Thermal Behaviour of a Carbonatic Clay: A Multi-Analytical Approach
Abstract
1. Introduction
2. Materials and Analytical Methods
3. Results
3.1. Raw Clay
3.1.1. Optical Microscopy
3.1.2. X-Ray Powder Diffraction
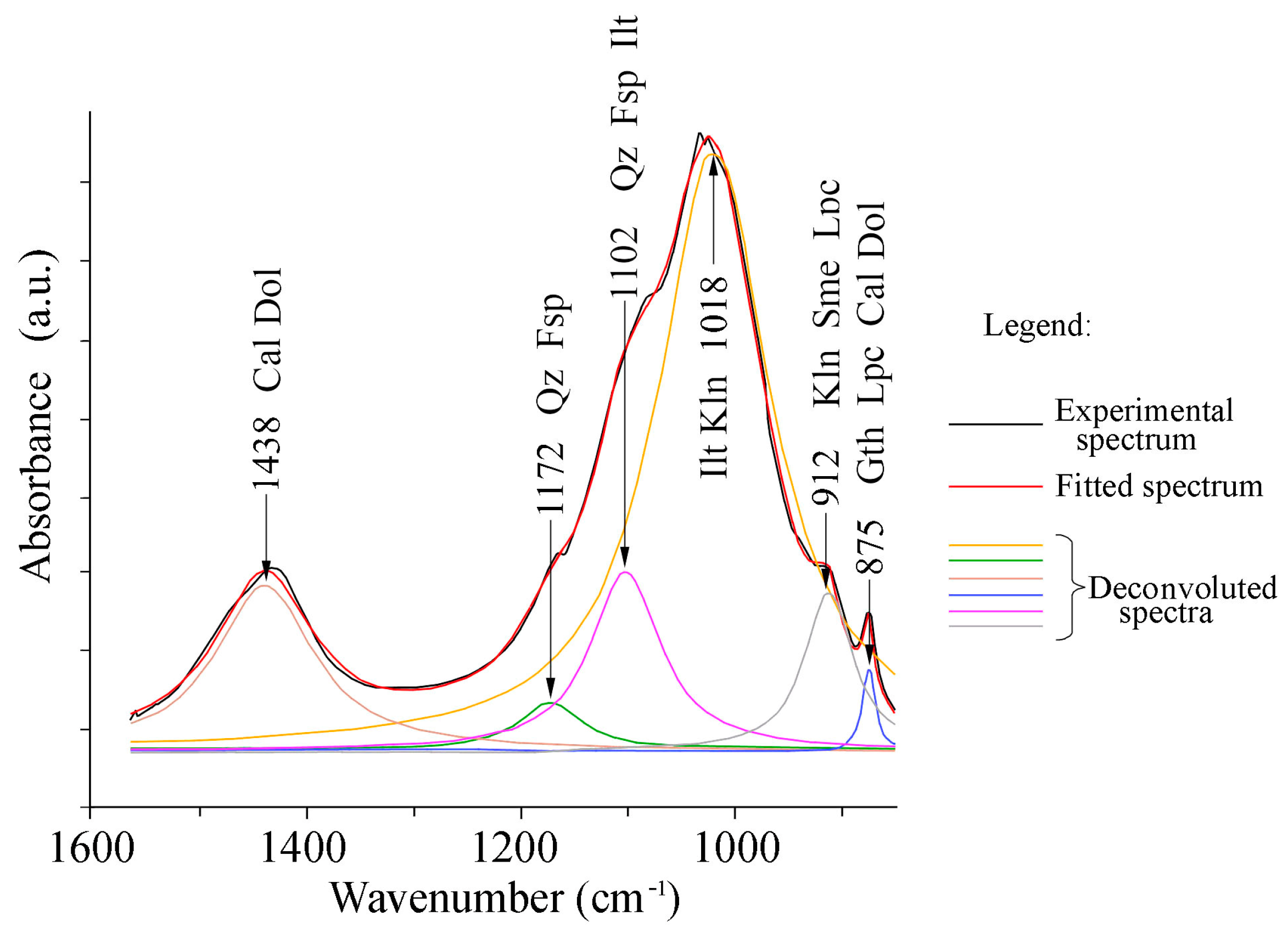
3.1.3. FTIR Spectroscopy
3.2. Heated Clay
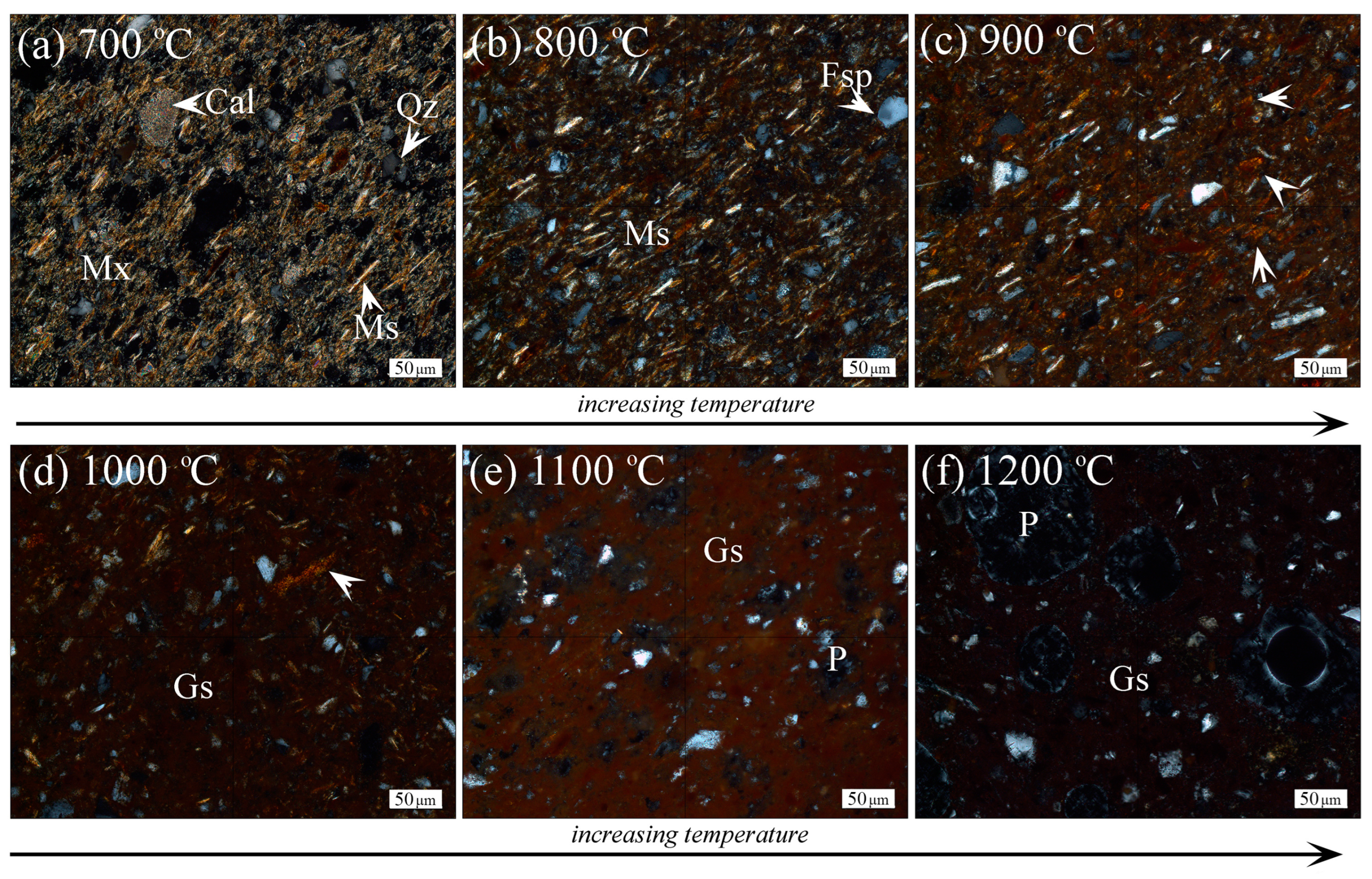
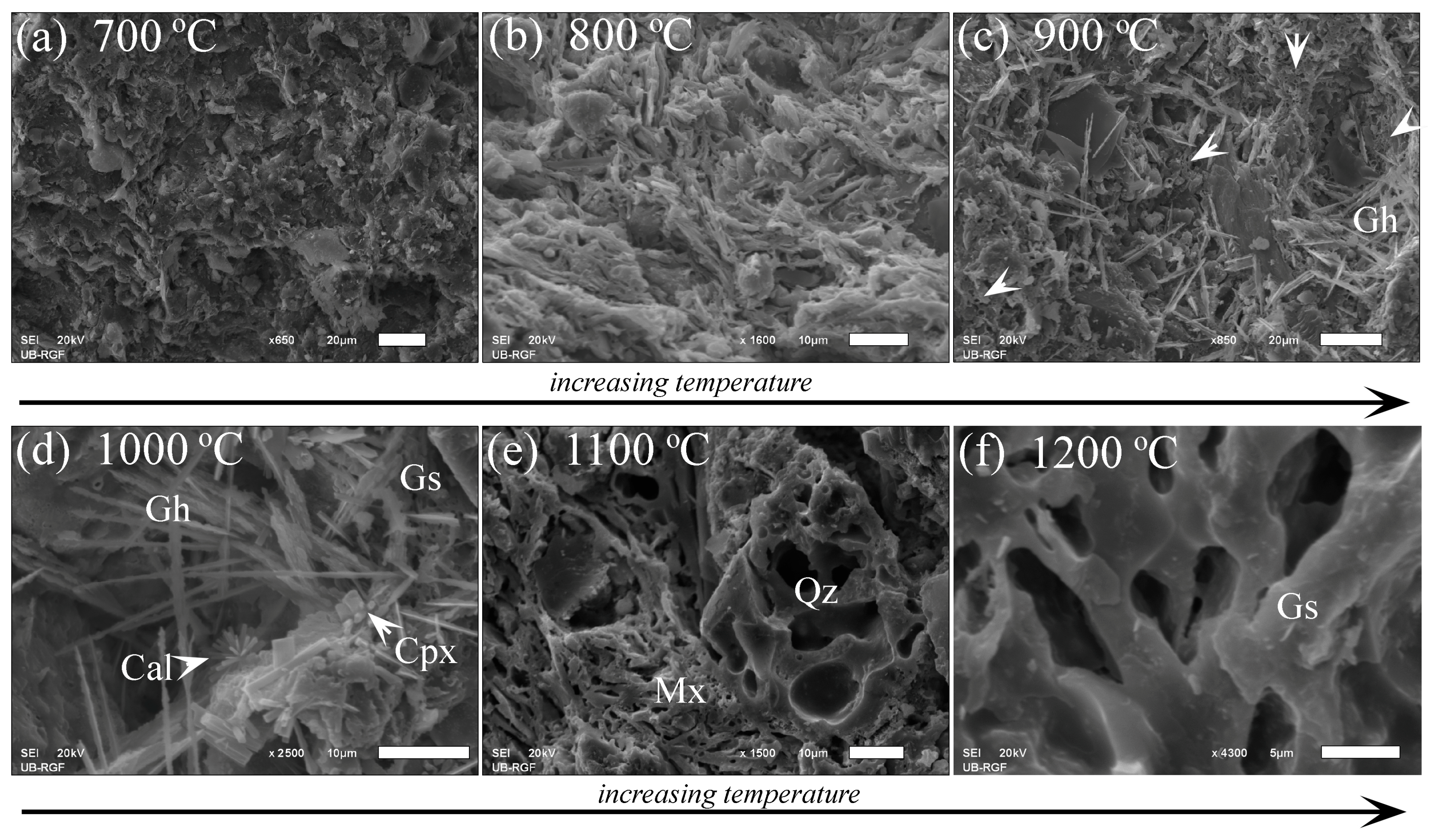
3.2.1. Optical Microscopy
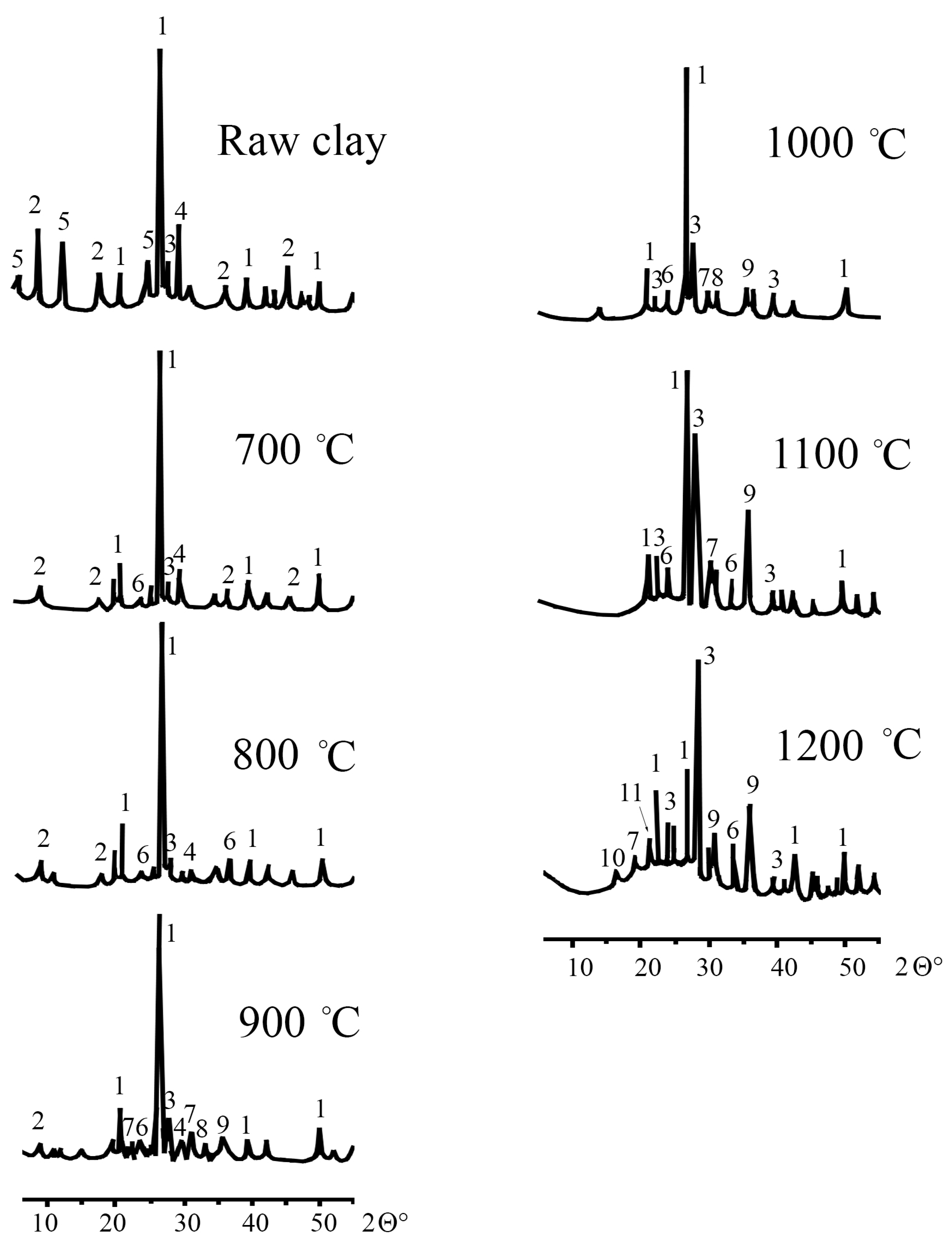
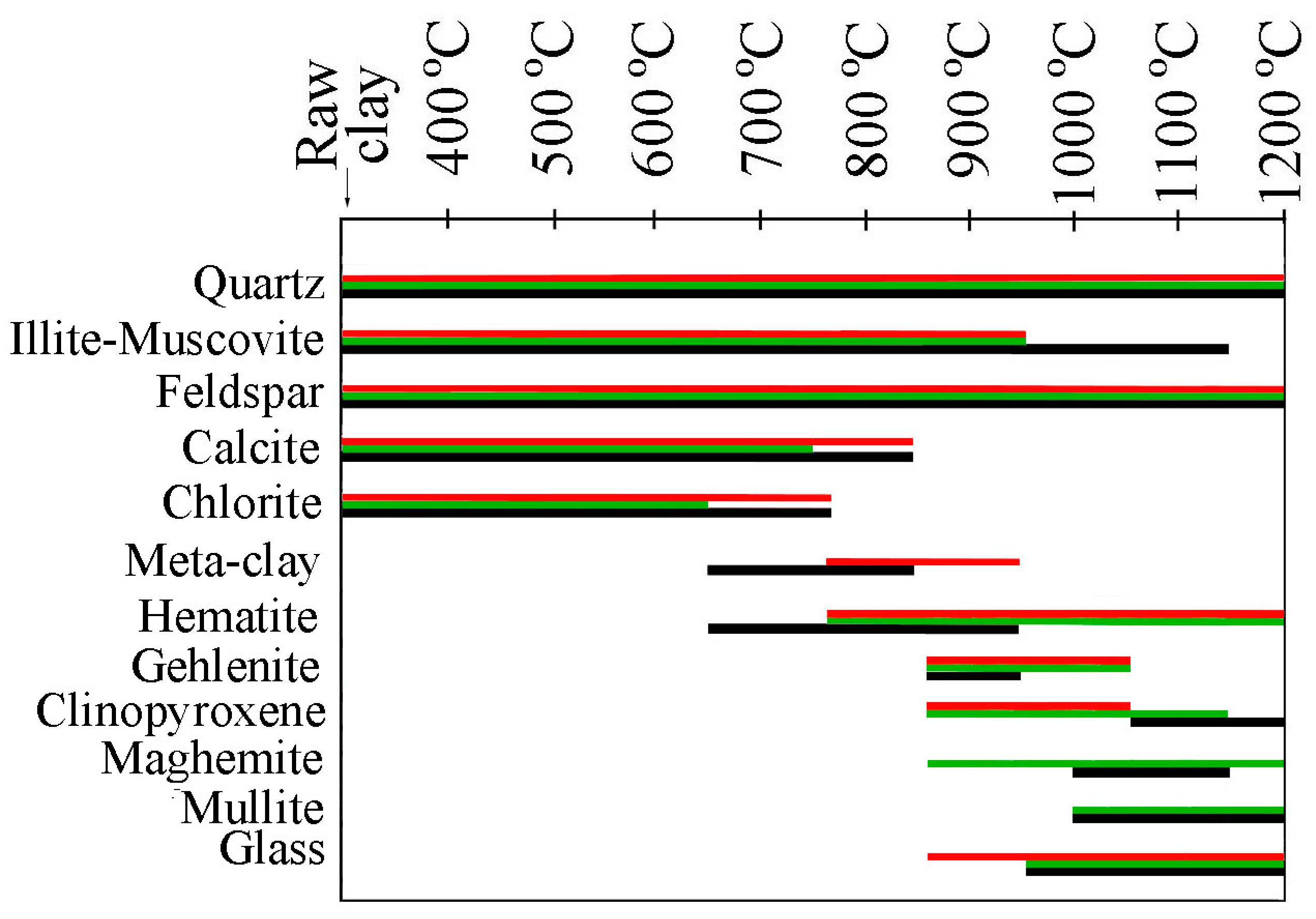
3.2.2. X-Ray Powder Diffraction
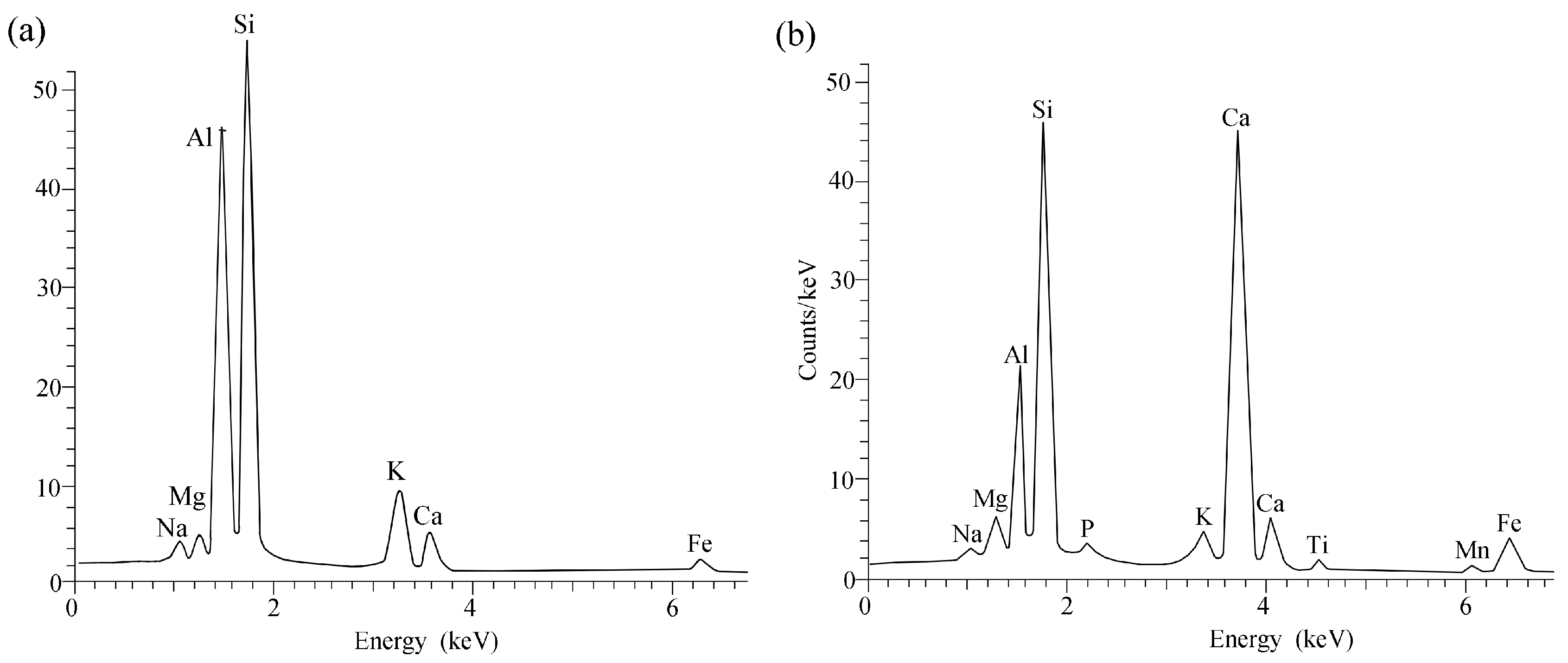
3.2.3. SEM-EDX
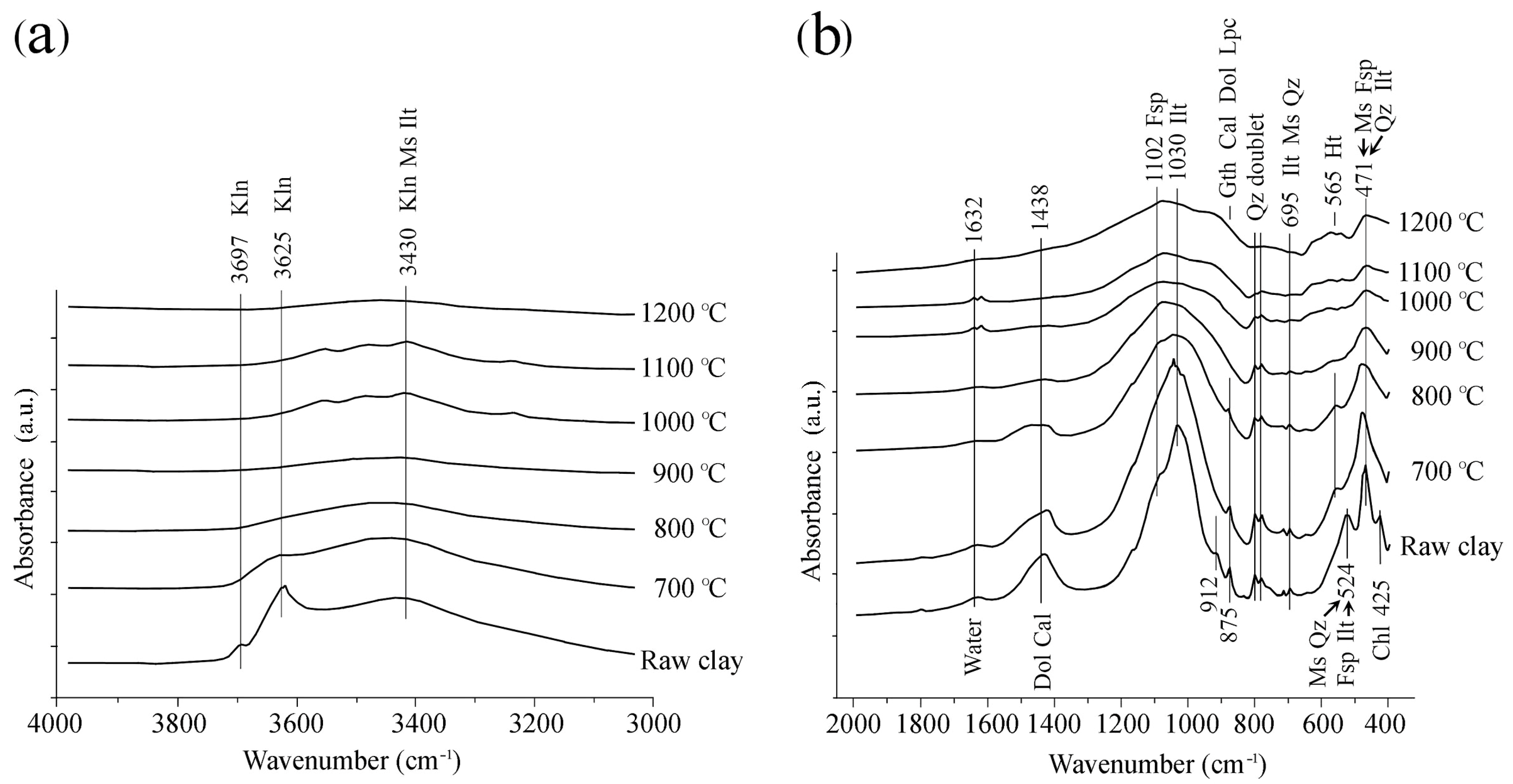
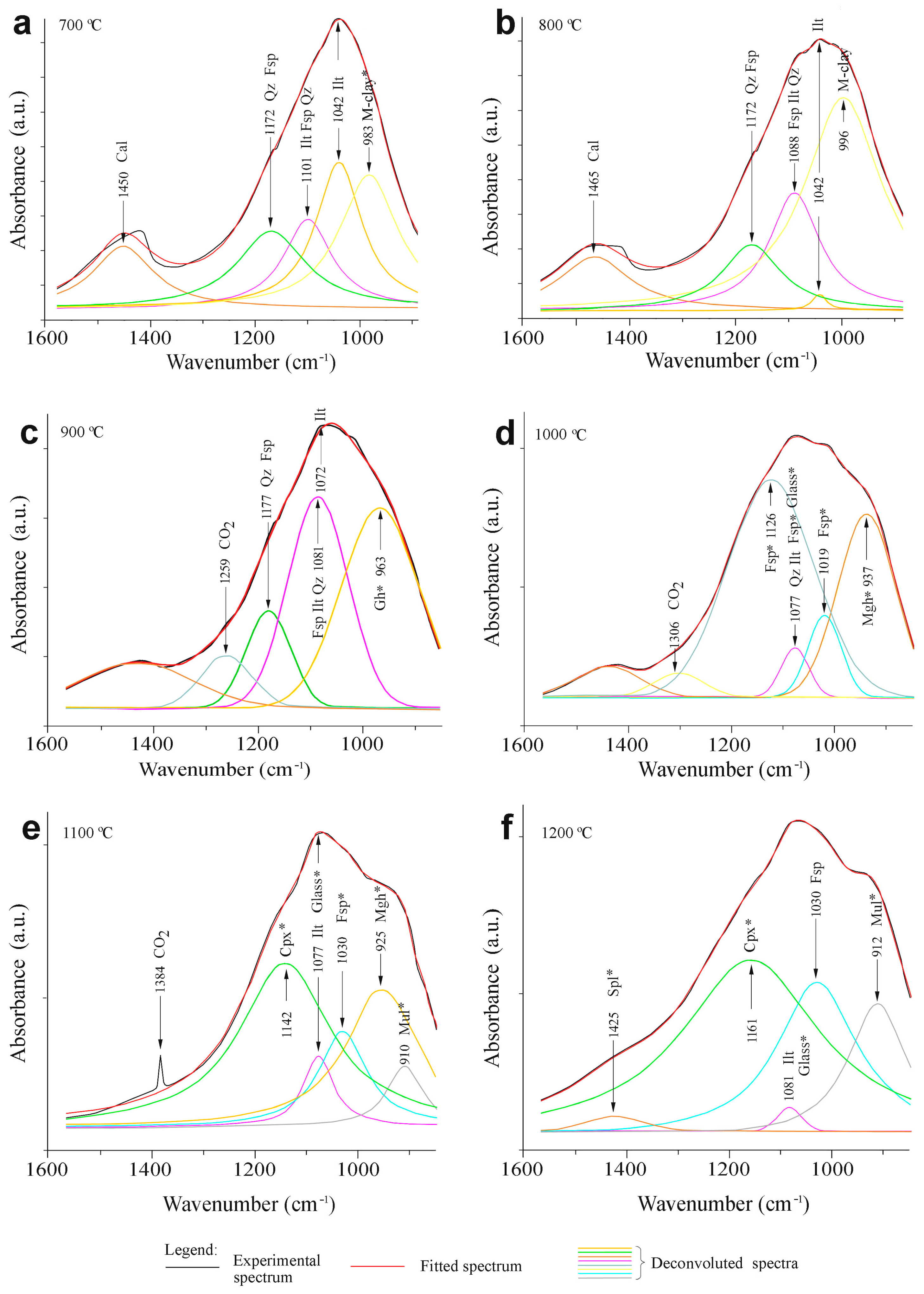
3.2.4. FTIR Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cm | clay minerals |
| Qz | quartz |
| Cbm | micrite carbonate |
| Cbs | sparite carbonate |
| Ms | muscovite |
| Fsp | feldspar |
| Cal | calcite |
| Ilit-Ms | illite-muscovite |
| Chl | chlorite |
| Bt | biotite |
| Dol | dolomite |
| Kln | kaolinite |
| Gth | goethite |
| Lpc | lepidocrocite |
| Gs | glass |
| P | pore |
| Mx | matrix |
| Gh | gehlenite |
| Crs | α-cristobalite |
| Cpx | clinopyroxene |
| Mgh | maghemite |
| Ht | hematite |
| Ilt | illite |
| Mul | mullite |
| Spl | spinel |
| M-clay | meta-clay |
| Fspp | primary feldspar |
| Fsph | newly-formed feldspar |
References
- Vandiver, P.B.; Soffer, O.; Klima, B.; Svoboda, J. The origins of ceramic technology at Dolni Vĕstonice, Czechoslovakia. Science 1989, 246, 1002–1008. [Google Scholar] [CrossRef]
- Shelach, G. On the invention of pottery. Science 2012, 336, 1644–1645. [Google Scholar] [CrossRef]
- Maniatis, Y. The emergence of ceramic technology and its evolution as revealed with the use of scientific techniques. In From Mine to Microscope: Advances in the Study of Ancient Technology; Shortland, A.J., Freestone, I.C., Rehren, T., Eds.; Oxbow Books: Oxford, UK, 2009; pp. 11–27. [Google Scholar]
