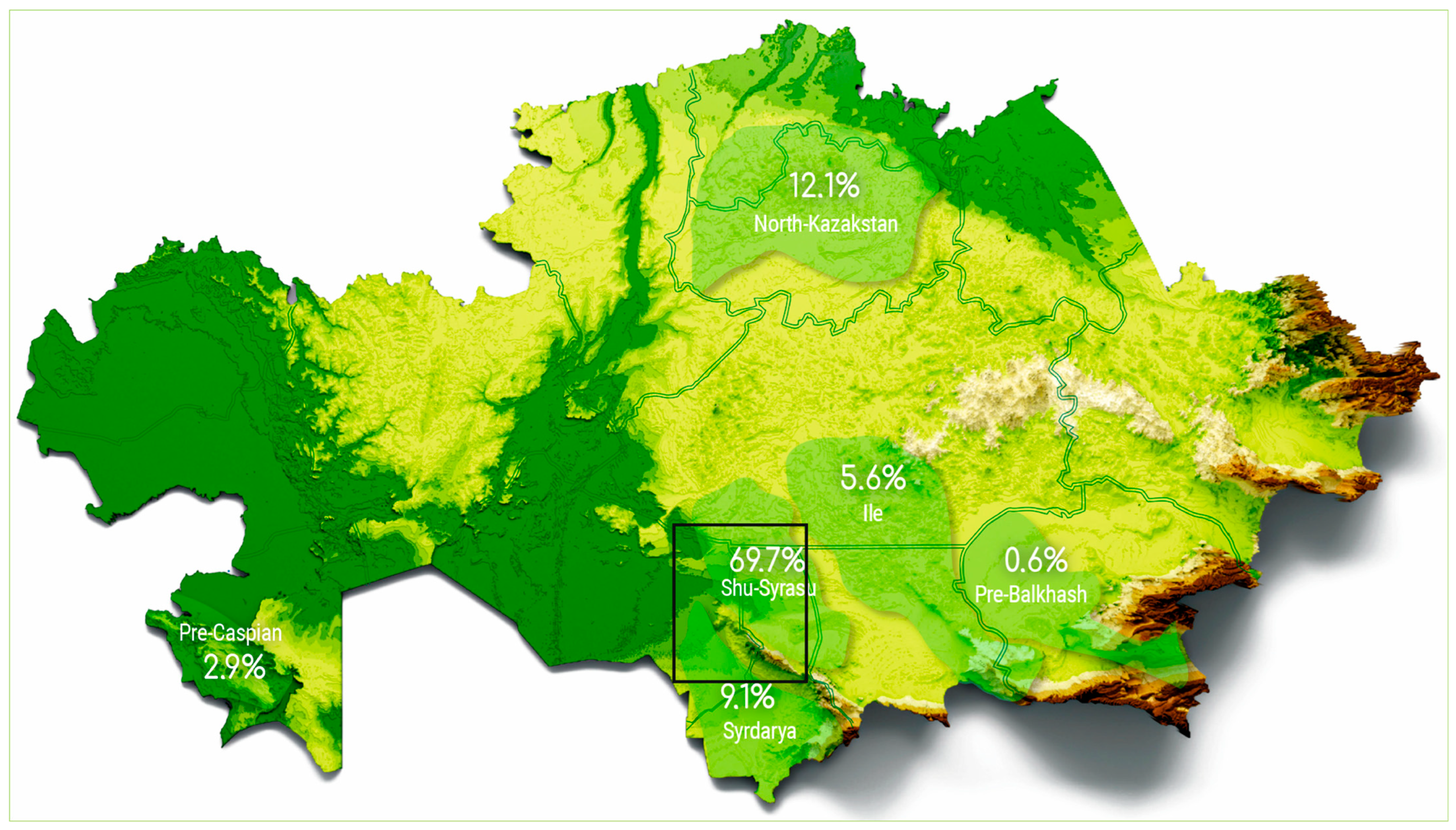
2. Materials and Methods
2.1. Mineralogy of Ores
2.2. X-ray Phase Studies of Sedimentation
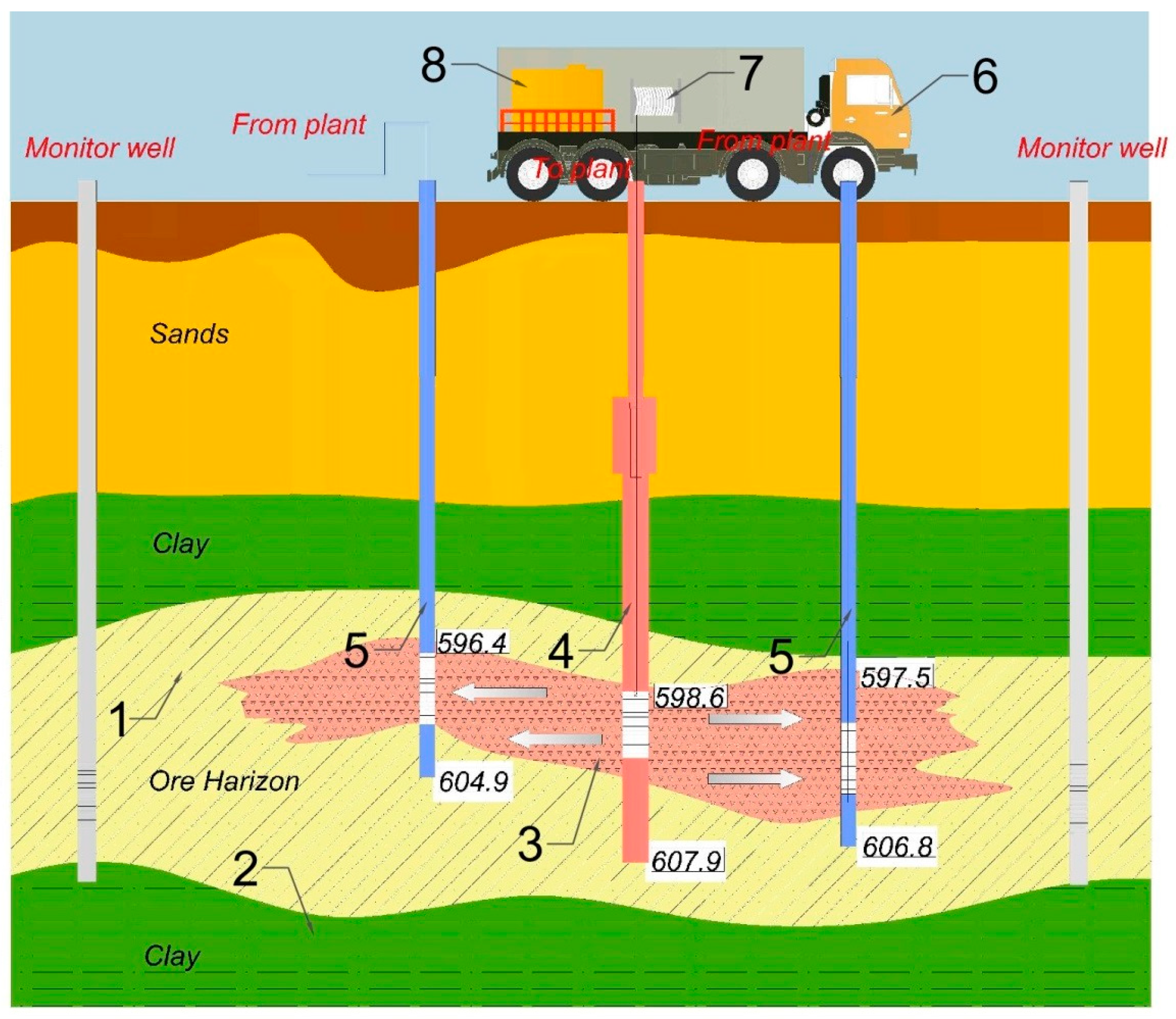
2.3. Development of a Method for Chemical Treatment of Wells
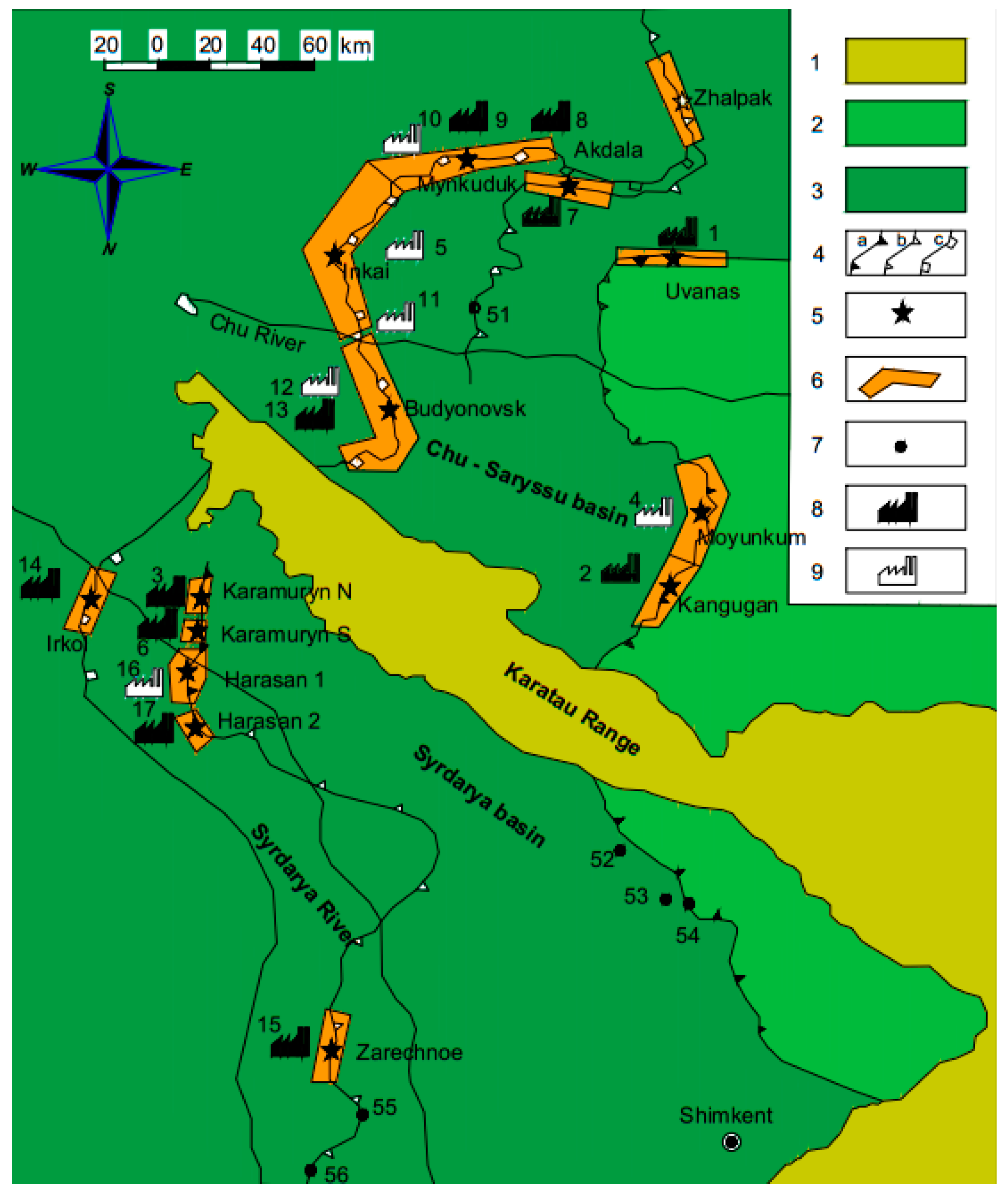
2.4. Experimental Trials on Geotechnological Wells and Blocks
3. Results and Discussion
3.1. Determination of Mineralogy of Ores and Sedimentation
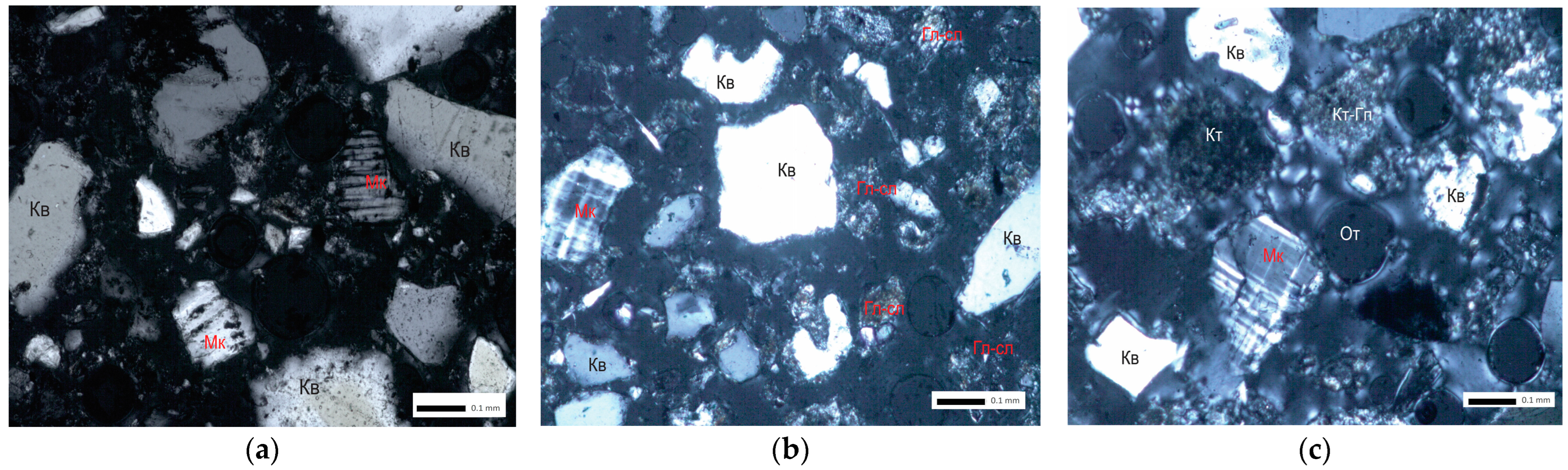
3.2. Microscopic Studies of Ores
3.3. The Mechanism of Intensification of the Uranium Leaching Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakia, S.A.; Rashad, M.M.; Mohamed, S.A.; Mira, H.E.; Abd el Wahab, G.M. Kinetics of uranium leaching process using sulfuric acid for Wadi Nasib ore, South western Sinai, Egypt. Aswan Univ. J. Environ. Stud. 2020, 1, 171–182. [Google Scholar]
- Khawassek, Y.M.; Taha, M.H.; Eliwa, A.A. Kinetics of leaching process using sulfuric acid for Sella uranium ore material, South Eastern desert, Egypt. Int. J. Nucl. Energy Sci. Eng. 2016, 6, 62–73. [Google Scholar] [CrossRef]
- Kenzhetaev, Z.; Nurbekova, M.; Togizov, K.; Abdraimova, M.; Toktaruly, B. Methods for intensification of borehole uranium mining at the fields with low filtration characteristics of ores. Min. Miner. Depos. 2021, 15, 95–101. [Google Scholar] [CrossRef]
