Abstract
CO2 mineral carbonation is an important method to sequester carbon dioxide (CO2) in the form of stable mineral carbonates for permanent storage. The slow kinetics of carbonation, especially for iron-rich olivine, is the major challenge for potential application. This work proposes methods to accelerate the mineral carbonation process of different materials in the general mineral grouping of divalent metals–olivine for simultaneous nickel and cobalt recovery. It is found that nickel-olivine is facile for mineral carbonation compared to ferrous and magnesium olivine. Ferrous olivine is the most difficult form of olivine to carbonate as illustrated in both thermodynamics and experimental test results. The increase in iron content in olivine inhibits the CO2 mineral carbonation process by forming an iron-silica-rich passivation interlayer. The use of a reducing gas or reagent can enhance the mineral carbonation of olivine probably through hindering oxidation of Fe(Ⅱ). The addition of sodium nitrilotriacetate (NTA) as a metal complexing agent is much more efficient for the acceleration than usage of a reducing atmosphere. The combination of sodium bicarbonate/CO2 gas supply and NTA can enhance the diffusion of all divalent metal ions from the reacting olivine surface, thereby limiting the formation of the passivation interlayer. Meanwhile, highly selective nickel and cobalt leaching can be simultaneously achieved along with the CO2 mineral carbonation, 94% nickel, and 92% cobalt leaching as well as 47% mineral carbonation versus only 10% iron and 1% magnesium leached in 2 h. This work provides a novel direction to achieve critical metals recovery with accelerated mineral carbonation process.
1. Introduction
CO2 mineral carbonation, also referred as CO2 mineralization, is an important method for carbon capture, utilization, and storage (CCUS) to sequester CO2 in the form of stable mineral carbonates [1,2,3,4,5,6,7]. There are abundant resources suitable for mineral carbonation, including olivine-based ultramafic mine wastes [8,9,10,11] and nickel laterites [12,13]. In the near future, with increasing demands for various materials and critical metals for global energy transition, there will be increasing amounts of mine or alkaline wastes [14,15,16]. These waste materials may have gone through energy-intensive size reduction steps (crushing and grinding) and thus be suitable for mineral carbonation processes through passive carbonation [17] or through an accelerated aqueous carbonation pathway [10,11,18,19,20,21]. In addition, some critical metals present in the waste materials may be recovered through direct aqueous mineral carbonation [12,22,23,24,25,26,27]. However, the slow kinetics of carbonation and the corresponding high capital and operational costs may be major concerns to limit the potential application [5,28,29]. It is desirable to accelerate the mineral carbonation process with efficient critical metal recovery for potential economic feasibility [9,22,26].
CO2 mineral carbonation is a natural weathering process. The rate of carbonation may be accelerated by increasing temperature [18,20], CO2 partial pressure [11,18,20,30], or introducing chemical reagents including sodium chloride [18,31,32], sodium bicarbonate [18,20,32,33], or reducing gas [1,21,34]. The effects of temperature may be associated with CO2 partial pressure (pCO2). For example, the optimum temperature for maximum mineral carbonation of olivine was 175 °C at pCO2 = 34.5 bar [18,20] but then shifted to 185 °C at pCO2 = 150 bar [30]. The deviation from the optimum temperature resulted in a decrease of mineral carbonation efficiency [18,30]. Sodium bicarbonate is much more effective than sodium chloride for acceleration of CO2 mineral carbonation, increasing by around 60% versus by about 20% in 5 h, respectively. Sodium bicarbonate is believed to work as a buffer together with dissolved CO2 to maintain the pH value at 7.2~8.0 and as a catalyst to transfer protons and carbonate ions for olivine dissolution and divalent metals carbonate precipitation [18]. Serpentine (hydrated silicate mineral) can be activated by converting to olivine with heat pre-treatment and thus become suitable as well for accelerated direct aqueous mineral carbonation [10,30]. Since olivine or converted olivine from serpentine may contain divalent metals in various contents that replace magnesium in the crystal structure, an increase in the iron (Fe(II)) content would detrimentally affect the mineral carbonation rate [34]. The feasibility of CO2 mineral carbonation is crucial and even more for iron-silicate mining wastes [35]. Fortunately, use of reducing gas in the carbonation system can accelerate the mineral carbonation process by preventing competitive oxidation of Fe(II) [34]. The suitable reducing gas may include H2 [34] or H2S [1,21]. In addition, using a suitable chelating ligand (e.g., 2,2-bipyridine) may also prevent the oxidation of Fe(II) and facilitate the precipitation of iron carbonates [36].
Furthermore, the critical metals nickel and cobalt may also replace magnesium in the olivine crystal structure and thus may be recovered through acceleration of olivine mineral carbonation—for example, nickel sulfidization [21,25] or selective metal extraction [12,22,23,26,27,37,38,39] during CO2 mineral carbonation. The benefits from the recovered critical metals during mineral carbonation may offset the capital and operational costs and potentially make CO2 mineralization economically feasible [9]. This work investigates the measures to accelerate mineral carbonation of olivine-based materials and to enhance the recovery of critical metals. To simultaneously achieve mineral carbonation and selective metal extraction, disodium ethylenediaminetetraacetate (EDTA) together with sodium bicarbonate are introduced to the carbonation system by stabilizing nickel and cobalt from olivine as complex ions in solution and precipitating magnesium and iron as mineral carbonates [12].
2. Materials and Methods
2.1. Materials
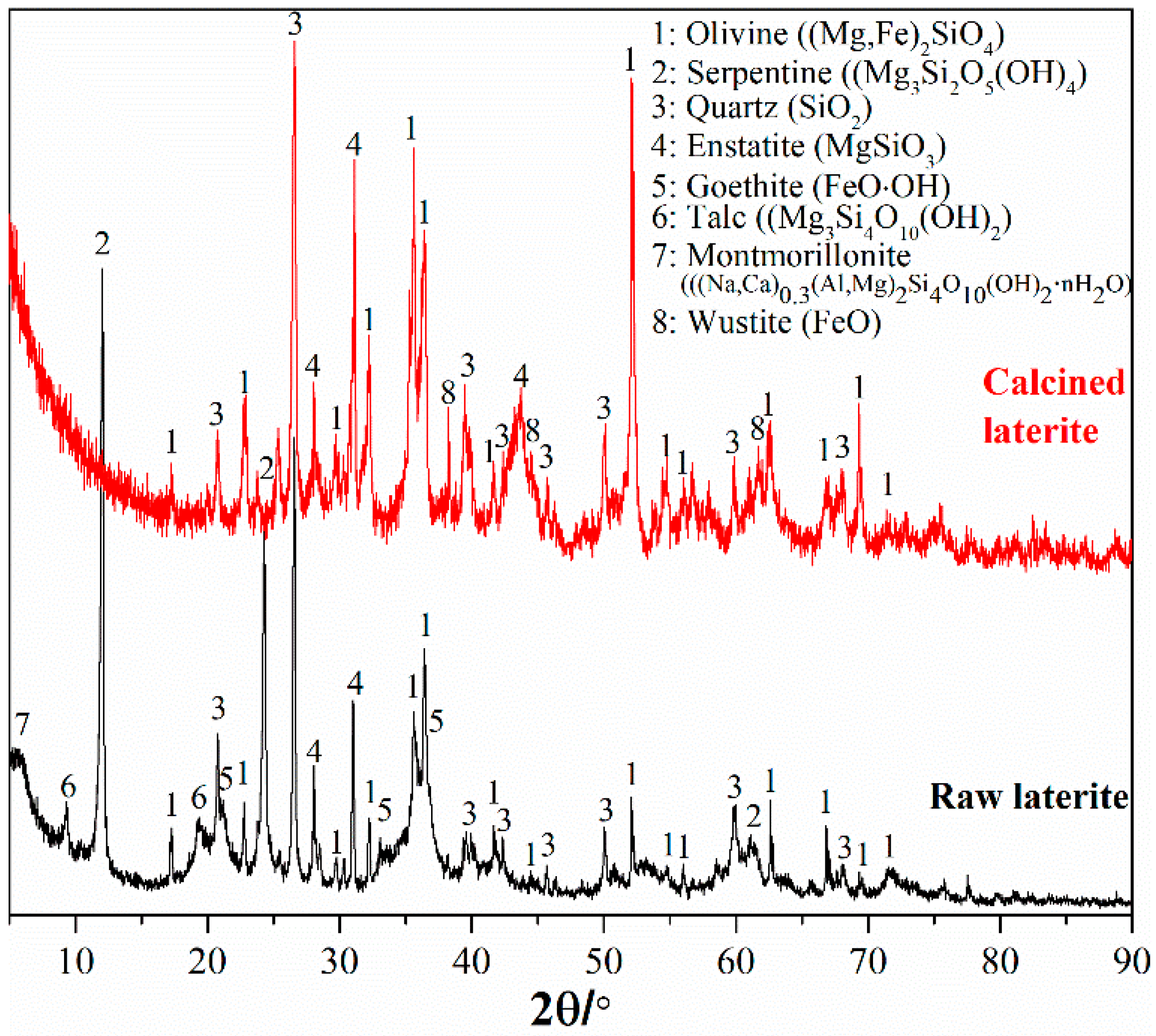
A saprolite laterite sample, weathered magnesium-/iron-silicate hydrate laterite, was used in this study. The chemical and mineral composition are shown in Table 1 and Figure 1. This laterite contains 2.28% nickel and 0.063% cobalt as critical metals for recovery. The corresponding theoretical mineral carbonation capacity based on total magnesium, iron, and calcium content [18,20] is 363 kg CO2 per ton of laterite. Since the dominant mineral for this laterite is serpentine (Figure 1), a heat pre-treatment [39] is required for activation to convert the hydrated silicate minerals to reactive olivine. Meanwhile, a reducing gas is also required to avoid oxidation of Fe(II) in hydrated silicate minerals and convert ferric (hydr)oxide (goethite, Figure 1) to reactive ferrous oxide during the heat pre-treatment. Therefore, this laterite was calcined at 700 °C in a tube electric furnace with a gas mixture of 5% CO and 95% N2 replenished at 300 mL/min flow rate and 1 h treatment. The mineral composition of the calcined laterite is also shown in Figure 1. All the hydrate silicate minerals including serpentine converted to olivine. Goethite also converted to reactive ferrous oxide (wustite). Therefore, the previous fundamental studies about olivine mineral carbonation [10,12,18,20,26,27,34] can be a suitable basis for carbonation of the calcined laterite.