- Maniatis, Y.; Simopoulos, A.; Kostikas, A. Moessbauer study of the effect of calcium content on iron oxide transformations in fired clays. J. Am. Cer. Soc. 1981, 64, 263–269. [Google Scholar] [CrossRef]
- Müller, N.S.; Vekinis, G.; Kilikoglou, V. Impact resistance of archaeological ceramics: The influence of firing and temper. J. Archaeol. Sci. Rep. 2006, 7, 519–525. [Google Scholar] [CrossRef]
- Tămăşan, M.; Mocuţa, H.; Ionescu, C.; Simon, V. Structural changes induced in mineral clays by high temperature heat treatments. Stud. Univ. Babes Bolyai Phys. 2009, 54, 3–8. [Google Scholar]
- Ionescu, C.; Hoeck, V.; Gruian, C.; Simon, V. Insights into the EPR characteristics of heated carbonate-rich illitic clay. Appl. Clay Sci. 2014, 97–98, 138–145. [Google Scholar] [CrossRef]
- Tite, M.S.; Kilikoglou, V.; Vekinis, G. Strength, toughness and thermal shock resistance of ancient ceramics, and their influence on technological choice. Archaeometry 2001, 43, 301–324. [Google Scholar] [CrossRef]
- Hein, A.; Müller, N.; Day, P.M.; Kilikoglou, V. Thermal conductivity of archaeological ceramics: The effect of inclusions, porosity and firing temperature. Thermochim. Acta 2008, 480, 35–42. [Google Scholar] [CrossRef]
- Gliozzo, E. Ceramic technology. How to reconstruct the firing process. Archaeol. Antropol. Sci. 2020, 12, 260. [Google Scholar] [CrossRef]
- Neff, H.; Bishop, L.; Sayre, E.V. Simulation approach to the problem of tempering in compositional studies of archaeologial ceramics. J. Archaeol. Sci. 1988, 15, 159–172. [Google Scholar] [CrossRef]
- Grifa, C.; Cultrone, G.; Langella, A.; Mercurio, M.; De Bonis, A.; Sebastián, E.; Morra, V. Ceramic replicas of archaeological artefacts in Benevento area (Italy): Petrophysical changes induced by different proportions of clays and temper. Appl. Clay Sci. 2009, 46, 231–240. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De la Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Miner. 2001, 13, 621–634. [Google Scholar] [CrossRef]