- Rakishev, B.R.; Bondarenko, V.I.; Matev, M.M.; Kenzhetaev, Z.S. Influence of chemical reagent complex on intensification of uranium well extraction. Nauk. Visnyk Natsionalnoho Hirnychoho Univ. 2019, 6, 25–30. [Google Scholar] [CrossRef]
- Yusupov, K.A.; Aliev, S.B.; Dzhakupov, D.A.; Elzhanov, E.A. Application of ammonium bifluoride for chemical treatment of wells in underground uranium leaching. Gorn. Zhurnal 2017, 4, 57–60. [Google Scholar] [CrossRef]
- Rakishev, B.R.; Mataev, M.M.; Kenzhetaev, Z.S. System Intensification of Borehole Uranium Mining; Publishing house “Polytech” KazNITU Named after. K.I. Satpayeva: Almaty, Kazakhstan, 2022; 281p. [Google Scholar]
- Atia, B.M.; Gado, M.A.; Cheira, M.F. Kinetics of uranium and iron dissolution by sulfuric acid from Abu Zeneima ferruginous siltstone, Southwestern Sinai, Egypt. Euro-Mediterr. J. Environ. Integr. 2018, 3, 39. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Song, Q.; Zhou, Z.; Yang, S. Exploration and mining evaluation system and price prediction of uranium resources. Min. Miner. Depos. 2018, 12, 85–94. [Google Scholar] [CrossRef]
- Rakishev, B.R.; Mataev, M.M.; Kenzhetaev, Z.S. Analysis of mineralogical composition of sediments in in-situ leach mining of uranium. MIAB 2019, 7, 123–131. [Google Scholar] [CrossRef]