Table 1.
Chemical composition of the laterite sample, wt%.
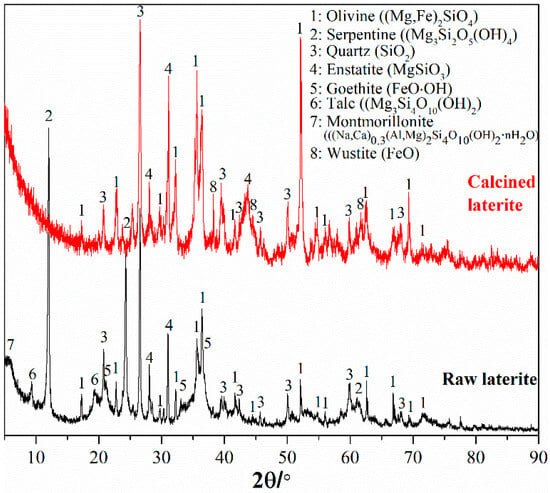
Figure 1.
Mineral composition of the raw laterite sample and the heat pre-treated laterite.
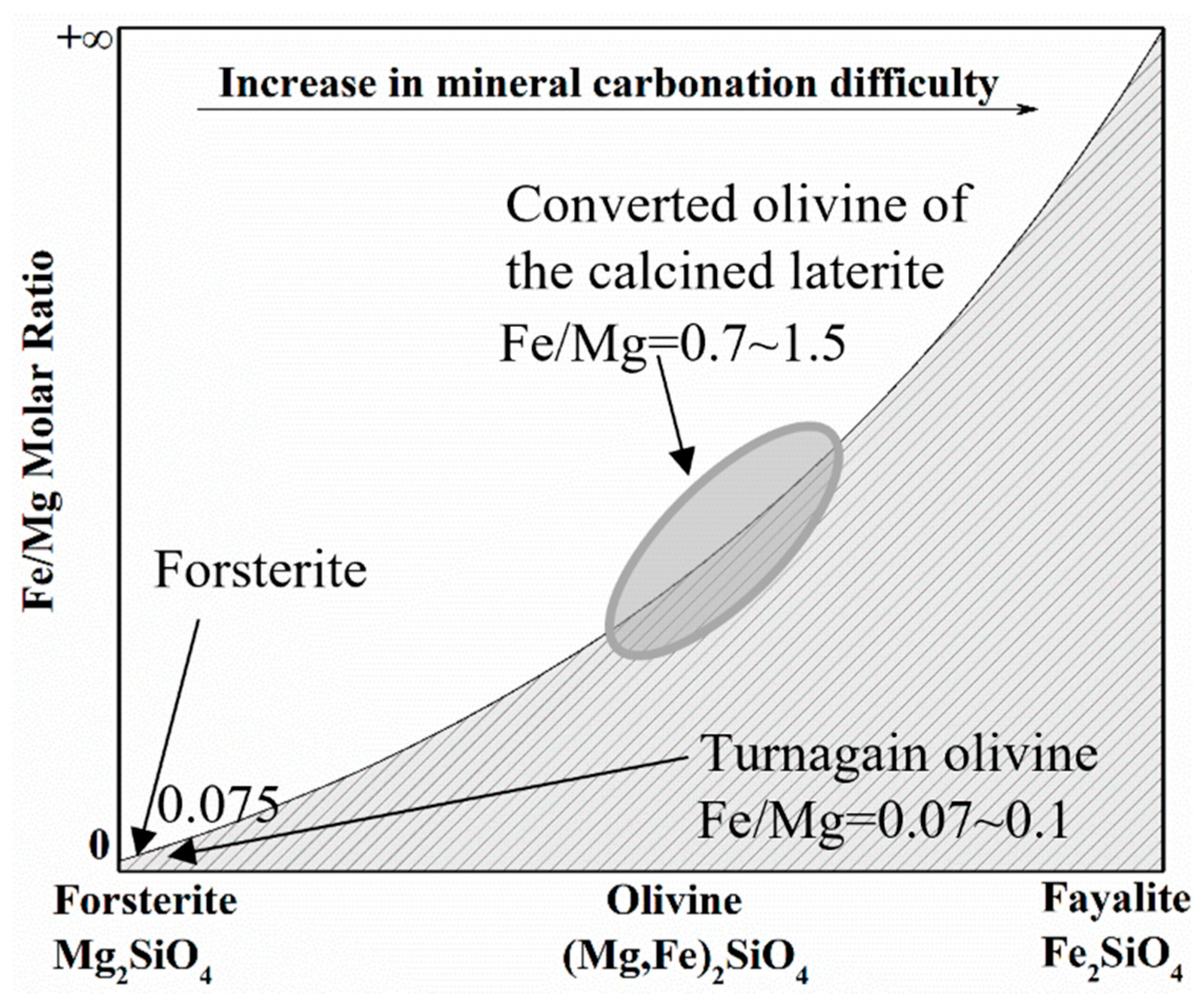
The iron/magnesium molar ratio in the converted olivine of the calcined laterite is high at 0.7~1.5 compared to around 0.07~0.1 for previously used forsterite [18] and Turnagain deposit samples [10]. The iron/magnesium molar ratio was determined by scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) analysis. As shown in Figure 2, based on the work of Wood et al. [34], the mineral carbonation of the iron-rich olivine in the calcined laterite is expected to be difficult. Therefore, a reducing atmosphere, consisting of CO gas (>99% purity, Praxair, Vancouver, BC, Canada) or sodium sulfite solution (≥98% purity, Sigma-Aldrich, Oakville, ON, Canada), was introduced to the mineral carbonation system to accelerate the reaction. CO2 or CO gas was continually dispersed into the solution throughout the mineralization reaction. A complexing reagent, nitrilotriacetate sodium salt (NTA, >99% purity, Sigma-Aldrich, Oakville, ON, Canada), was also used to accelerate the mineral carbonation as an alternative method [22,26,39].
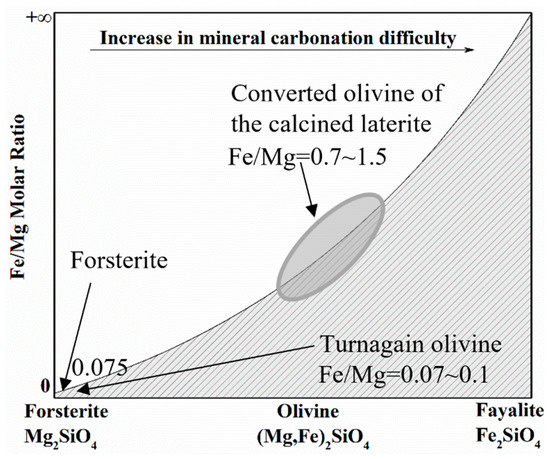
Figure 2.
Schematic diagram for effects of iron/magnesium molar ratio in olivine on CO2 mineral carbonation.
2.2. Methods
Based on our previous work on mineral carbonation of olivine [18], the mineral carbonation of the calcined laterite was carried out in a 600 mL stainless autoclave (Model 5103) at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate (NaHCO3), and 0.5 wt% solid content. The solid content (0.5 wt%) was far lower than the solid content applied in industries (~30–40 wt%) due to the availability of material from the heat pre-treatment through the laboratory furnace (RSRB 80-750/11, Nabertherm, Lilienthal, Germany). Based on the previous study [12], EDTA was preloaded with the carbonation system at the optimized molar ratio EDTA/Ni(total) = 1.2 (13 mM EDTA) to selectively stabilize released nickel and cobalt from olivine into an aqueous solution. After mineral carbonation, the slurry was then filtered to obtain solid residue for LECO carbon analysis (LECO CS3200, LECO Corporation, St. Joseph, MI, USA) to calculate the mineral carbonation efficiency and to separate aqueous solution for inductively coupled plasma atomic emission spectroscopy analysis (ICP-AES) to calculate the metal extraction efficiency. As described by the previous study [12], the mineral carbonation efficiency was the percentage of practical mineralized CO2 over the theoretical mineral carbonation capacity based on the contents of magnesium, iron, and calcium in the feed material; the metal extraction efficiency was the percentage of extracted metal in aqueous solution over the total metal in the feed material. The relative error of the mineral carbonation and metal extraction efficiency was determined by at least three replicated tests at less than ±2%. All the experimental conditions have been summarized in Table 2. To investigate the mechanism of mineral carbonation of the pretreated laterite, X-ray diffraction (XRD, Rigaku Multiflex, Tokyo, Japan) and SEM-EDX (Quanta 650, FEI Company, Hillsboro, OR, US) were used to analyze the outer surface and transverse surface of reacted laterite residue. The SEM-EDX analysis for the transverse surface of particles followed the same procedures as our previous study on CO2 mineral carbonation of forsterite [18].

Table 2.
Summary of experimental conditions for simultaneous nickel and cobalt recovery with accelerated mineral carbonation.