- Heimann, R. Firing technologies and their possible assessment by modern analytical method. In Archaeological Ceramics; Olin, J.S., Franklin, A.D., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 89–96. [Google Scholar]
- Shepard, A.O. Ceramics for the Archaeologist. Carnegie Institute: Washington, DC, USA, 1976; p. 414. [Google Scholar]
- Maggetti, M. Phase analysis and its significance for technology and origin. In Archaeological Ceramics; Olin, J.S., Franklin., A.D., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 121–133. [Google Scholar]
- Degryse, P.; Braekmans, D. Petrography: Optical Microscopy. In The Oxford Handbook of Archaeological Ceramic Analysis; Hunt, A.M.W., Ed.; Oxford University Press: Oxford, UK, 2017; pp. 288–304. [Google Scholar]
- Shoval, S. Mineralogical changes upon heating calcitic and dolomitic marl rocks. Thermochim. Acta 1988, 135, 243–252. [Google Scholar] [CrossRef]
- Shoval, S. Using FT-IR spectroscopy for study of calcareous ancient ceramics. Opt. Mater. 2003, 24, 117–122. [Google Scholar] [CrossRef]
- Heimann, R. X-ray powder diffraction (XRPD). In The Oxford Handbook of Archaeological Ceramic Analysis; Hunt, A.M.W., Ed.; Oxford University Press: Oxford, UK, 2017; pp. 288–304. [Google Scholar] [CrossRef]
- Miras, A.; Galán, E.; González, I.; Romero-Baena, A.; Martín, D. Mineralogical evolution of ceramic clays during heating. An ex/in situ X-ray diffraction method comparison study. Appl. Clay Sci. 2018, 161, 176–183. [Google Scholar] [CrossRef]
- Tite, M.S.; Maniatis, Y. Examination of ancient pottery using the scanning electron microscope. Nature 1975, 257, 122–123. [Google Scholar] [CrossRef]
- Maniatis, Y.; Tite, M.S. Technological examination of Neolithic-Bronze Age pottery from central and southeast Europe and from the Near East. J. Archaeol. Sci. 1981, 8, 59–76. [Google Scholar] [CrossRef]