- Panfilov, M.; Uralbekov, B.; Burkitbayev, M. Reactive transport in the underground leaching of uranium: Asymptotic analytical solution for multi-reaction model. Hydrometallurgy 2016, 160, 60–72. [Google Scholar] [CrossRef]
- Rakishev, B.; Mataev, M.M.; Kenzhetayev, Z.; Togizov, K. Improving the Efficiency of Downhole Uranium Production Using Oxygen as an Oxidizer. Minerals 2022, 12, 1005. [Google Scholar] [CrossRef]
- Beleckij, V.I.; Bogatkov, L.K.; Volkov, N.I. Spravochnik po Geotehnologii Urana; Energoatomizdat: Moskva, Russia, 1997.
- Rakishev, B.; Mataev, M.M.; Kenzhetayev, Z.; Altaybayev, B.; Shampikova, A. Research into leaching of uranium from core samples in tubes using surfactants. Min. Miner. Depos. 2020, 14, 97–104. [Google Scholar] [CrossRef]
- Kenzhetaev, Z.; Kuandykov, T.; Togizov, K.; Abdraimova, M.; Nurbekova, M. Selection of rational parameters for opening and drilling of technological wells underground uranium leaching. News NAS RK Ser. Geol. Tech. Sci. 2022, 3, 115–127. [Google Scholar] [CrossRef]
- Kuandykov, T.; Nauryzbayeva, D.; Yelemessov, K.; Karmanov, T.; Kakimov, U.; Kolga, A. Development and justification of a hydro-impulse method for increasing ore permeability in conditions of uranium borehole production. News NAS RK Ser. Geol. Tech. Sci. 2020, 6, 126–133. [Google Scholar] [CrossRef]
