Natural Mineral Materials for Enhanced Performance in Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Zinc Anode Materials
3. Separators
4. Cathode Materials
5. Electrolytes
6. Conclusions
- (1)
- Multifunctional Mineral Design: Future studies should focus on engineering minerals with dual or multi-functional properties. For instance, montmorillonite could be chemically modified to simultaneously act as a Zn2+-selective membrane and a host to load catalysts for suppressing hydrogen evolution. Integrating redox-active mineral phases (e.g., MnO2-bearing clays) into cathodes could enhance energy density while stabilizing Mn3+/Mn⁴+ redox couples. Such multifunctionality would streamline battery architecture and reduce reliance on auxiliary additives, improving cost-effectiveness.
- (2)
- Scalable and Sustainable Synthesis: To authentically realize the environmental benignity of natural minerals, the extraction and processing must conform to green chemistry principles. Innovations in low-energy mineral purification (e.g., bioleaching, mechanochemical activation) and scalable coating techniques (e.g., roll-to-roll deposition for separators) are desirable. Additionally, leveraging abundant, underutilized minerals could reduce dependency on rare resources. Life cycle assessments should guide the development of closed-loop systems for mineral recycling, minimizing environmental footprints.
- (3)
- Comprehensive and Deeper Understanding: While remarkable progress has been achieved in leveraging mineral materials for AZIBs, concerted efforts are still required to deepen insights into this field. For instance, numerous studies have relied on binders like PVDF to integrate minerals with battery components, yet the impact of these polymers is underexplored. In addition, the complicated micro-nano architectures of natural minerals warrant deeper analysis of their interaction with active materials, electrolytes, and even side-reaction products. Notably, beyond the benefits from minerals, their potential adverse impacts—such as the clogging of mineral-derived nanopores by side products or electrolyte components—also need to be systematically evaluated. A deeper understanding will clarify mineral functionalities and guide the design of advanced AZIBs.
- (4)
- Advanced Characterization and Modeling: A deeper understanding of mineral-electrolyte-electrode interactions requires cutting-edge characterization tools. Operando techniques such as synchrotron X-ray tomography and in situ Raman spectroscopy can map dynamic processes like Zn2+ transport in halloysite nanotubes or Mn dissolution at kaolinite-modified interfaces. Computational modeling, including density functional theory (DFT) and molecular dynamics (MD), could predict ion migration barriers in mineral frameworks and optimize interfacial charge distribution. These insights will accelerate the rational design of mineral-enhanced systems.
- (5)
- Synergistic Integration with Emerging Technologies: Combining mineral materials with advanced manufacturing methods could unlock novel architectures. For example, 3D-printed dickite scaffolds might guide spatially controlled Zn deposition, while AI-driven material discovery could identify optimal mineral-polymer hybrids for flexible electrolytes. Hybrid systems integrating mineral-coated anodes with solid-state electrolytes or redox mediators could further enhance energy density and safety.
- (6)
- Long-Term Stability and Real-World Testing: Rigorous validation under practical conditions is imperative. Testing should include high areal capacity electrodes (>5 mAh cm−2), wide temperature ranges (−20°C to 60°C), and prolonged cycling (>10,000 cycles). Accelerated aging protocols and failure mode analysis will clarify degradation mechanisms in mineral-based components. Field trials in grid-scale storage or electric vehicles will provide actionable feedback for iterative improvements.
- (7)