- Shoval, S. Fourier transform infrared spectroscopy (FT-IR) in archaeological ceramic analysis. In The Oxford Handbook of Archaeological Ceramic Analysis; Hunt, A.M.W., Ed.; Oxford University Press: Oxford, UK, 2017; pp. 288–304. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vibr. Spectr. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- De Benedetto, G.E.; Laviano, R.; Sabbatini, L.; Zambonin, P.G. Infrared spectroscopy in the mineralogical characterization of ancient pottery. J. Cult. Herit. 2002, 3, 177–186. [Google Scholar] [CrossRef]
- Barone, G.; Crupi, V.; Galli, S.; Majolino, D.; Migliardo, P.; Venuti, V. Spectroscopic investigation of Greek ceramic artefacts. J. Mol. Str. 2003, 651–653, 449–458. [Google Scholar] [CrossRef]
- Barone, G.; Crupi, V.; Longo, F.; Majolino, D.; Mazzoleni, P.; Tanasi, D.; Venuti, V. FT-IR spectroscopic analysis to study the firing processes of prehistoric ceramics. J. Mol. Struct. 2011, 993, 147–150. [Google Scholar] [CrossRef]
- Shoval, S.; Beck, P. Thermo-FTIR spectroscopy analysis as a method of characterizing ancient ceramic technology. J. Therm. Anal. Calorim. 2005, 82, 609–616. [Google Scholar]
- Kaufhold, S.; Hein, M.; Dohrmann, R.; Ufer, K. Quantification of the mineralogical composition of clays using FTIR spectroscopy. Vibr. Spectr. 2012, 59, 29–39. [Google Scholar] [CrossRef]
- Germinario, C.; Cultrone, G.; De Bonis, A.; Izzo, F.; Langella, A.; Mercurio, M.; Morra, V.; Santoriello, A.; Siano, S.; Grifa, C. The combined use of spectroscopic techniques for the characterisation of Late Roman common wares from Benevento (Italy). Measurement 2018, 114, 515–525. [Google Scholar] [CrossRef]
- Răileanu, G.; Marinescu, F.; Popescu, A. Geological Map of Romania, 1:200000 Scale; Geological Institute of Romania: Bucharest, Romania, 1968. [Google Scholar]
- Hoeck, V.; Ionescu, C.; Metzner-Nebelsick, C.; Nebelsick, L.D. Mineralogy of the ceramic slags from the Bronze Age funerary site in Lăpuş, NW Romania. Geol. Quat. 2012, 56, 649–664. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. Available online: https://www.icdd.com (accessed on 8 October 2024).