- Rakishev, B.; Yazikov, E.G.; Mataev, M.M.; Kenzhetayev, Z. Studies of uranium leaching from core sample in tubes using an oxidizer. Gorn. Zhurnal 2021, 9, 84–89. [Google Scholar] [CrossRef]
- Kenzhetaev, Z.; Togizov, K.; Abdraimova, M.; Nurbekova, M. Selecting the rational parameters for restoring filtration characteristics of ores during borehole mining of uranium deposits. Min. Miner. Depos. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Gorbatenko, O.A. Repair and Restoration Work on Geotechnological Wells of Uranium PSV Enterprises: Textbook; Demekhova, Y.V., Ed.; NAC Kazatomprom: Almaty, Kazakhstan, 2017; 194p. [Google Scholar]
- Golik, V.I.; Dmitrak, Y.V.; Brigida, V.S. Impact of duration of mechanochemical activation on enhancement of zinc leaching from polymetallic ore tailings. Nauk. Visnyk Natsionalnoho Hirnychoho Univ. 2020, 5, 47–54. [Google Scholar] [CrossRef]
- Filipov, A.P.; Nesterov, Y.V. Redox Processes and Intensification of Metal Leaching; Publishing House “Ore and Metals”: Moscow, Russia, 2009; 543p. [Google Scholar]
- Rakishev, B.R.; Matayev, M.M.; Kenzhetayev, Z.S.; Shampikova, A.H.; Toktaruly, B. Innovative methods for intensifying borehole production of uranium in ores with low filtration characteristics. News NAS RK Ser. Geol. Tech. Sci. 2020, 6, 213–219. [Google Scholar] [CrossRef]