- Circular Economy Approaches: Developing circular strategies for mineral recovery and reuse is critical. For instance, spent montmorillonite separators could be regenerated via acid washing, while dissolved Mn2+ from cathodes might be adsorbed and recycled using mineral adsorbents. Upcycling mineral waste (e.g., mining byproducts) into battery components could further align AZIBs with circular economy principles.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AZIBs | Aqueous zinc-ion batteries |
| ATP | Attapulgite |
| BT | Bentonite |
| CEI | Cathode-electrolyte interphase |
| CTAB | Cetyltrimethylammonium bromide |
| DFT | Density functional theory |
| DL | Diatomite |
| GF | Glass fiber |
| HCCE | High-concentration colloidal electrolyte |
| HER | Hydrogen evolution reaction |
| HNTs | Halloysite nanotubes |
| KL | Kaolinite |
| MD | Molecular dynamics |
| MMT | Montmorillonite |
| MPS | 3-(methacryloyloxy)propyltrimethoxysilane |
| PAM | Polyacrylamide |
| PCS | Polyvinyl alcohol/sulfonated cellulose/sepiolite |
| SA | Sodium alginate |
| SEI | Solid electrolyte interphase |
| WiME | Water-in-montmorillonite |
| WiSCE | Water-in-swelling clay |
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kittner, N.; Lill, F.; Kammen, D.M. Energy Storage Deployment and Innovation for the Clean Energy Transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design Strategies for High-Energy-Density Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200598. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Biomedical Applications of Cationic Clay Minerals. RSC Adv. 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable Role of Biochar-Inherent Mineral Constituents in Its Environmental Applications: A Review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Bhagawat, L.I.; Patil, V.S.; Kale, B.B.; Sonawane, S.H.; Bhanvase, B.A.; Pinjari, D.V.; Ashokkumar, M. Sonoprocessing of LiFePO4 Nanoparticles and Nanocomposites for Cathode Material in Lithium Ion Batteries. Polym. Compos. 2016, 37, 1874–1880. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Wang, C. Investigation of Electrochemical Performance of Montmorillonite Clay as Li-Ion Battery Electrode. Sustain. Mater. Technol. 2019, 19, e00086. [Google Scholar] [CrossRef]
- Liu, F.; Chuan, X. Recent Developments in Natural Mineral-Based Separators for Lithium-Ion Batteries. RSC Adv. 2021, 11, 16633–16644. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, F.; Pan, Q.; Deng, D.; Liu, L.; Chen, B. A Three-Dimensional NiCo-LDH Array Modified Halloysite Nanotube Composite for High-Performance Battery-Type Supercapacitor. J. Alloys Compd. 2021, 884, 161162. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Liu, S.; Yang, H. Nitrogen-Doped Three-Dimensional Porous Carbon Anode Derived from Hard Halloysite Template for Sodium-Ion Batteries. Appl. Clay Sci. 2021, 200, 105916. [Google Scholar] [CrossRef]
- Shinde, S.K.; Ghodake, G.S.; Maile, N.C.; Yadav, H.M.; Jagadale, A.D.; Jalak, M.B.; Kadam, A.A.; Ramesh, S.; Bathula, C.; Kim, D.-Y. Designing of Nanoflakes Anchored Nanotubes-like MnCo2S4/Halloysite Composites for Advanced Battery like Supercapacitor Application. Electrochim. Acta 2020, 341, 135973. [Google Scholar] [CrossRef]
- Diggle, J.W.; Despic, A.R.; Bockris, J.O. The Mechanism of the Dendritic Electrocrystallization of Zinc. J. Electrochem. Soc. 1969, 116, 1503. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, 1903778. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, X.; Zhou, Q.; Li, T.; Zhao, Y.; Niu, Z.; Wu, Y.; Zhou, H.; Wang, M.; Zhang, X.; et al. Recent Advances in Aqueous Zinc–Sulfur Batteries: Overcoming Challenges for Sustainable Energy Storage. J. Mater. Chem. A 2023, 11, 18029–18045. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.; Zhu, C. Interfacial Parasitic Reactions of Zinc Anodes in Zinc Ion Batteries: Underestimated Corrosion and Hydrogen Evolution Reactions and Their Suppression Strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Li, D.; Cao, L.; Deng, T.; Liu, S.; Wang, C. Design of a Solid Electrolyte Interphase for Aqueous Zn Batteries. Angew. Chem. Int. Ed. 2021, 60, 13035–13041. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Manthiram, A. High-Capacity Zinc-Ion Storage in an Open-Tunnel Oxide for Aqueous and Nonaqueous Zn-Ion Batteries. J. Mater. Chem. A 2016, 4, 18737–18741. [Google Scholar] [CrossRef]