3. Results and Discussion
3.1. Thermodynamics
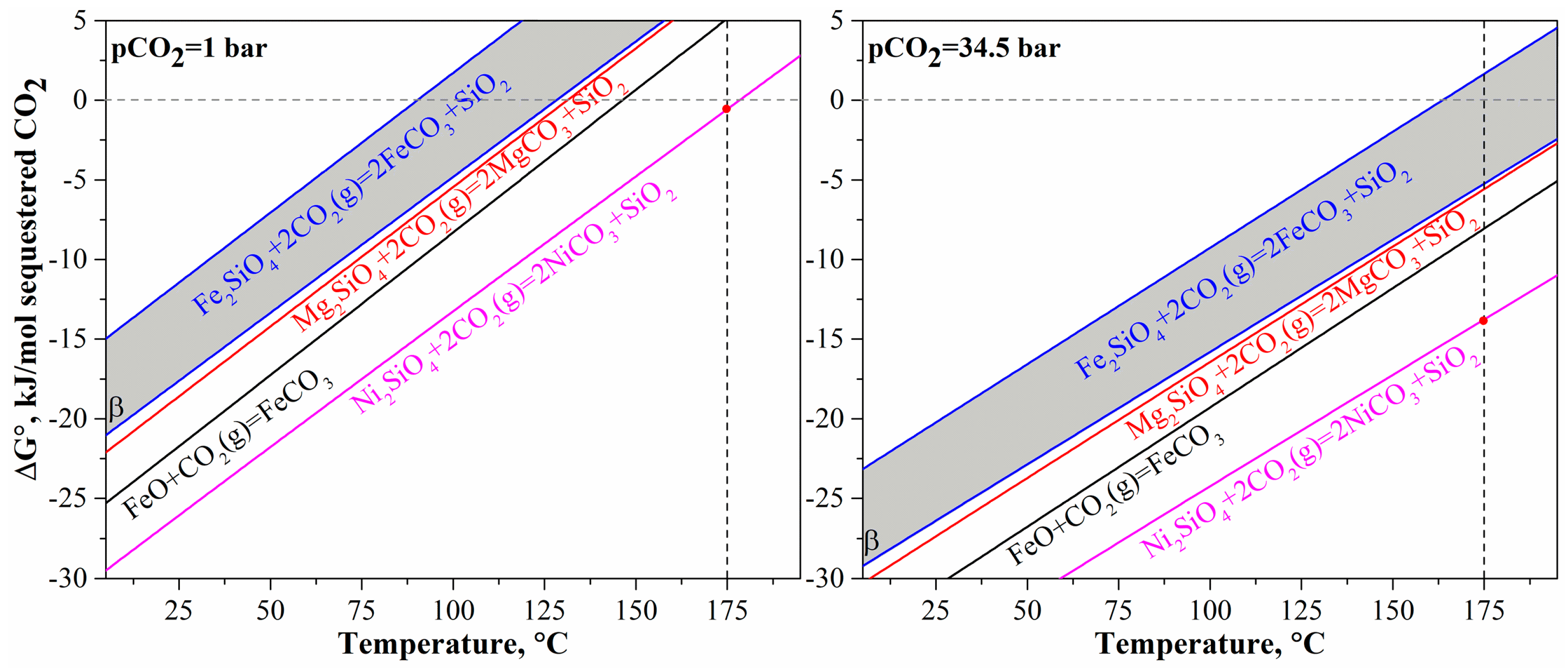
As discovered in the previous study [12], mineral carbonation provides the condition for simultaneous nickel and cobalt recovery from olivine. Prior to mineral carbonation tests, it is necessary to analyze the thermodynamics for the overall reaction of olivine mineral carbonation. It is well-known for the overall carbonation reaction that CO2 is stabilized by olivine to form mineral carbonates and to generate silica. However, there may be various divalent metals (Mg, Fe(II), Ni) in the olivine crystal structure, and the different metals contained in olivine have different thermodynamic behaviors as shown in Figure 3. The thermodynamic data are collected from the database of HSC Chemistry 7.1 (Outotec, Finland). Generally, nickel olivine (Ni2SiO4) is much more thermodynamically favorable for mineral carbonation than the other olivine types including magnesium olivine (Mg2SiO4, forsterite) and ferrous olivine (Fe2SiO4, fayalite), even at pCO2 = 1 bar and 175 °C. In contrast, fayalite is the least favorable in the thermodynamics for mineral carbonation. This order of thermodynamic favorability is in accordance with the experimental results of Wood et al. [34] that showed an increase in iron content in olivine would decrease the mineral carbonation efficiency. The mineral carbonation of ferrous oxide (wustite), which can be formed through calcination/reduction of laterites, is between magnesium silicate and nickel silicate in thermodynamics. The effects of temperature and pCO2 on the mineral carbonation of olivine are also shown in Figure 3. An increase in temperature makes the carbonation of all types of olivine and ferrous oxide less favorable. The mineral carbonation of all materials is favorable at room temperature even at PCO2 = 1 bar but gradually becomes unfavorable at 91~129 °C, 132 °C, 147 °C, and 179 °C for Fe2SiO4, Mg2SiO4, FeO, and Ni2SiO4, respectively. The detrimental change may be because of the decreasing solubility of CO2 with temperature (less than 200 °C). The increase in pCO2 from 1 bar to 34.5 bar can then make the reaction thermodynamically favorable at elevated temperatures, although mineral carbonation of ferrous olivine may still be unfavorable at a temperature of >165 °C. This may be the reason why high pCO2 is required for mineral carbonation of olivine at high temperatures [18,20,30]. Nevertheless, it can be concluded through the thermodynamic analysis that the increase in nickel content in olivine would make the olivine mineral carbonation more favorable whereas the increase in iron content would make the reaction less favorable. Since the converted olivine of the pre-treated laterite contains high iron content with Fe/Mg ratio at 0.7~1.5 (molar ratio) and nickel content at an average of >2 wt%, the CO2 mineral carbonation of the pre-treated laterite should be studied to determine the impact of the compositional variation in content of the different divalent metals.
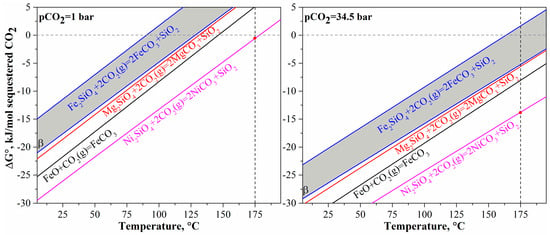
Figure 3.
Thermodynamics of CO2 mineral carbonation of olivine in Mg2SiO4, Fe2SiO4, Ni2SiO4, and wustite FeO.
3.2. Mineral Carbonation in Presence of EDTA
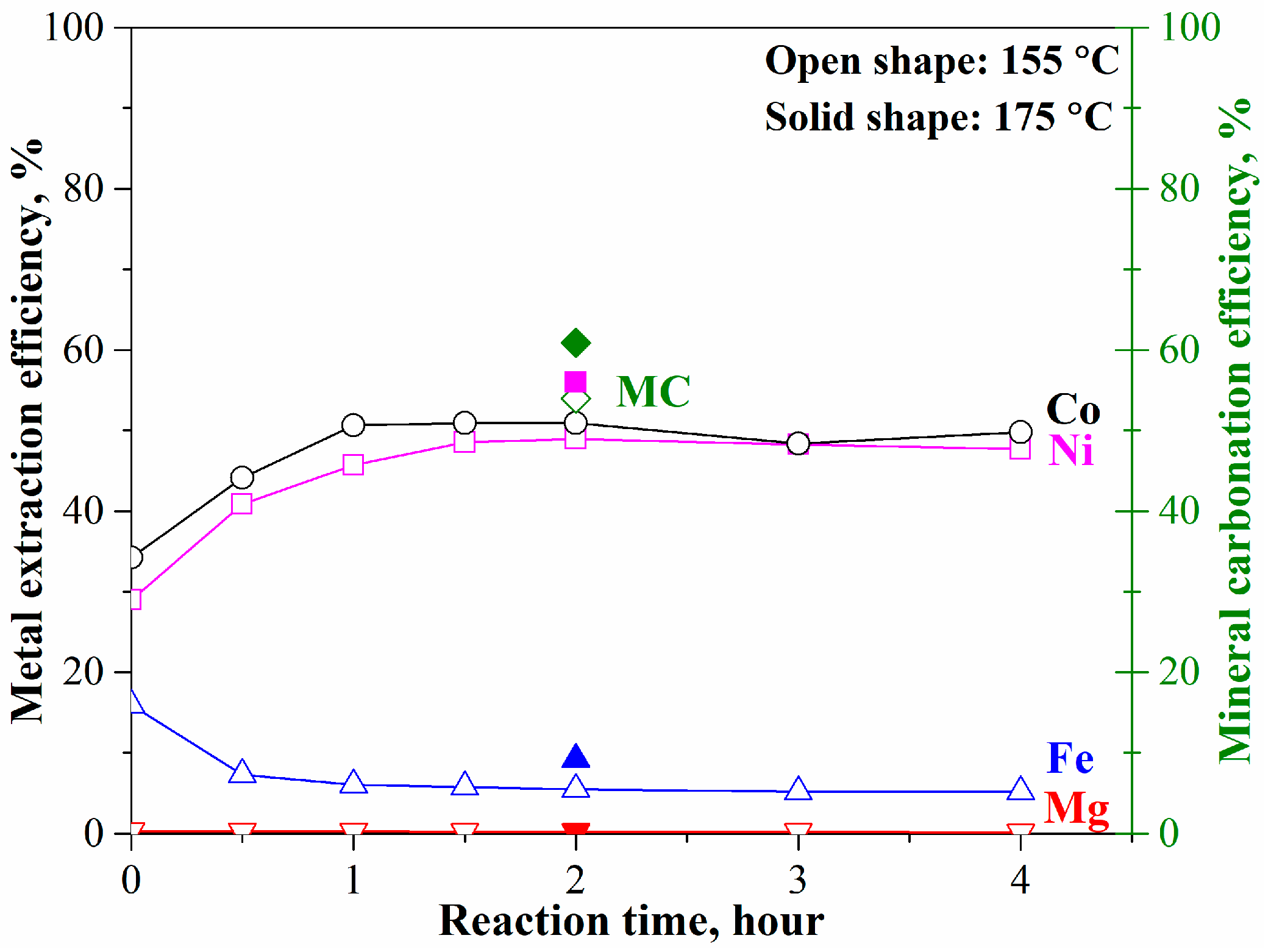
The mineral carbonation tests of the pre-treated laterite with converted olivine were carried out at 175 °C (or 155 °C), pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2. As shown in Figure 4, the leached nickel and cobalt at 155 °C gradually increased with time to 49% at 2 h and then remained unchanged. The corresponding mineral carbonation efficiency was 54% at 2 h. With temperature increasing to 175 °C, there were slight increases in metal extraction and mineral carbonation, to 56% for nickel and cobalt leaching efficiency and to 60% for mineral carbonation efficiency. The iron and magnesium leaching efficiency was less than 9% and 0.2%, respectively, because of the strong competition among the divalent elements and the lowest priority of iron– and magnesium–EDTA complex ions [12]. The test results indicate that the maximum mineral carbonation and nickel/cobalt leaching efficiency through the conventional carbonation method [10,18,20] would be just around 60% and 56% in 2 h, respectively. The further increase in reaction time did not enhance the mineral carbonation process. This result is different from the previous study on forsterite (Fe/Mg molar ratio = 0.075) [18].