- US National Institute of Standards and Technology Reference Material. Available online: www.nist.gov/programs-projects/powder-diffraction-srms (accessed on 8 October 2024).
- Moenke, H. Spektralanalyse von Mineralien und Gesteinen; Akademische Verlagsgesellschaft Geest & Portig K.G.: Leipzig, Germany, 1962; p. 222. [Google Scholar] [CrossRef]
- Moenke, H. Silica, the three-dimensional silicates, borosilicates, and beryllium silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of London: London, UK, 1974; pp. 365–382. [Google Scholar] [CrossRef]
- Farmer, V.C. Vibrational spectroscopy in mineral chemistry. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of London: London, UK, 1974; Monograph 4; pp. 1–10. [Google Scholar] [CrossRef]
- Farmer, V.C. The layer silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of London: London, UK, 1974; Monograph 4; pp. 331–363. [Google Scholar] [CrossRef]
- Freund, F. Ceramics and thermal transformations of minerals. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of London: London, UK, 1974; Monograph 4; pp. 465–482. [Google Scholar] [CrossRef]
- Kiefer, S.W. Thermodynamics and lattice vibrations of minerals: 2. Vibrational characteristics of silicates. Rev. Geophys. Space Phys. 1979, 17, 20–34. [Google Scholar] [CrossRef]
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of AIPEA and CMS nomenclature committees. Clay Miner. 1995, 30, 257–259. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Chester, R.; Elderfiel, H. An infrared study of clay minerals, 2. The identification of kaolinite-group clays in deep-sea sediments. Chem. Geol. 1973, 12, 281–288. [Google Scholar] [CrossRef]
- Rochester, C.H.; Topham, S.A. Infrared study of surface hydroxyl groups on goethite. J. Chem. Soc. Faraday Trans. 1 1979, 75, 591–602. [Google Scholar]
- Skogby, H.; David, R.; Bell, D.R.; Rossman, G.R. Hydroxide in pyroxene: Variations in the natural environment. Am. Miner. 1990, 75, 764–774. [Google Scholar]