- Yu, H.; Chen, D.; Zhang, T.; Fu, M.; Cai, J.; Wei, W.; Ji, X.; Chen, Y.; Chen, L. Insight on the Double-Edged Sword Role of Water Molecules in the Anode of Aqueous Zinc-Ion Batteries. Small Struct. 2022, 3, 2200143. [Google Scholar] [CrossRef]
- Wen, Q.; Fu, H.; Cui, R.; Chen, H.-Z.; Ji, R.-H.; Tang, L.-B.; Yan, C.; Mao, J.; Dai, K.-H.; Zhang, X.-H.; et al. Recent Advances in Interfacial Modification of Zinc Anode for Aqueous Rechargeable Zinc Ion Batteries. J. Energy Chem. 2023, 83, 287–303. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, Y.; Kang, L.; Du, W.; Gao, Y.; Sun, X.; Zhou, Y.; Li, X.; Li, H.; Jiang, F.; et al. Quasi-Isolated Au Particles as Heterogeneous Seeds to Guide Uniform Zn Deposition for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6490–6496. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Liu, W.; Ni, X.; Qing, P.; Zhao, Q.; Wei, W.; Ji, X.; Ma, J.; Chen, L. Uniform and Dendrite-Free Zinc Deposition Enabled by in Situ Formed AgZn3 for the Zinc Metal Anode. J. Mater. Chem. A 2021, 9, 8452–8461. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Yu, F.; Xu, G.; Li, Z.; Zhu, M.; Yue, Z.; Wu, M.; Liu, H.-K.; Dou, S.-X.; et al. Highly Reversible and Dendrite-Free Zn Electrodeposition Enabled by a Thin Metallic Interfacial Layer in Aqueous Batteries. Chem. Eng. J. 2021, 416, 128062. [Google Scholar] [CrossRef]
- Han, D.; Wu, S.; Zhang, S.; Deng, Y.; Cui, C.; Zhang, L.; Long, Y.; Li, H.; Tao, Y.; Weng, Z.; et al. A Corrosion-Resistant and Dendrite-Free Zinc Metal Anode in Aqueous Systems. Small 2020, 16, 2001736. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Fang, G.; Sun, H.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Suppressing By-Product via Stratified Adsorption Effect to Assist Highly Reversible Zinc Anode in Aqueous Electrolyte. J. Energy Chem. 2021, 55, 549–556. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A Hafnium Oxide-Coated Dendrite-Free Zinc Anode for Rechargeable Aqueous Zinc-Ion Batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-Life and Deeply Rechargeable Aqueous Zn Anodes Enabled by a Multifunctional Brightener-Inspired Interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Liu, H.; Amine, A.; Zhao, Q.; Song, Y.; Yang, J.; Wang, K.; Pan, F. Artificial Solid-Electrolyte Interface Facilitating Dendrite-Free Zinc Metal Anodes via Nanowetting Effect. ACS Appl. Mater. Interfaces 2019, 11, 32046–32051. [Google Scholar] [CrossRef]
- Feng, S.; Singh, R.K.; Fu, Y.; Li, Z.; Wang, Y.; Bao, J.; Xu, Z.; Li, G.; Anderson, C.; Shi, L.; et al. Low-Tortuous and Dense Single-Particle-Layer Electrode for High-Energy Lithium-Sulfur Batteries. Energy Environ. Sci. 2022, 15, 3842–3853. [Google Scholar] [CrossRef]
- Rong, Z.; Malik, R.; Canepa, P.; Sai Gautam, G.; Liu, M.; Jain, A.; Persson, K.; Ceder, G. Materials Design Rules for Multivalent Ion Mobility in Intercalation Structures. Chem. Mater. 2015, 27, 6016–6021. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, C.; Zhang, J.; Jiao, Y.; Li, M.; Lin, W.; Ran, L.; Clement, B.; Lyu, M.; Gentle, I.; et al. A Hydrophobic and Fluorophilic Coating Layer for Stable and Reversible Aqueous Zinc Metal Anodes. Chem. Eng. J. 2022, 446, 136607. [Google Scholar] [CrossRef]
- Wang, H.; Tang, W.; Ni, L.; Ma, W.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Synthesis of Silicon Nanosheets from Kaolinite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Phys. Chem. Solids 2020, 137, 109227. [Google Scholar] [CrossRef]