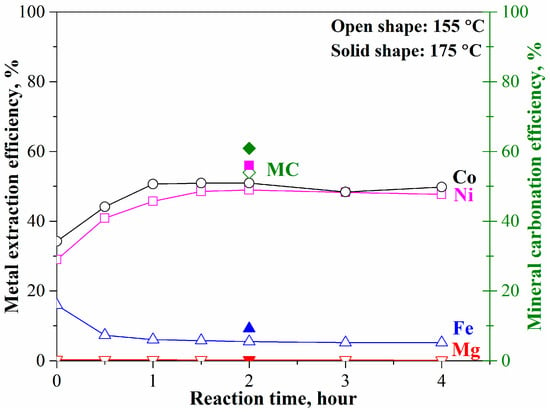
Figure 4.
Mineral carbonation and selective nickel and cobalt extraction from the pre-treated laterite at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2.
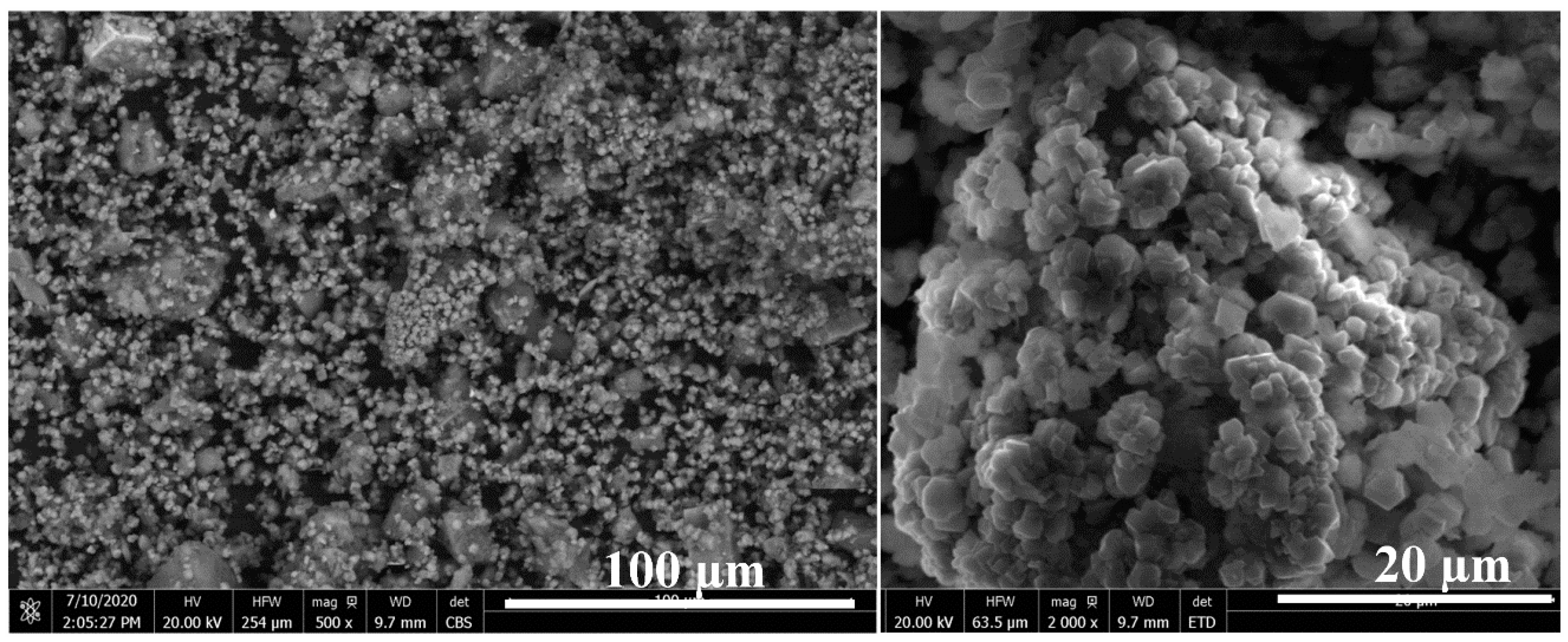
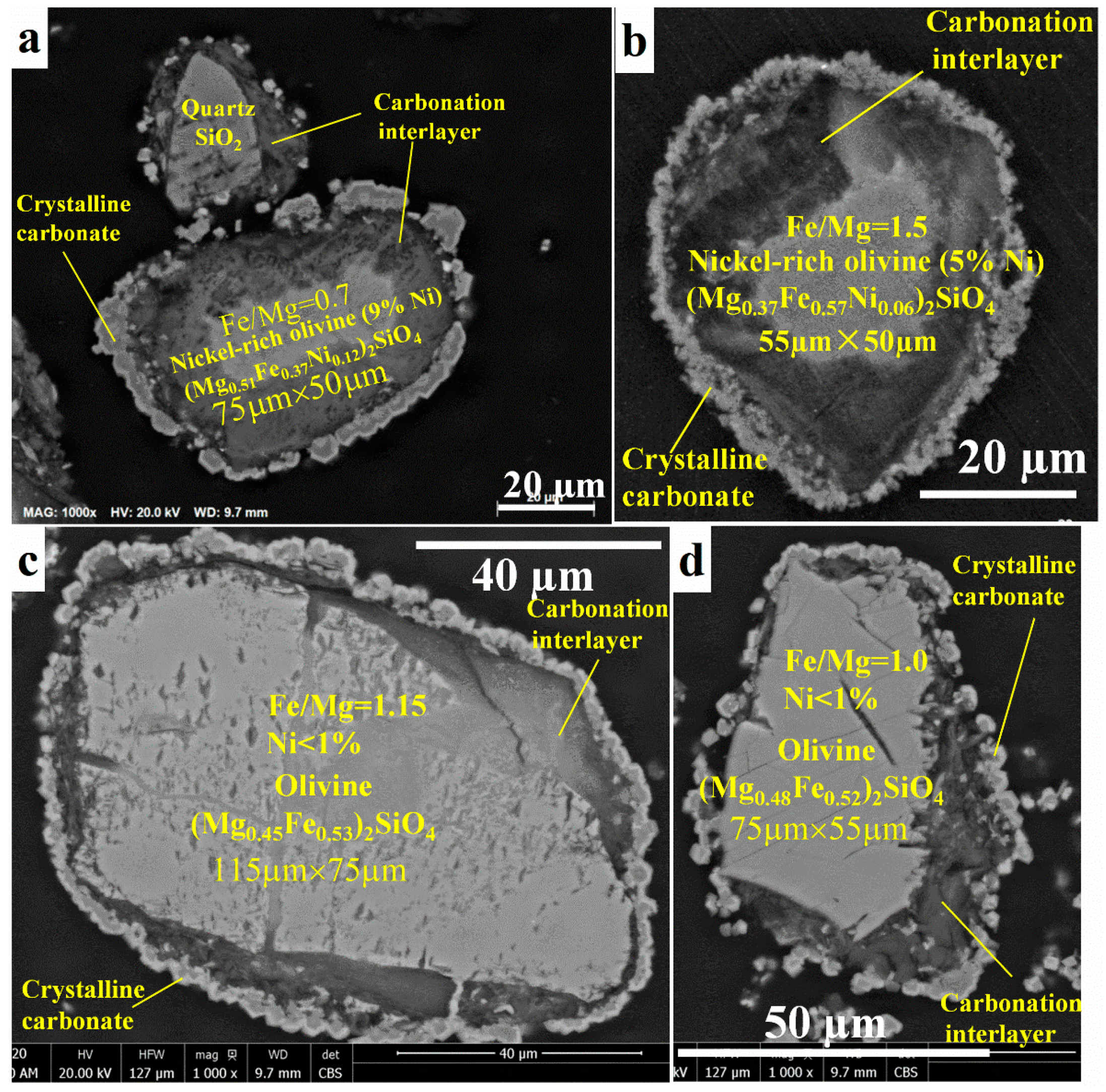
The SEM images of the outer surface of the reacted residue (Figure 5) confirm the formation of stable mineral carbonates that is in accordance with the previous work on forsterite [18]. The carbonates are in the form of magnesium and iron carbonate mixtures [18]. However, the SEM-EDX images of the transverse surface of the reacted olivine (Figure 6) show a passivation interlayer between the unreacted olivine core and the crystalline carbonate layer, while there was no passivation layer as a porous void for the reaction of forsterite at the identical conditions [18]. In detail, there are also two different reaction behaviors of olivine carbonation due to the different nickel content. The reaction rate of nickel-rich olivine containing more than 5 wt% nickel (Figure 6a,b) was much faster by presenting a smaller unreacted olivine core size than that of nickel-poor olivine containing less than 1 wt% nickel (Figure 6c,d). Despite the different iron content in olivine for which the Fe/Mg molar ratios were 0.7 for olivine containing 9 wt% nickel (Figure 6a) and 1.5 for olivine containing 5 wt% (Figure 6b), most of the olivine particle (more than 60%) has been reacted and left a small unreacted olivine core surrounded by a large passivation interlayer, followed by a crystalline carbonate layer. In contrast, most of the olivine containing <1 wt% nickel remained unreacted with a small passivation interlayer between the unreacted core and crystalline carbonate layer (Figure 6c,d), although the Fe/Mg molar ratio was around 1.1 in the range of 0.7~1.5. According to the volume ratio of the unreacted olivine core over the original olivine based on the SEM-EDX analysis, the mineral carbonation rates of the olivine containing >5 wt% nickel were roughly twice those of the mineral carbonation process of the olivine containing <1 wt% nickel, regardless of the Fe/Mg molar ratio. This indicates that the nickel content in olivine may be more important than iron content to affect the olivine mineral carbonation process. The higher the nickel content, the easier the olivine mineral carbonation.