- Weckler, B.; Lutz, H.D. Lattice vibration spectra. Part XCV. Infrared spectroscopic studies on the iron oxide hydroxides goethite (α), akaganéite (β), lepidocrocite (γ), and feroxyhite (δ). Eur. J. Solid State Inorg. Chem. 1998, 35, 531–544. [Google Scholar] [CrossRef]
- Vargas, M.A.; Diosa, J.E.; Mosquera, E. Data on study of hematite nanoparticles obtained from Iron(III) oxide by the Pechini method. Data Brief 2019, 25, 104183. [Google Scholar] [CrossRef]
- Deju, R.; Mazilu, C.; Stanculescu, I.; Tuca, C. Fourier transform infrared spectroscopic characterization of thermal treated kaolin. Rom. Rep. Phys. 2020, 72, 806. [Google Scholar]
- Sočo, E.; Domoń, A.; Papciak, D.; Michel, M.M.; Pająk, D.; Cieniek, B.; Azizi, M. Characteristics of Adsorption/Desorption Process on Dolomite Adsorbent in the Copper(II) Removal from Aqueous Solutions. Materials 2023, 16, 4648. [Google Scholar] [CrossRef]
- Shoval, S.; Yadin, E.; Panczer, G. Analysis of thermal phases in calcareous Iron Age pottery using FT-IR and Raman spectroscopy. J. Therm. Analys. Calorim. 2011, 104, 515–525. [Google Scholar] [CrossRef]
- Hlavay, J.; Jonas, K.; Elek, S.; Inczedy, J. Characterization of the particle size and the crystallinity of certain minerals by IR spectrophotometry and other instrumental methods—II. Investigations on quartz and feldspar. Clays Clay Miner. 1978, 26, 139–143. [Google Scholar] [CrossRef]
- Fabbri, B.; Gualtieri, S.; Shoval, S. The presence of calcite in archaeological ceramics. J. Eur. Ceram. Soc. 2014, 34, 1899–1911. [Google Scholar] [CrossRef]
- Delineau, T.; Allard, T.; Muller, J.-P.; Barges, O.; Yvon, J.; Cases, J.-M. FTIR Reflectance vs. EPR studies of structural iron in kaolinites. Clays Clay Miner. 1994, 42, 308–320. [Google Scholar] [CrossRef]
- Vaculícová, L.; Plevová, E. Identification of clay minerals and micas in sedimentary rocks. Acta Geodynam. Geomater. 2005, 2, 167–175. [Google Scholar]