- Pongsuk, P.; Pumchusak, J. Effects of Halloysite Nanotubes on Ionic Conductivity, Morphology, Crystallinity and Mechanical Properties of PEO-Based Solid Polymer Electrolyte. Mater. Today Proc. 2019, 17, 1956–1963. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Zhang, J. Design of advanced separators for high performance Li-S batteries using natural minerals with 1D to 3D microstructures. J. Colloid Interface Sci. 2022, 614, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Guo, B.; Jia, D. Newly Emerging Applications of Halloysite Nanotubes: A Review. Polym. Int. 2010, 59, 574–582. [Google Scholar] [CrossRef]
- Fan, L.; Lin, A.; Cao, L.; Gu, F.; Xiong, S.; Li, Z. Enabled Uniform Zn Stripping/Plating by Natural Halloysite Nanotube Coating with Opposite Charge for Aqueous Zn-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 5838–5846. [Google Scholar] [CrossRef]
- Xu, P.; Wang, C.; Zhou, Y.; Cheng, H. An interfacial coating with high corrosion resistance based on halloysite nanotubes for anode protection of zinc-ion batteries. J. Colloid Interface Sci. 2021, 602, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay Mineral Adsorbents for Heavy Metal Removal from Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C. Chitosan-poly(vinyl alcohol)/attapulgite nanocomposites for copper(II) ions removal: pH dependence and adsorption mechanisms. Colloids Surf. Physicochem. Eng. Asp. 2016, 500, 186–194. [Google Scholar] [CrossRef]
- Song, Q.; Li, A.; Shi, L.; Qian, C.; Feric, T.G.; Fu, Y.; Zhang, H.; Li, Z.; Wang, P.; Li, Z.; et al. Thermally Stable, Nano-Porous and Eco-Friendly Sodium Alginate/Attapulgite Separator for Lithium-Ion Batteries. Energy Storage Mater. 2019, 22, 48–56. [Google Scholar] [CrossRef]
- Sun, D.; Yu, D.; Lin, S.; Wang, Z.; Deng, F.; Zhou, X.; Ma, G.; Lei, Z. Porous Attapulgite Interfaces with Nanochannels That Enable Dendrite-Free Aqueous Zinc Ion Batteries. ACS Appl. Nano Mater. 2021, 4, 11723–11731. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; Zhao, Y.; Huang, K.; Tong, Z.; Liao, B.; Pang, H. Montmorillonite-Based Materials for Electrochemical Energy Storage. Green Chem. 2024, 26, 678–704. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents Based on Montmorillonite for Contaminant Removal from Water: A Review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Nan, Y.; Yang, S.; Li, B. Ultrafast Zinc–Ion–Conductor Interface toward High-Rate and Stable Zinc Metal Batteries. Adv. Energy Mater. 2021, 11, 2100186. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Liao, D.; Wu, Y.; Yu, Y.; Hu, C. Highly Zn2+-conductive and robust modified montmorillonite protective layer of electrodes toward high-performance rechargeable zinc-ion batteries. Energy Storage Mater. 2022, 51, 212–222. [Google Scholar] [CrossRef]
- Hong, L.; Wu, X.; Ma, C.; Huang, W.; Zhou, Y.; Wang, K.-X.; Chen, J.-S. Boosting the Zn-Ion Transfer Kinetics to Stabilize the Zn Metal Interface for High-Performance Rechargeable Zn-Ion Batteries. J. Mater. Chem. A 2021, 9, 16814–16823. [Google Scholar] [CrossRef]
- Liu, M.; Hu, A.; Yan, Z.; Chen, J.; He, M.; Zhou, B.; Pan, Y.; Fan, Y.; Gao, D.; Long, J. Enhancing Zn2+ Diffusion for Dendrite-Free Zinc Anodes via a Robust Zincophilic Clay Mineral Coating. Chem. Eng. J. 2024, 479, 147410. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, P.; Jiang, Y.; Sun, M.; Liu, D.; Yu, L. Preparation and Characterization of Linear Low-Density Polyethylene/Dickite Nanocomposites Prepared by the Direct Melt Blending of Linear Low-Density Polyethylene with Exfoliated Dickite. J. Appl. Polym. Sci. 2011, 120, 1736–1743. [Google Scholar] [CrossRef]
- Xue, B.; Yang, K.; Wang, X.; Chi, Q.; Jiang, Y. The Role of Potassium Chlorate on Expansion of Dickite Layers and the Preparation of a Novel TiO2 Impregnated Dickite Photocatalyst Using Expanded Dickite as Carrier. RSC Adv. 2016, 6, 9803–9811. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Ma, Z.; Ma, Z.; Xue, B. An artificial layer structure-modulated “high selective ion flux” coating for aqueous zinc ion battery anodes. Mater. Today Chem. 2024, 38, 102113. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Yang, K.; Wang, H.; Zhang, Z.; Xue, B. Dickite nanolayers for ultrathin anode coatings in highly stable zinc-ion batteries. Appl. Clay Sci. 2024, 261, 107553. [Google Scholar] [CrossRef]