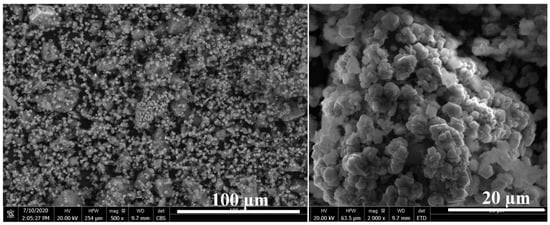
Figure 5.
Outer surface of reacted laterite after mineral carbonation at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2.
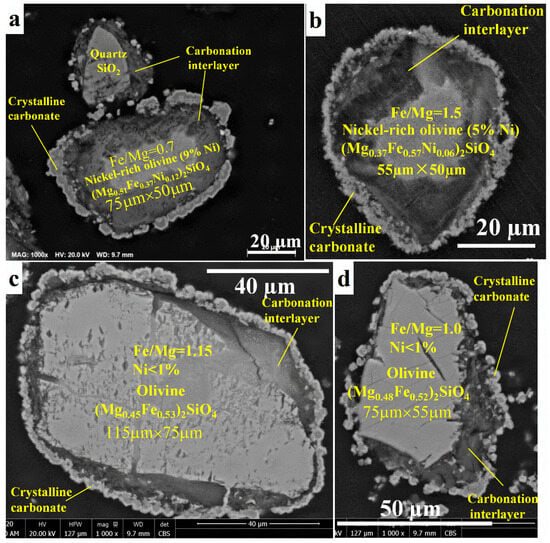
Figure 6.
Transverse surface of reacted olivine particles after mineral carbonation at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2: (a,b) nickel-rich olivine (containing >5 wt% nickel) and (c,d) nickel-poor olivine (containing <1 wt% nickel).
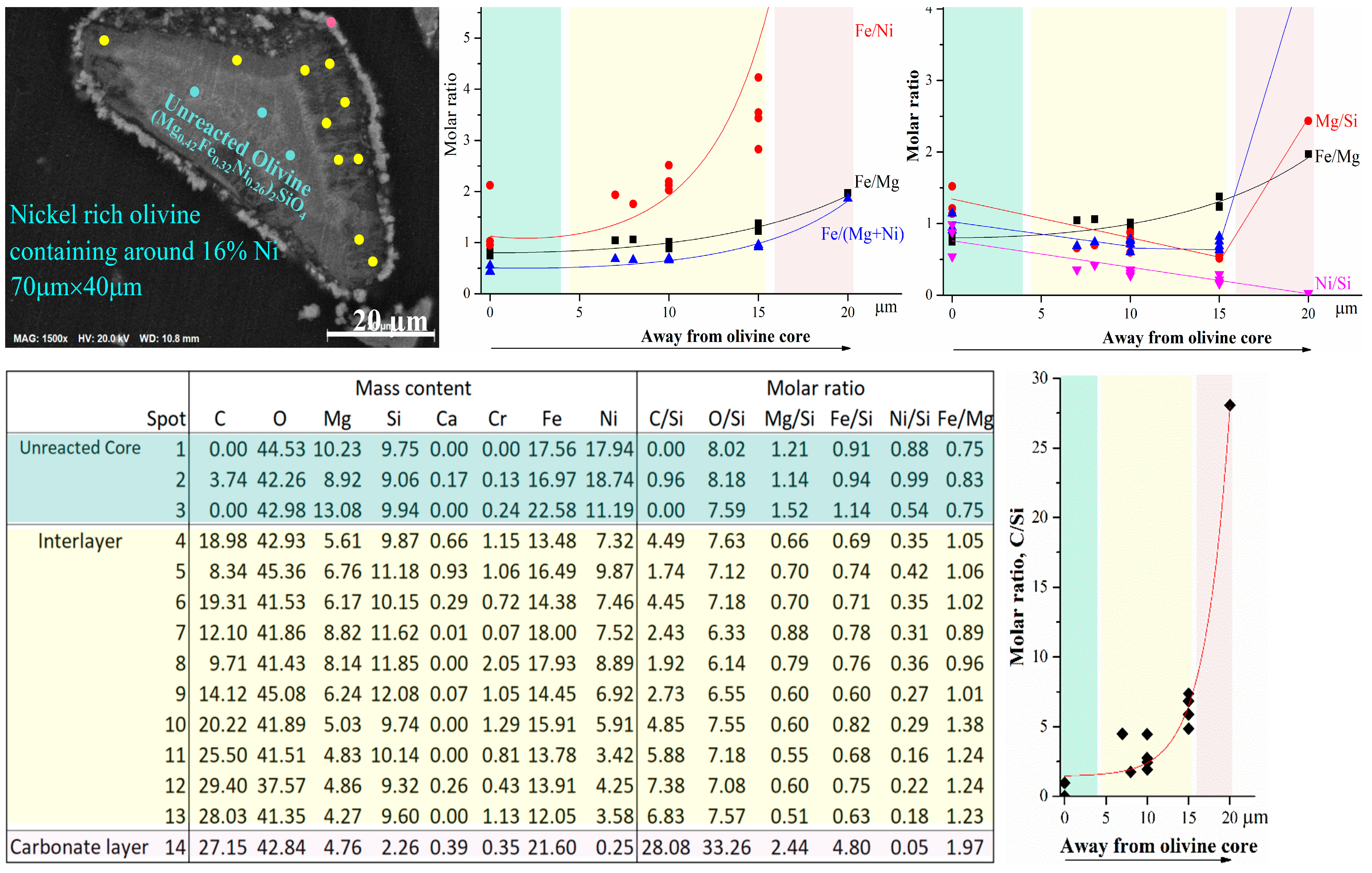
To investigate the formation of the passivation interlayer during mineral carbonation, a detailed SEM-EDX analysis was carried out (as shown in Figure 7) by showing the change in chemical composition of different layers. The molar ratio of iron over the other elements may reveal the reasons. With distance away from the unreacted olivine core, the molar ratio of iron/nickel (Fe/Ni) dramatically increased. This is mainly due to the considerable decrease in nickel content with distance, although iron content also slightly decreased. Nickel was not detected in the crystalline carbonate layer (the precision of the SEM-EDX instrument in analyzing the elements was less than 1 wt% and the analysis volume of the EDX instrument for each spot was around 3 µm3) due to the stabilization by EDTA as complex ions in solution. The molar ratio of iron/magnesium (Fe/Mg) also gradually increased with increase in the distance, but the trend was much milder than that of Fe/Ni. Similar to the change in nickel, the magnesium content also gradually decreased with a stronger trend than the iron content. Correspondingly, the molar ratio of Fe/(Mg + Ni) also gradually increased with distance. The changes in Ni, Mg, and Fe are in accordance with the thermodynamic analysis (Figure 3). Ni olivine was the easiest for mineral carbonation, followed by Mg olivine and then Fe olivine. The changes in molar ratio of divalent metals/silicon (Si) with distance away from the unreacted olivine core can further confirm the effects of the divalent metals on the passivation layer. Although the Fe/Mg molar ratio gradually increases with distance, both Mg/Si and Fe/Si ratio gradually decreased in the passivation interlayer, followed by a significant increase in the crystalline carbonate layer. Silicon remained in the passivation interlayer compared to diffusion of Mg and Fe from olivine to the carbonate layer. The Ni/Si molar ratio proportionally decreased with distance and reached 0 at the carbonate layer. Ni also gradually diffused throughout the passivation interlayer but with a stronger rate than Mg and then Fe and subsequently into aqueous solution as a Ni-EDTA complex ions. As a result, the passivation layer mainly contained Fe and Si and thus was considered as silica-iron-rich layer. In contrast, the carbon content (C/Si molar ratio) exponentially increased with distance (Figure 7), which reveals the gradual diffusion of (bi)carbonate ions from the bulk solution to the surface of the unreacted olivine core to facilitate olivine dissolution and the mineral carbonation process [18].