- Maggetti, M. Mineralogisch-petrographische Untersuchung des Scherbenmaterials der urnenfelderzeitlichen Siedlung Elchinger Kreuz, Ldkr. Neu-Ulm/Donau. Kat. Prähistorischen Staatssamml. 1979, 19, 141–172. [Google Scholar]
- Ionescu, C.; Hoeck, V.; Ghergari, L. Electron microprobe analysis of ancient ceramics: A case study from Romania. Appl. Clay Sci. 2011, 53, 466–475. [Google Scholar] [CrossRef]
- Gliozzo, E.; Fortina, C.X.; Memmi Turbanti, I.; Turchiano, M.; Volpe, G. Cooking and painted ware from San Giusto (Lucera, Foggia): The production cycle, from the supply of raw materials to the commercialization of products. Archaeometry 2005, 47, 13–29. [Google Scholar] [CrossRef]
- Ionescu, C.; Hoeck, V.; Simon, V. Effect of the temperature and the heating time on the composition of an illite-rich clay: An XRPD study. Studia Univ. Babeș-Bolyai Phys. 2011, 56, 69–78. [Google Scholar]
- Duminuco, P.; Messiga, B.; Riccardi, M.P. Firing process of natural clays: Some microtextures and related phase compositions. Thermochim. Acta 1998, 321, 185–190. [Google Scholar] [CrossRef]
- Ionescu, C.; Hoeck, V. Firing-induced transformations in Copper Age ceramics from NE Romania. Eur. J. Miner. 2011, 23, 937–958. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Vendrell-Saz, M. The colours of Ca-rich ceramic pastes: Origin and characterization. Appl. Clay Sci. 1998, 13, 187–202. [Google Scholar] [CrossRef]
- Carroll, D.L.; Kemp, T.F.; Bastow, T.J.; Smith, M.E. Solid-state NMR characterisation of the thermal transformation of a Hungarian white illite. Solid State Nucl. Magn. Reson. 2005, 28, 31–43. [Google Scholar] [CrossRef]
- Drachman, S.R.; Roc, G.E.; Smith, M.E. Solid state NMR characterisation of the thermal transformation of Fuller‘s Earth. Solid State Nucl. Magn. Reson. 1997, 9, 257–267. [Google Scholar] [CrossRef]
- Amadori, M.L.; Pallante, P.; Fermo, P.; Emami, M.A.; Chaverdi, A.A.; Callieri, P.; Matin, E. Advances in Achaemenid brick manufacturing technology: Evidence from the monumental gate at Tol-e Ajori (Fars, Iran). Appl. Clay Sci. 2018, 152, 131–142. [Google Scholar] [CrossRef]