- Wang, M.-S.; Fan, L.-Z.; Huang, M.; Li, J.; Qu, X. Conversion of Diatomite to Porous Si/C Composites as Promising Anode Materials for Lithium-Ion Batteries. J. Power Sources 2012, 219, 29–35. [Google Scholar] [CrossRef]
- Chunyuan, W.; Dubin, H.; Botao, S.; Hongfei, C. Diatomite-Layer Modified Zinc Anode and Its Application in Zinc Ion Batteries. Bull. Chin. Ceram. Soc. 2020, 39, 909–1915. [Google Scholar]
- Li, F.; Zhou, C.; Zhang, J.; Gao, Y.; Nan, Q.; Luo, J.; Xu, Z.; Zhao, Z.; Rao, P.; Li, J.; et al. Mullite Mineral-Derived Robust Solid Electrolyte Enables Polyiodide Shuttle-Free Zinc-Iodine Batteries. Adv. Mater. 2024, 36, 2408213. [Google Scholar] [CrossRef]
- Gao, J.; Xie, X.; Liang, S.; Lu, B.; Zhou, J. Inorganic Colloidal Electrolyte for Highly Robust Zinc-Ion Batteries. Nano Micro. Lett. 2021, 13, 69. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Ren, J.; Li, P.; Zhang, K.; Wu, T.; Wang, L. Synergistic effect of organic-inorganic hybrid electrolyte for ultra-long Zn–I2 batteries. Int. J. Hydrogen Energy 2023, 48, 21985–21995. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Fu, L.; Liu, S.; Yang, H. A novel and improved hydrophilic vanadium oxide-based cathode for aqueous Zn-ion batteries. Electrochim. Acta 2020, 354, 136721. [Google Scholar] [CrossRef]
- Yang, Y.; Chuan, X.; Li, J.; Liu, F.; Li, A. Synthesis and Properties of Halloysite Templated Tubular MoS2 as Cathode Material for Rechargeable Aqueous Zn-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 6052–6059. [Google Scholar] [CrossRef]
- Liu, S.; Han, Q.; He, C.; Xu, Z.; Huang, P.; Cai, L.; Chen, H.; Zheng, H.; Zhou, Y.; Wang, M.; et al. Ion-Sieving Separator Functionalized by Natural Mineral Coating toward Ultrastable Zn Metal Anodes. ACS Nano 2024, 18, 25880–25892. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Zhang, Q.; Zhou, J.; Cai, Z.; Pan, A. Fabrication of an Inexpensive Hydrophilic Bridge on a Carbon Substrate and Loading Vanadium Sulfides for Flexible Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36676–36684. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Y.; Wang, C.; Cao, Z.; Cheng, H. Conductive Halloysite Nanotubes/Polypyrrole Cathodes Prepared by One-Step in Situ Polymerization for Zinc-Ion Batteries. Polym. Bull. 2024, 81, 1117–1129. [Google Scholar] [CrossRef]
- Xu, P.; Wang, C.; Zhao, B.; Zhou, Y.; Cheng, H. A high-strength and ultra-stable halloysite nanotubes-crosslinked polyacrylamide hydrogel electrolyte for flexible zinc-ion batteries. J. Power Sources 2021, 506, 230196. [Google Scholar] [CrossRef]
- Tian, S.; Hwang, T.; Malakpour Estalaki, S.; Tian, Y.; Zhou, L.; Milazzo, T.; Moon, S.; Wu, S.; Jian, R.; Balkus, K.; et al. A Low-Cost Quasi-Solid-State “Water-in-Swelling-Clay” Electrolyte Enabling Ultrastable Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300782. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Nan, Q.; Wen, H.; Xu, Z.; Zhang, J.; Zhao, Z.; Li, J.; Xing, Z.; Rao, P.; et al. Simultaneous Inhibition of Vanadium Dissolution and Zinc Dendrites by Mineral-Derived Solid-State Electrolyte for High-Performance Zinc Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202412006. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Han, M.; Li, S.; Zhi, J.; Chen, P. Morphological regulation of Mn2+ deposition products enables long lifespan of aqueous zinc batteries. Electrochim. Acta 2024, 479, 143878. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, M.; Fu, B.; Li, M.; Liang, S.; Fang, G. Effective Proton Conduction in Quasi-Solid Zinc-Manganese Batteries via Constructing Highly Connected Transfer Pathways. Angew. Chem. Int. Ed. 2024, 64, e202417049. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, R.; An, Y.; Liu, W.; Kang, F. “Water-in-Montmorillonite” Quasi-Solid-State Electrolyte for Ultralow Self-Discharge Aqueous Zinc-Ion Batteries. Energy Mater. Devices 2024, 2, 9370047. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Xu, H.; Jiang, Y.; Li, F.; Xue, B. An Expanded Clay-Coated Separator with Unique Microporous Structure for Enhancing Electrochemical Performance of Rechargeable Hybrid Aqueous Batteries. J. Solid State Electrochem. 2019, 23, 215–226. [Google Scholar] [CrossRef]