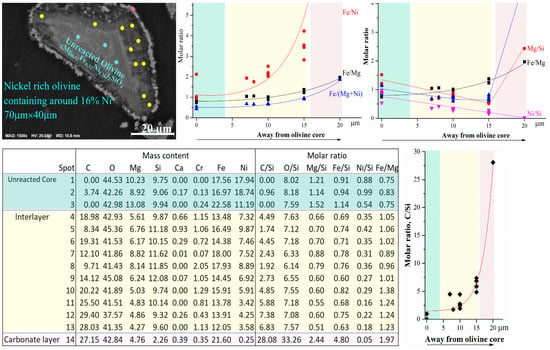
Figure 7.
SEM-EDX analysis of passivation layer of the reacted olivine after mineral carbonation.
Therefore, there may be two major pathways to enhance the mineral carbonation process. The first one is to use a reducing atmosphere to prevent the potential oxidation of Fe(II) [34] and thus to facilitate the Fe diffusion away from the passivation interlayer into aqueous solution and make it available for carbonation. In this case, introducing CO or sodium sulfite into the mineral carbonation system can be tested for the acceleration of the carbonation reaction. The second one is to use a suitable complexing ligand to enhance the dissolution of Fe and Mg away from the passivation interlayer and olivine core so that the unreacted olivine can continue to react. In the following context, a complexing ligand NTA was also used to enhance the olivine dissolution and the corresponding mineral carbonation process.
3.3. Mineral Carbonation Accelerated by Reducing Atmosphere
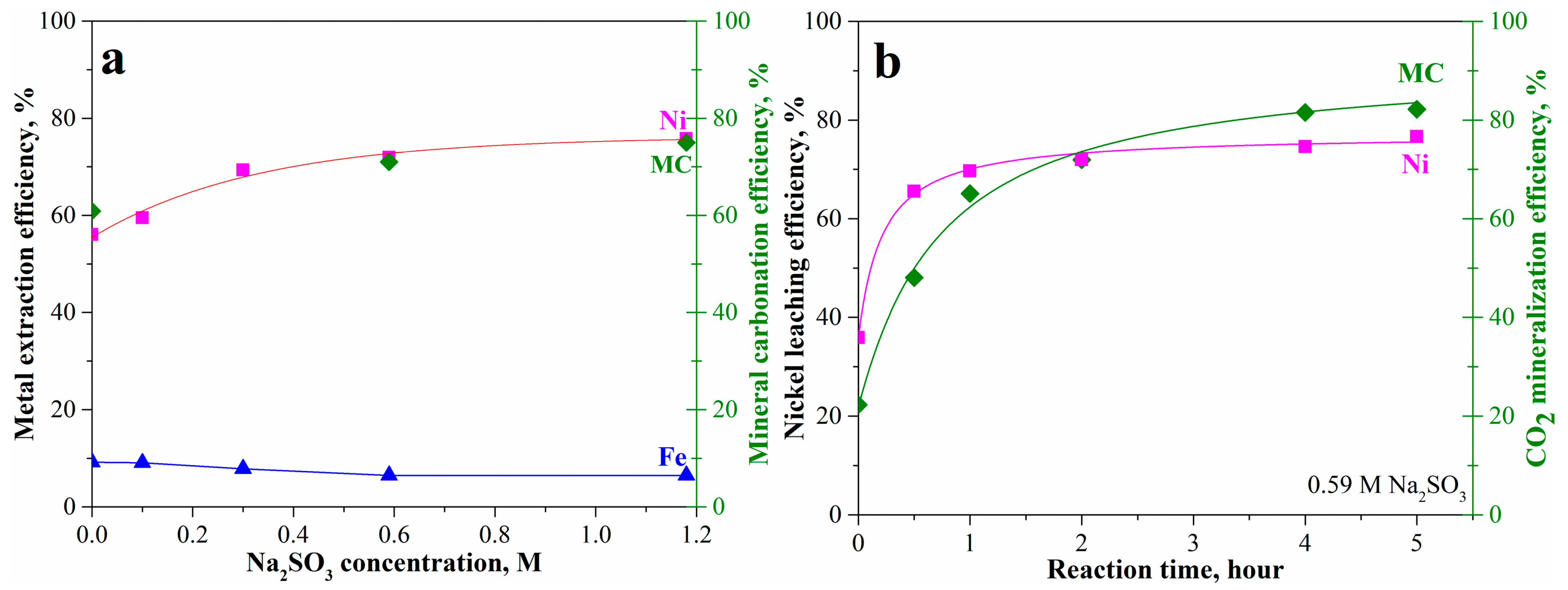
As inspired by the preceding SEM-EDX analysis, our previous work [21], and Wood et al. [34], usage of a reducing atmosphere can potentially enhance the mineral carbonation of olivine containing high iron content. Herein, CO gas and sodium sulfite soluble salt additions were used. It is obvious that introducing sodium sulfite can increase the mineral carbonation and the corresponding nickel extraction efficiency as shown in Figure 8. With increase in sodium sulfite to 0.59 M, the nickel leaching efficiency gradually increased from 56% to 72% together with 72% mineral carbonation efficiency in 2 h (Figure 8a). The further increase in sodium sulfite to 1.18 M slightly increased nickel leaching and mineral carbonation to 75%. Correspondingly, the iron leaching efficiency gradually decreased from 9% to 6%. At 0.59 M sodium sulfite, the nickel leaching and mineral carbonation rapidly increased within the first 2 h followed by slightly increasing to 77% and 82% at 5 h, respectively, as shown in Figure 8b. This shows that the mineral carbonation has been significantly enhanced by the addition of sodium sulfite, although the passivation interlayer still existed. The increase in sodium sulfite concentration can benefit the mineral carbonation process.
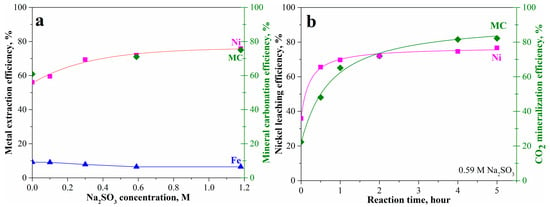
Figure 8.
Mineral carbonation and nickel leaching of the pretreated laterite accelerated by sodium sulfite at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2: (a) effects of sodium sulfite concentration and (b) reaction with time.
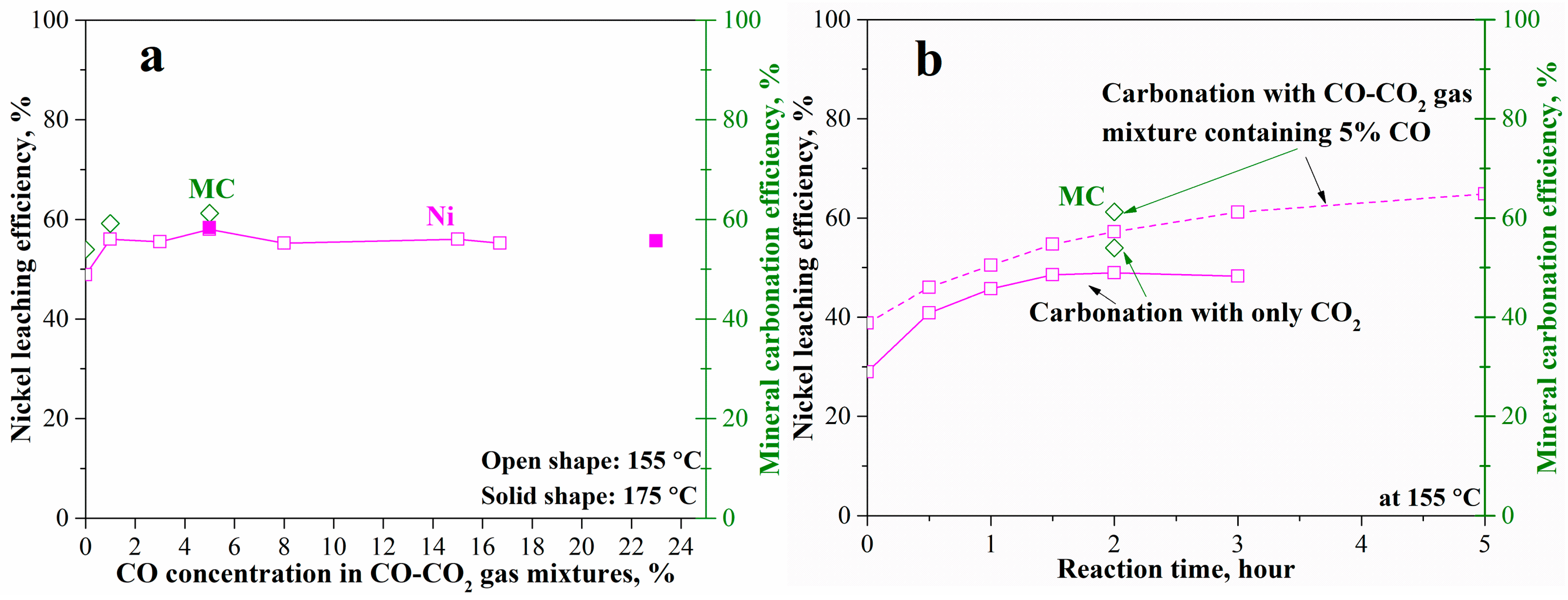
The effects of CO concentration in the CO-CO2 (pCO2 = 34.5 bar) gas mixtures on the mineral carbonation and nickel leaching are shown in Figure 9. The introduction of CO even at 1% increased the nickel leaching and mineral carbonation efficiency at 155 °C from 49% and 53% to 57% and 59% (Figure 9a), respectively. However, the increase of CO concentration (to around 20% in the CO-CO2 gas mixture) has no further beneficial effects. Furthermore, the increase in temperature to 175 °C did not obviously increase the mineral carbonation process as shown in Figure 9a, due to the high iron content in olivine. Compared to sodium sulfite, usage of CO produced a lower enhancement probably due to the much lower solubility compared with CO2. The solubility of CO in water at 175 °C even at 13.8 bar CO pressure was still less than 0.01 M. Therefore, the increase in CO concentration (% in CO-CO2 gas mixtures with pCO2 maintaining at 34.5 bar) did not obviously increase the aqueous CO concentration (M) in the carbonation system, although the introduction of CO resulted in a reducing atmosphere. The effects of a reducing atmosphere by using CO are shown in the nickel leaching efficiency with time in detail (Figure 9b). With the increase in reaction time, the nickel leaching efficiency gradually increased to 57% in 2 h and 65% in 5 h at 155 °C. In contrast, the nickel leaching without using CO remained unchanged at 49% after 2 h. The reducing atmosphere can enhance the diffusion of divalent metals throughout the passivation interlayer. However, the enhancement by either CO or sodium sulfite is not strong enough as the mineral carbonation and nickel leaching still need further improvements. Therefore, using a suitable complexing ligand was tested as an alternative enhancement method as shown in the following context.