- Kosunov, A.O.; Shavanda, V.V.; Kopbaeva, M.P.; Myrzabek, K.A.; Mataev, M.M.; Kenzhetaev, Z.S.; Bishimov, K.E.; Lipinsky, V.K.; Tretyakov, S.Y. Mobile Chemical Treatment Unit Technological Wells. Innovation Patent of the Republic of Kazakhstan No. 2153, 9 September 2016. bulleten No. 8, 28 April 2017. [Google Scholar]




| Composition | Volume of Leaching Solution, m3 | H2SO4, kg | HF, kg | H2O2, kg | Average Inter-Repair Cycle, Days | Uranium Content in PS, mg/L |
|---|---|---|---|---|---|---|
| H2SO4 + HF | 3 | 150 | 300 | 600 | 16 | 10 |
| Mineral | Formula | Syrdarya Depression | Shu-Sarysu Depression | |||
|---|---|---|---|---|---|---|
| Santonian Stage | Maastrichtian Stage | Campanian Stage | Inkuduk Stage | Mynkuduk Stage | ||
| Quartz | SiO2 | 90.8 | 54.7 | 66.3 | 79 | 96 |
| Smectite | KAl2(AlSi3O10) (OH)2 | - | 27.0 | - | ||
| K-feldspar | KAlSi3O8 | 9.2 | 10.1 | 5.7 | 15 | 3 |
| Kaolinite | Al2(Si2O5)(OH)4 | - | 6.7 | 11.6 | 6 | |
| Gypsum | CaSO4 2(H2O) | - | - | 16.4 | ||
| Albite | NaAlSi3O8 | 1 | ||||
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)2(Mg,Fe)3(OH)6 | |||||
| Hematite | Fe2O3 | |||||
| Mineral | Formula | Syrdarya Depression | Shu-Sarysu Depression | |||
|---|---|---|---|---|---|---|