- Zong, Y.; He, H.; Wang, Y.; Wu, M.; Ren, X.; Bai, Z.; Wang, N.; Ning, X.; Dou, S.X. Functionalized Separator Strategies toward Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300403. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and Performance Evaluation of Lithium-Ion Battery Separators. Nat. Energy 2018, 4, 16–25. [Google Scholar] [CrossRef]
- Cao, P.; Zhou, H.; Zhou, X.; Du, Q.; Tang, J.; Yang, J. Stabilizing Zinc Anodes by a Cotton Towel Separator for Aqueous Zinc-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 8350–8359. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Tian, Q.; Chen, J.; Xu, X.; Wong, C.-P. Cotton-Derived Cellulose Film as a Dendrite-Inhibiting Separator to Stabilize the Zinc Metal Anode of Aqueous Zinc Ion Batteries. Energy Storage Mater. 2022, 44, 57–65. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Yang, T.; Yu, L.; Wei, N.; Tian, Z.; Cai, J.; Lv, J.; Shao, Y.; Rümmeli, M.H.; et al. Directly Grown Vertical Graphene Carpets as Janus Separators toward Stabilized Zn Metal Anodes. Adv. Mater. 2020, 32, 2003425. [Google Scholar] [CrossRef]
- Su, Y.; Liu, B.; Zhang, Q.; Peng, J.; Wei, C.; Li, S.; Li, W.; Xue, Z.; Yang, X.; Sun, J. Printing-Scalable Ti3C2TX MXene-Decorated Janus Separator with Expedited Zn2+ Flux toward Stabilized Zn Anodes. Adv. Funct. Mater. 2022, 32, 2204306. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Huang, Z.; Zeng, Z.; Zhang, Y.; Wang, Z. Recyclable Nanopaper Separators with Uniform Sub-20 Nm Nanopores for High-Power and High-Capacity Zinc Metal Anodes. Electrochim. Acta 2022, 430, 141081. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The Rise of Organic Electrode Materials for Energy Storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible Aqueous Zinc/Manganese Oxide Energy Storage from Conversion Reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Wei, Z.-K.; Hua, X.-Z.; Xiao, K.; Zhou, X.-L.; Ye, Z.-G. An Investigation on Electrochemical Performance of Supercapacitor Electrode Materials Prepared by MnO2 with Four Different Crystal Forms. J. Electrochem. 2015, 21, 150408. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese Sesquioxide as Cathode Material for Multivalent Zinc Ion Battery with High Capacity and Long Cycle Life. Electrochim. Acta 2017, 229, 422–428. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, G.; Zhou, J.; Guo, J.; Wang, Z.; Wang, C.; Li, J.; Tang, Y.; Liang, S. Binder-Free Stainless steel@Mn3O4 Nanoflower Composite: A High-Activity Aqueous Zinc-Ion Battery Cathode with High-Capacity and Long-Cycle-Life. J. Mater. Chem. A 2018, 6, 9677–9683. [Google Scholar] [CrossRef]
- Hao, J.; Mou, J.; Zhang, J.; Dong, L.; Liu, W.; Xu, C.; Kang, F. Electrochemically Induced Spinel-Layered Phase Transition of Mn3O4 in High Performance Neutral Aqueous Rechargeable Zinc Battery. Electrochim. Acta 2018, 259, 170–178. [Google Scholar] [CrossRef]
- Zavalij, P.Y.; Whittingham, M.S. Structural Chemistry of Vanadium Oxides with Open Frameworks. Acta Crystallogr. Sect. B 1999, 55, 627–663. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z. Design Strategies for Vanadium-Based Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 Aqueous Hybrid-Ion Battery with High Voltage Platform and Long Cycle Life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Hu, J.; Liu, J.; Miao, L.; Jiang, J. An In Situ Artificial Cathode Electrolyte Interphase Strategy for Suppressing Cathode Dissolution in Aqueous Zinc Ion Batteries. Small Methods 2021, 5, 2100094. [Google Scholar] [CrossRef]