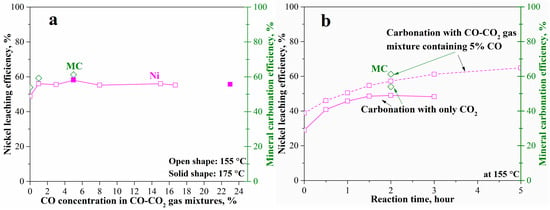
Figure 9.
Mineral carbonation and nickel leaching of the pre-treated laterite accelerated by CO-CO2 gas mixtures at 175 °C, pCO2 = 34.5 bar, 1.5 molal sodium bicarbonate, and EDTA/Ni = 1.2: (a) effects of CO concentration and (b) reaction with time.
3.4. Mineral Carbonation Accelerated by Complexation
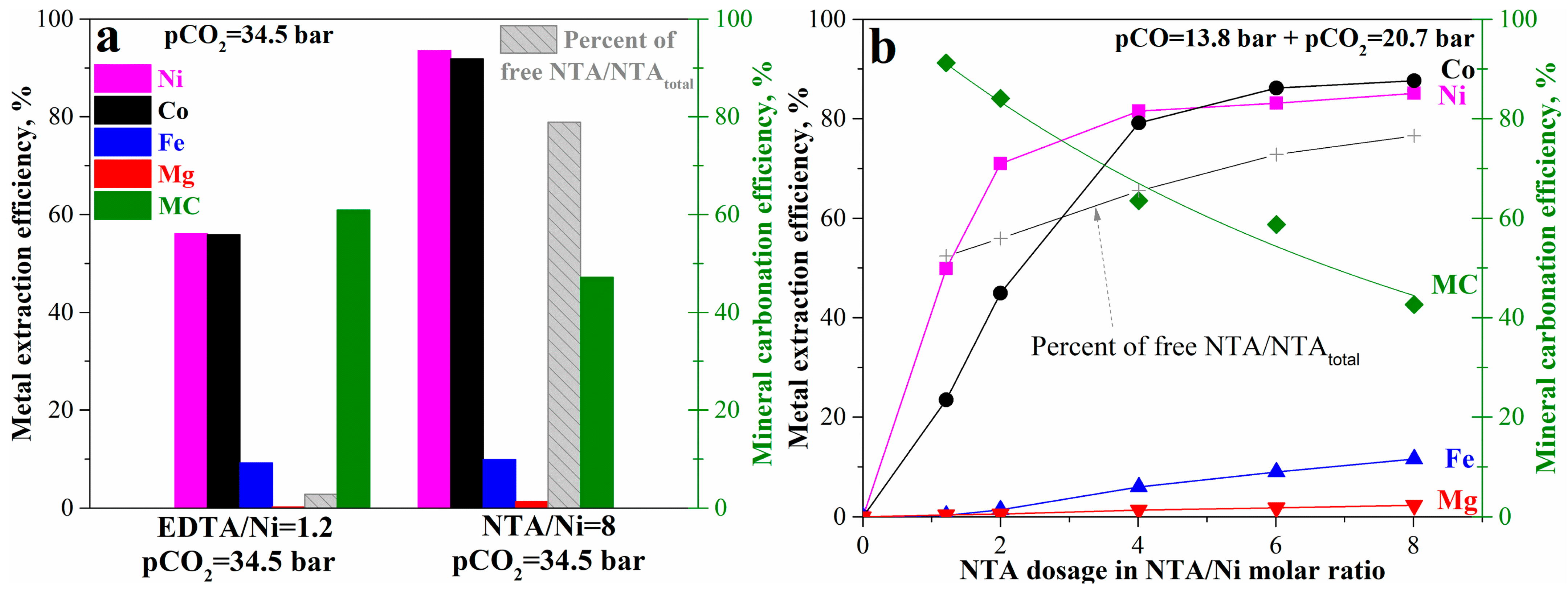
An alternative method to enhance the mineral carbonation and nickel extraction process is to add a suitable complexing ligand. Herein, NTA was used to replace EDTA. As shown in Figure 10a, usage of NTA at NTA/Ni = 8 (molar ratio) can significantly increase nickel and cobalt extraction efficiency from 56% at EDTA/Ni = 1.2 to 94% and 92%, respectively, while there was no obvious change in iron and magnesium leaching efficiency. Based on the metal extraction efficiency (which usually corresponds to mineral carbonation efficiency based on our previous study [12], the mineral carbonation process should have been close to complete, also at around 94%. However, the mineral carbonation efficiency at NTA/Ni = 8 was only 47%, lower than 60% at EDTA/Ni = 1.2. The difference between expectation and the experimental results may be due to the competition between NTA complexation and carbonate precipitation. Different from EDTA, NTA has a similar strength for metal-complex ions to the corresponding metal carbonates [24,26,37]. As a result, the majority of the added NTA (NTA/Ni = 8) was maintained as free NTA ions (HNTA2−), occupying 79%, while the free EDTA ions (H2EDTA2−) accounted for only 2% of the added EDTA. EDTA was too strong, so almost all the EDTA complexed the released divalent metal ions from mineral carbonation and lost the ability to further bind the divalent metals in the passivation layer. In contrast, the dominant free NTA ions that remained in the bulk solution kept the complexing ability on divalent metals in the passivation layer and the unreacted olivine core. The divalent metals were significantly released from olivine by complexation and diffused into an aqueous solution as NTA complex ions followed by precipitation as carbonates and free NTA ions, except that nickel– and cobalt–NTA complex ions remained due to the greatest stability among the divalent metals–NTA complex ions. On the other hand, the substantial free NTA ions may also prevent the metal carbonate precipitation because of the competition with complex formation. As a result, the high nickel and cobalt leaching efficiency and relatively mild mineral carbonation efficiency showed up simultaneously. Around 19.6 kg nickel per t sample can be recovered together with 207 kg CO2 gross sequestered per t sample. Therefore, the gross amount of sequestered CO2 during metal production can reach 10.6 tCO2/t nickel recovered. With further consideration of the equivalent CO2 (CO2-eq) emissions due to energy consumption, heat pre-treatment, and processing [22], the potential net value can still reach 3.2 tCO2/t nickel recovered. In terms of carbon intensity, 0.7 t CO2-eq emitted per t CO2 sequestered can be reached, i.e., negative carbon emission. It is significant for similar deposits to reach sustainable productions with carbon neutrality.
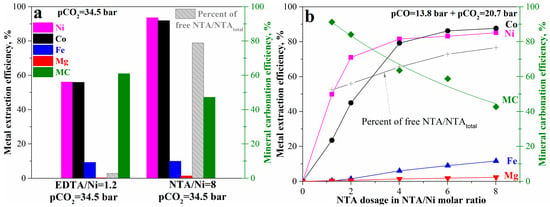
Figure 10.
Mineral carbonation and nickel and cobalt leaching of the pre-treated laterite accelerated by NTA: (a) comparison between NTA and EDTA and (b) effects of NTA dosage.
The effects of NTA concentration at pCO = 13.8 bar and pCO2 = 20.7 bar (Figure 10b) can confirm this explanation. With increase in NTA dosage, nickel and cobalt extraction efficiency significantly increased while mineral carbonation efficiency gradually decreased. At NTA/Ni = 1.2, the same dosage as EDTA/Ni, the mineral carbonation efficiency has reached 91% where nickel and cobalt extraction efficiency were 50% and 23%, respectively. The corresponding free NTA ions also accounted for 52% of the total added NTA. The percentage of free NTA ions gradually increased with the increase of NTA/Ni to 77% at NTA/Ni = 8, while the mineral carbonation efficiency gradually decreased to 43%. In addition, the iron and magnesium extraction also slightly increased with the increase in NTA dosage to 11% and 1.8% at NTA/Ni = 8, respectively. The comparison of mineral carbonation and metal extraction at between pCO2 = 34.5 bar and pCO = 13.8 bar + pCO2 = 20.7 bar at NTA/Ni = 8 can also illustrate the competition. With pCO2 increase from 20.7 bar to 34.5 bar regardless of pCO, the mineral carbonation efficiency increased from 43% to 47% and nickel and cobalt extraction efficiency increased from 87% to around 92%, while iron and magnesium extraction efficiency decreased from 11.6% and 1.8% to 9.8% and 1.4%, respectively. Increase in pCO2 can benefit the carbonate precipitation for carbonation efficiency and make the competition with complex formation stronger. Introducing CO was not significant anymore during the mineral carbonation with complexation by using NTA. The enhancement by the complexing reagent NTA can cover the effects of reductive CO gas. Considering the significant benefits of nickel and cobalt (much higher price than carbon tax) with high extraction rate, the enhancement by a suitable ligand (e.g., NTA) may be the best method for the mineral carbonation of olivine. By utilizing the strong competition between NTA complexation and carbonation precipitation through adjusting NTA concentration or CO2 pressure, the process can also shift the competition more to complex ions or carbonate precipitates as needed by the practical situations.