| Santonian Stage | Maastrichtian Stage | Campanian Stage | Inkuduk Stage | Mynkuduk Stage | ||
| Quartz | SiO2 | - | - | 35.6 | 78.2 | 89.9 |
| Gypsum | CaSO4 2(H2O) | 100 | 100 | 16.7 | ||
| Calcite | Ca(CO3) | - | - | 8.9 | ||
| Albite | NaAlSi3O8 | - | - | 33.9 | 6.3 | 4.4 |
| Microcline | KAlSi3O8 | - | - | 4.9 | 15.4 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togizov, K.; Kenzhetaev, Z.; Temirkhanova, R.; Muzapparova, A.; Omirgali, A.; Altaibayev, B. The Influence of the Physicochemical Characteristics of Ores on the Efficiency of Underground Well Leaching of Uranium Deposits in Kazakhstan. Minerals 2024, 14, 381. https://doi.org/10.3390/min14040381
Togizov K, Kenzhetaev Z, Temirkhanova R, Muzapparova A, Omirgali A, Altaibayev B. The Influence of the Physicochemical Characteristics of Ores on the Efficiency of Underground Well Leaching of Uranium Deposits in Kazakhstan. Minerals. 2024; 14(4):381. https://doi.org/10.3390/min14040381
Chicago/Turabian StyleTogizov, Kuanysh, Zhiger Kenzhetaev, Raushan Temirkhanova, Akerke Muzapparova, Armanbek Omirgali, and Bagdat Altaibayev. 2024. "The Influence of the Physicochemical Characteristics of Ores on the Efficiency of Underground Well Leaching of Uranium Deposits in Kazakhstan" Minerals 14, no. 4: 381. https://doi.org/10.3390/min14040381