- Guo, S.; Liang, S.; Zhang, B.; Fang, G.; Ma, D.; Zhou, J. Cathode Interfacial Layer Formation via in Situ Electrochemically Charging in Aqueous Zinc-Ion Battery. ACS Nano 2019, 13, 13456–13464. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Mohammed, A.K.; Shuaib, D.T. Potential of Using Kaolin as a Natural Adsorbent for the Removal of Pollutants from Tannery Wastewater. Heliyon 2019, 5, e02923. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Li, T.; Liu, J.; Gong, D.; Xiao, J.; Shen, L.; Ding, B.; Zhang, X. Fabrication of the Oxygen Vacancy Amorphous MnO2/Carbon Nanotube as Cathode for Advanced Aqueous Zinc-Ion Batteries. Energy Technol. 2021, 9, 2000769. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Wang, S.; Han, J.; Zhao, Y.; Huang, Y.; Qin, J. Mechanochemical Reactions of MnO2 and Graphite Nanosheets as a Durable Zinc Ion Battery Cathode. Appl. Surf. Sci. 2020, 534, 147630. [Google Scholar] [CrossRef]
- Guo, C.; Tian, S.; Chen, B.; Liu, H.; Li, J. Constructing α-MnO2@PPy Core-Shell Nanorods towards Enhancing Electrochemical Behaviors in Aqueous Zinc Ion Battery. Mater. Lett. 2020, 262, 127180. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lv, R.; Na, B.; Wang, B.; He, Y. Nanostructured Polypyrrole Composite Aerogels for a Rechargeable Flexible Aqueous Zn-Ion Battery with High Rate Capabilities. Energy Technol. 2019, 7, 1801092. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.; Ma, Y.; Bin, D.; Wang, Y.; Xia, Y. Recent Progress of Rechargeable Batteries Using Mild Aqueous Electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Gui, Y.; Lei, Y.; Fan, B.A. Investigation on the Effect of Different Mild Acidic Electrolyte on ZIBs Electrode/Electrolyte Interface and the Performance Improvements with the Optimized Cathode. Front. Chem. 2020, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, G.; Trócoli, R.; Bani Hashemi, A.; La Mantia, F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochim. Acta 2016, 222, 74–83. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Zhang, X.; Wang, G.; Yan, X. A Rechargeable Aqueous Zinc/Sodium Manganese Oxides Battery with Robust Performance Enabled by Na2SO4 Electrolyte Additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, J.; Wang, F.; Liu, X.; Zhang, Q.; Liu, J.; Kaghazchi, P.; Ma, D.; Chi, Z.; Wang, L. Tuning Surface Energy of Zn Anodes via Sn Heteroatom Doping Enabled by a Codeposition for Ultralong Life Span Dendrite-Free Aqueous Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 27085–27095. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, Y.; Zhang, D.; Luo, D.; Wu, H.; Wang, X.; Yang, C.; Zhang, L.; Yang, X.; Qin, J. Regulating Electrode/Electrolyte Interface with Additives towards Dendrite-Free Zinc-Ion Batteries. ChemElectroChem 2024, 11, e202400064. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The Colloidal and Rheological Properties of Bentonite Suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Meng, J.; Zuo, C.; Wu, B.; Li, C.; Sun, W.; Mai, L. Comprehensive Understandings of Hydrogen Bond Chemistry in Aqueous Batteries. Adv. Mater. 2024, 36, 2308628. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Hong, Y.; Yu, Z.; Chen, J.; Zheng, J. Ultrahigh-Rate and Ultralong-Life Aqueous Batteries Enabled by Special Pair-Dancing Proton Transfer. Sci. Adv. 2023, 9, eadf4589. [Google Scholar] [CrossRef]
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-Ion Batteries: Recent Progresses and Challenges. Small 2019, 15, 1804760. [Google Scholar] [CrossRef]
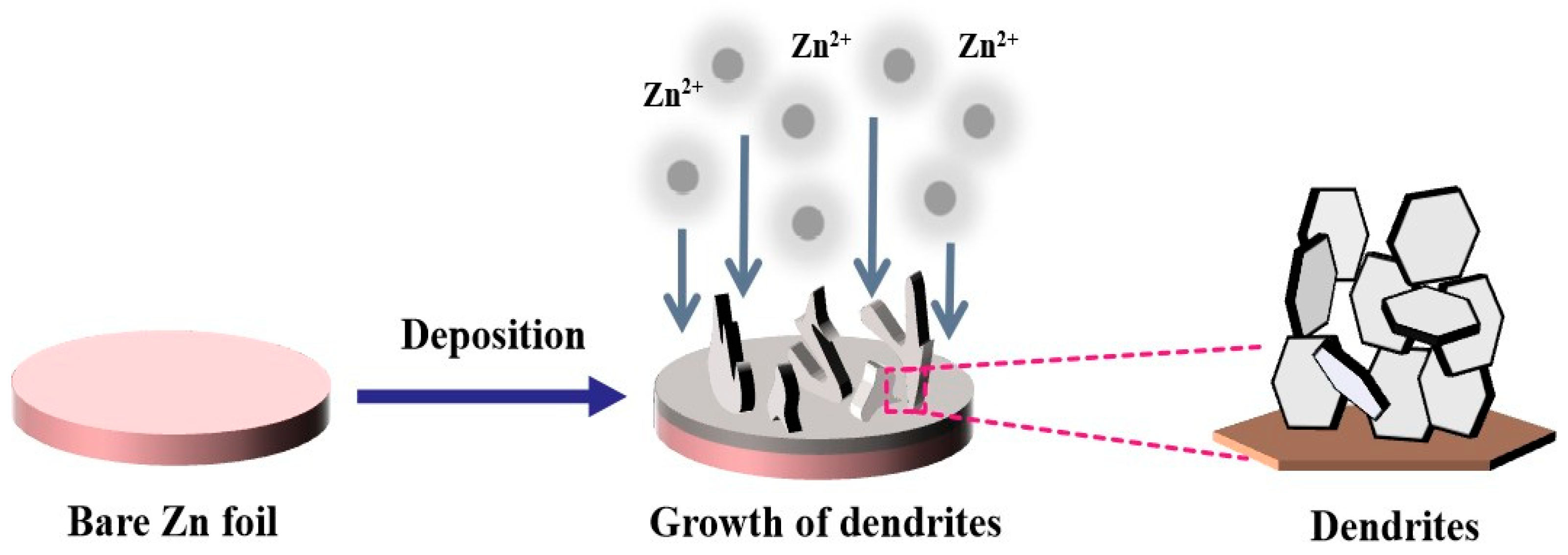
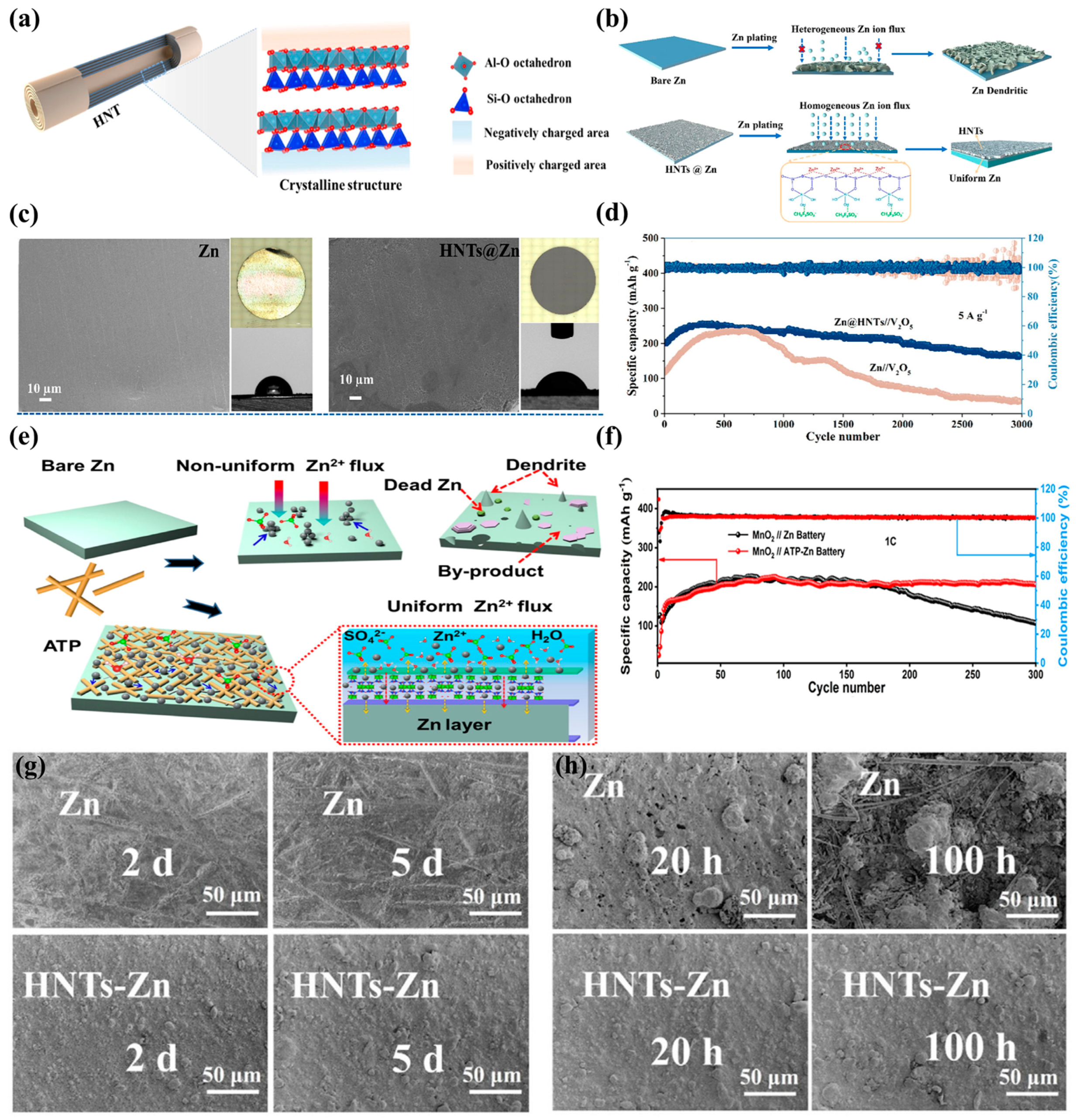


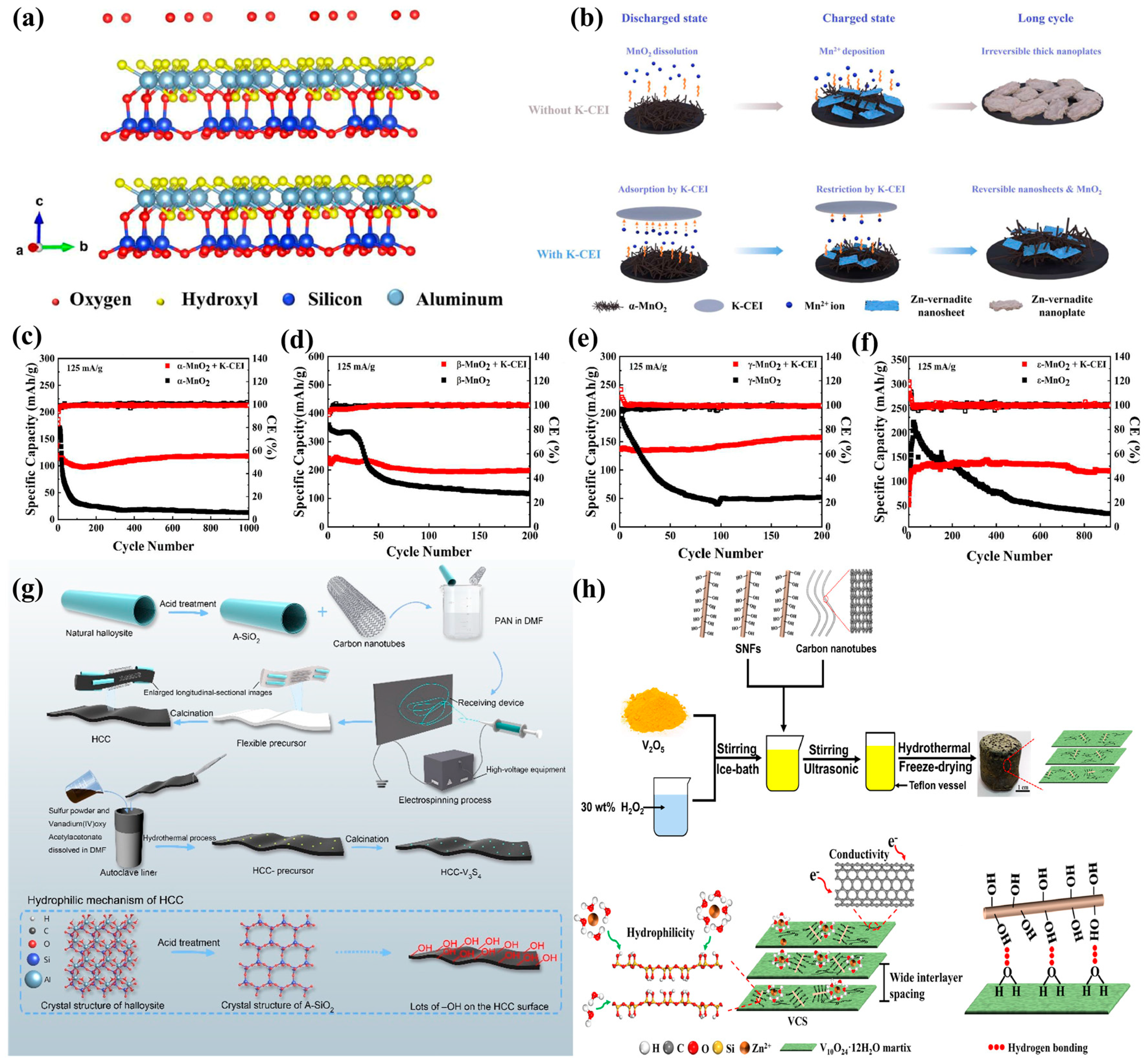


| Type | Anode | Cathode | Separator | Electrolyte | Zn//Zn Symmetric Battery Performance | Full Battery Performance | Ref |
|---|---|---|---|---|---|---|---|
| Attapulgite (1D) | ATP-Zn | MnO2 | GF | 2 M ZnSO4 + 0.1 M MnSO4 | 1600 h at 0.25 mA cm−2 for 0.05 mAh cm−2 | 210 mAh g−1 after 300 cycles at 1 C | [46] |