4. Conclusions
This work investigated the CO2 mineral carbonation and concurrent critical nickel and cobalt extraction from olivine-dominant heat-pretreated saprolite sample and the corresponding methods to accelerate the CO2 mineral carbonation process. It is discovered for the first time that nickel (and cobalt) olivine was the priority for mineral carbonation and metal extraction in both thermodynamics and experimental tests. Iron olivine was the least reactive for mineral carbonation. Due to the different behaviors of various divalent metals–olivine, there was a silica-iron-rich passivation interlayer formed during the simultaneous mineral carbonation and metal extraction between the unreacted olivine core and crystalline carbonate layer. Nickel was the easiest to extract from olivine during the mineral carbonation process, followed by magnesium and then iron. Usage of reducing conditions, e.g., sodium sulfite or CO gas, can enhance the CO2 mineral carbonation process. Using NTA as a complexing ligand was a much better method to significantly accelerate the mineral carbonation and simultaneous metal extraction process. Nickel and cobalt extraction efficiency from the heat pre-treated laterite (where olivine was the major mineral) can reach 94% and 92% in 2 h, respectively, together with 47% mineral carbonation efficiency. The competition between NTA complexation and carbonate precipitation contributed to the major enhancement of the integrated process. This work provides a novel direction to accelerate mineral carbonation and to utilize the CO2 mineralization for critical metals recovery.
Author Contributions
Conceptualization, F.W. and D.D.; methodology, F.W. and D.D.; validation, F.W.; formal analysis, F.W. and D.D.; investigation, F.W.; resources, D.D.; data curation, F.W.; writing—original draft preparation, F.W.; writing—review and editing, D.D. and F.W.; supervision, D.D.; project administration, D.D.; funding acquisition, F.W. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mitacs and LeadFX Inc., grant number IT26205.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Acknowledgments
Berend Wassink, Edouard Asselin, Wenying Liu, and Leili Tafaghodi are specially thanked for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.; Oelkers, E.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Alfredsson, H.A.; Wolff-boenisch, D.; et al. Rapid Carbon Mineralization for Permanent Disposal of Anthropogenic Carbon Dioxide Emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Burton, K.W.; Snæbjörnsdóttir, S.O.; Sigfússon, B.; Aradóttir, E.S.; Gunnarsson, I.; Alfredsson, H.A.; Mesfin, K.G.; Oelkers, E.H.; Gislason, S.R. Rapid CO2 Mineralisation into Calcite at the CarbFix Storage Site Quantified Using Calcium Isotopes. Nat. Commun. 2019, 10, 1983. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Dipple, G.M. Serpentinite Carbonation for CO2 Sequestration. Elements 2013, 9, 115–121. [Google Scholar] [CrossRef]
- Sandalow, D.; Aines, R.; Friedmann, J.; Mccormick, C.; Power, I.; Schmidt, B.; Siobhan, W. Carbon Mineralization Roadmap Draft (ICEF Innovation Roadmap Project, October, 2021). 2021. Available online: https://www.osti.gov/biblio/1829577 (accessed on 25 July 2024).
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- The National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019; ISBN 9780309484527. [Google Scholar]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial Global Carbon Uptake by Cement Carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Hitch, M.; Dipple, G.M. Economic Feasibility and Sensitivity Analysis of Integrating Industrial-Scale Mineral Carbonation into Mining Operations. Miner. Eng. 2012, 39, 268–275. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D. Status of CO2 Mineralization and Its Utilization Prospects. Miner. Miner. Mater. 2022, 1, 4. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetic Evaluation of Mineral Carbonation of Natural Silicate Samples. Chem. Eng. J. 2021, 404, 126522. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Trytten, L.; Hitchins, T. Application and Optimization of a Quantified Kinetic Formula to Mineral Carbonation of Natural Silicate Samples. Miner. Eng. 2021, 161, 106712. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D. Carbon Mineralization with Concurrent Critical Metal Recovery from Olivine. Proc. Natl. Acad. Sci. USA 2022, 119, e2203937119. [Google Scholar] [CrossRef]
- Zappala, L.C.; Balucan, R.D.; Vaughan, J.; Steel, K.M. Development of a Nickel Extraction-Mineral Carbonation Process: Analysis of Leaching Mechanisms Using Regenerated Acid. Hydrometallurgy 2020, 197, 105482. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline Residues and the Environment: A Review of Impacts, Management Practices and Opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Renforth, P. The Negative Emission Potential of Alkaline Materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Watari, T.; McLellan, B.C.; Giurco, D.; Dominish, E.; Yamasue, E.; Nansai, K. Total Material Requirement for the Global Energy Transition to 2050: A Focus on Transport and Electricity. Resour. Conserv. Recycl. 2019, 148, 91–103. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon Mineralization: From Natural Analogues to Engineered Systems. Rev. Mineral Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and Mechanism of Mineral Carbonation of Olivine for CO2 Sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.B.; Jarvis, M.; Hitchins, T. The Technology of CO2 Sequestration by Mineral Carbonation: Current Status and Future Prospects. Can. Metall. Q. 2018, 57, 46–58. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T.; Dyson, D. Quantifying Kinetics of Mineralization of Carbon Dioxide by Olivine under Moderate Conditions. Chem. Eng. J. 2019, 360, 452–463. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T.; Trytten, L. CO2 Mineralization and Concurrent Utilization for Nickel Conversion from Nickel Silicates to Nickel Sulfides. Chem. Eng. J. 2021, 406, 126761. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D. An Integrated Process of CO2 Mineralization and Selective Nickel and Cobalt Recovery from Olivine and Laterites. Chem. Eng. J. 2023, 451, 139002. [Google Scholar] [CrossRef]
- Dreisinger, D.; Wang, F. Concerted Mineral Carbonation and Selective Leaching from Laterites. PCT/CA2021/051445, 21 April 2022. [Google Scholar]
- Wang, F.; Dreisinger, D.; Barr, G.; Wang, F.; Dreisinger, D.; Barr, G. Accelerated CO2 Mineralization and Simultaneous Critical Metal Recovery from Ultramafic Tailings. In Proceedings of the 62nd Conference of Metallurgists, COM 2023, Toronto, ON, Canada, 21–24 August 2023; Springer: Cham, Switzerland, 2023; pp. 45–53. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Barr, G.; Martin, C. Utilization of Copper Nickel Sulfide Mine Tailings for CO2 Sequestration and Enhanced Nickel Sulfidization. In Proceedings of the Minerals, Metals and Materials Series; Springer: Cham, Switzerland, 2022; pp. 227–239. [Google Scholar]
- Wang, F.; Dreisinger, D.; Xiao, Y. Accelerated CO2 Mineralization and Utilization for Selective Battery Metals Recovery from Olivine and Laterites. J. Clean. Prod. 2023, 393, 136345. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D. Enhanced CO2 Mineralization and Selective Critical Metal Extraction from Olivine and Laterites. Sep. Purif. Technol. 2023, 321, 124268. [Google Scholar] [CrossRef]
- Olajire, A.A. A Review of Mineral Carbonation Technology in Sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 Disposal by Means of Silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex Situ Aqueous Mineral Carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, W.K.; Dahlin, D.C.; Nilsen, D.N.; Walters, R.P. Carbon Dioxide Sequestration by Direct Aqueous Mineral Carbonation. In Proceedings of the 25th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 5–8 March 2001; DOE/ARC-2001-029. Available online: http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=897123 (accessed on 25 July 2024).
- Gadikota, G.; Matter, J.; Kelemen, P.; Park, A.-H.A. Chemical and Morphological Changes during Olivine Carbonation for CO2 Storage in the Presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679–4693. [Google Scholar] [CrossRef]
- Wang, F. Mineral Carbonation to Sequester CO2 with Concurrent Metal Sulfidization. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. [Google Scholar]
- Wood, C.E.; Qafoku, O.; Loring, J.S.; Chaka, A.M. Role of Fe(II) Content in Olivine Carbonation in Wet Supercritical CO2. Environ. Sci. Technol. Lett. 2019, 6, 592–599. [Google Scholar] [CrossRef]
- Reynes, J.F.; Mercier, G.; Blais, J.F.; Pasquier, L.C. Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine. Minerals 2021, 11, 343. [Google Scholar] [CrossRef]
- Reynes, J.F.; Mercier, G.; Blais, J.F.; Pasquier, L.C. Carbon Dioxide Sequestration by Mineral Carbonation via Iron Complexation Using Bipyridine Chelating Ligands. Dalton Trans. 2023, 52, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dreisinger, D. Laterites. In Energy Technology 2023, The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2023; pp. 63–74. [Google Scholar]
- Wang, F.; Dreisinger, D. The Extraction of Nickel and Cobalt from Laterite Ores with Concurrent Carbon Sequestration. In Proceedings of the 61st Conference of Metallurgists, COM 2022, Montreal, QC, Canada, 21–24 August 2022; Springer: Cham, Swizerland, 2023; pp. 797–809. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Xiao, Y. Pre-Treatment through Reductive Calcination for CO2 Mineralization and Selective Battery Metal Extraction from Laterites. Sep. Purif. Technol. 2024, 340, 126818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).