| Mullite (1D) | Zn | AC@I2 | / | Zn-ML | 140 h at 0.5 mA cm−2 for 0.1 mAh cm−2 | 127.4 mAh g−1 after 3000 cycles at 0.5 A g−1 | [59] |
| Palygorskite (1D) | Zn | α-MnO2 | GF | HCCE | / | 212 mAh g−1 after 1000 cycles at 0.5 A g−1 | [60] |
| Sepiolite (1D) | Zn | AC@I2 | GF | PCS | 2000 h at 1 mA cm−2 for 1 mAh cm−2 | 82.95% retention after 10,000 cycles at 5 A g−1 | [61] |
| Sepiolite | Zn | VCS | GF | 2 M ZnSO4 | / | 92.7% retention after 1000 cycles at 10 A g−1 | [62] |
| HNTs (1D) | Zn | MoS2 | GF | 3 M Zn (CF3SO3)2 | / | 74% retention after 800 cycles at 0.2 A g−1 | [63] |
| HNTs | Zn | MnO2 | HNT-GF | 2 M ZnSO4 + 0.1 M MnSO4 | 3000 h at 1 mA cm−2 for 1 mAh cm−2 | 93.4% retention after 1000 cycles at 2 A g−1 | [64] |
| HNTs | CFC−Zn | HCC-V3S4 | GF/D | 2 M ZnSO4 | / | 95% retention after 200 cycles at 0.5 A g−1 | [65] |
| HNTs | HNTs@Zn | V2O5 | GF | 1 M Zn (CF3SO3)2 | 2000 h at 0.2 mA cm−2 for 0.2 mAh cm−2 | 82% retention after 3000 cycles at 5 A g−1 | [41] |
| HNTs | Zn | HNTs-PPy | GF | 2 M ZnSO4 | / | 87.4% retention after 500 cycles at 0.5 A g−1 | [66] |
| HNTs | HNTs-Zn | MnO2 | GF | 2 M ZnSO4 | 650 h at 0.5 mA cm−2 for 0.5 mAh cm−2 | 79% retention after 400 cycles at 3 C | [42] |
| HNTs | Carbon cloth and CNF | MnO2 | hydrogel | M-HNTs/PAM | 1200 h at 4.4 mA cm−2 for 1.1 mAh cm−2 | 92.7% retention after 1000 cycles at 10 C | [67] |
| Bentonite (2D) | Zn | NVO | GF | WiSCE | 1000 h at 1 mA cm−2 for 1 mAh cm−2 | 88.29% retention after 5000 cycles at 3 A g−1 | [68] |
| Kaolin (2D) | Zn | NH4V4O10 | KL-Zn | KL-Zn | 2200 h at 0.2 mA cm−2 for 0.1 mAh cm−2 | 241.6 mAh g−1 after 500 cycles at 1 A g−1 | [69] |
| Kaolin | Zn | K-CEI-α-MnO2 | AGM | 1.8 M ZnSO4 + 0.2 M MnSO4 | / | 85% retention after 200 cycles at 0.05 A g−1 | [70] |
| Montmorillonite (2D) | MMT-Zn | MMT-MnO2 | GF | 2 M ZnSO4 + 0.1 M MnSO4 | 1000 h at 10 mA cm−2 for 45 mAh cm−2 | 191.5 mAh g−1 after 1100 cycles at 2 C | [49] |
| Montmorillonite | ZnOMMT-Zn | ZnOMMT-MnO2 | GF | 2 M ZnSO4 + 0.2 M MnSO4 | 1100 h at 1 mA cm−2 for 1 mAh cm−2 | 205 mAh g−1 after 700 cycles at 1 A g−1 | [50] |
| Montmorillonite | UMMT-Zn | V2O5 | / | 2 M ZnSO4 | 1300 h at 6 mA cm−2 for 3 mAh cm−2 | 254 mAh g−1 after 4000 cycles at 10 A g−1 | [52] |
| Montmorillonite | Zn-Mont | MnO2 | / | 2 M ZnSO4 + 0.1 M MnSO4 | 900 h at 1 mA cm−2 for 0.5 mAh cm−2 | 85.4% retention after 1000 cycles at 2 C | [51] |
| Montmorillonite | Cu@Zn | α-MnO2 | / | Pro-Pr3+ -ZnMT | / | 92.2% retention after 800 cycles at 0.8 mA cm−2 | [71] |
| Montmorillonite | Zn | MnOOH | / | WIME | 1800 h at 0.5 mA cm−2 for 0.25 mAh cm−2 | / | [72] |
| Dickite (2D) | DE-Zn | MnO2 | GF | 2 M ZnSO4+ 0.1 M MnSO4 | 5500 h at 0.5 mA cm−2 for 0.1 mAh cm−2 | 144 mAh g−1 after 750 cycles at 0.15 A g−1 | [56] |
| Dickite | DCU-Zn | MnO2 | GF | 2 M ZnSO4+ 0.1 M MnSO4 | 1000 h at 0.5 mA cm−2 | 144 mAh g−1 after 3000 cycles at 3 A g−1 | [55] |
| Dickite | Zn | LiMn2O4 | DUC | 2 M ZnSO4 +1 M Li2SO4 | / | / | [73] |
| Diatomite (3D) | DL-Zn | Mn3O4 | GF | 2 M ZnSO4+ 0.1 M MnSO4 | 200 h at 10 mA cm−2 for 2 mAh cm−2 | 80% retention after 400 cycles at 5 C | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Zheng, Q.; Wang, K.; Hu, Y. Natural Mineral Materials for Enhanced Performance in Aqueous Zinc-Ion Batteries. Minerals 2025, 15, 328. https://doi.org/10.3390/min15040328
Chen P, Zheng Q, Wang K, Hu Y. Natural Mineral Materials for Enhanced Performance in Aqueous Zinc-Ion Batteries. Minerals. 2025; 15(4):328. https://doi.org/10.3390/min15040328
Chicago/Turabian StyleChen, Peilin, Qinwen Zheng, Ke Wang, and Yingmo Hu. 2025. "Natural Mineral Materials for Enhanced Performance in Aqueous Zinc-Ion Batteries" Minerals 15, no. 4: 328. https://doi.org/10.3390/min15040328
APA StyleChen, P., Zheng, Q., Wang, K., & Hu, Y. (2025). Natural Mineral Materials for Enhanced Performance in Aqueous Zinc-Ion Batteries. Minerals, 15(4), 328. https://doi.org/10.3390/min15040328