- Bordia, R.K.; Kang, S.-J.L.; Olevsky, E.A. Current understanding and future research directions at the onset of the next century of sintering science and technology. J. Am. Cer. Soc. 2017, 100, 2314–2352. [Google Scholar] [CrossRef]
- Gál, Á.; Ionescu, C.; Bajusz, M.; Codrea, V.A.; Hoeck, V.; Barbu-Tudoran, L.; Simon, V.; Mureşan-Pop, M.; Csók, Z. Composition, technology and provenance of Roman pottery from Napoca (Cluj-Napoca, Romania). Clay Miner. 2018, 53, 621–641. [Google Scholar] [CrossRef]
- Shoval, S.; Gaft, M.; Beck, P.; Kirsh, Y. Thermal behaviour of limestone and monocrystalline calcite tempers during firing and their use in ancient vessels. J. Therm. Analys. 1993, 40, 263–273. [Google Scholar] [CrossRef]
- Berna, F.; Behar, A.; Shahack-Gross, R.; Berg, J.; Boaretto, E.; Gilboa, A.; Sharon, I.; Shalev, S.; Shilstein, S.; Yahalom-Mack, N.; et al. Sediments exposed to high temperatures: Reconstructing pyrotechnological processes in late Bronze and Iron Age Strata at Tel Dor (Israel). J. Archaeol. Sci. 2007, 34, 358–373. [Google Scholar] [CrossRef]
- Guggenheim, S.; Chang, Y.-W.; van Groos, K. Muscovite dehydroxylation: High-temperature studies. Am. Mineral. 1987, 72, 537–550. [Google Scholar]
- Abbott, R.N. Energy calculations on the dehydroxylation of muscovite. Can. Miner. 1994, 32, 87–92. [Google Scholar]
- Padeletti, G.; Fermo, P. A scientific approach to the attribution problem of renaissance ceramic productions based on chemical and mineralogical markers. Appl. Phys. A 2010, 100, 771–784. [Google Scholar] [CrossRef]
- Viani, A.; Cultrone, G.; Sotiriadis, K.; Ševčík, R.; Šašek, P. The use of mineralogical indicators for the assessment of firing temperature in fired-clay bodies. Appl. Clay Sci. 2018, 163, 108–118. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Guarini, G.; Marsigli, M.; Venturi, I. Evolluzione della microstruttura durante la cottura rapida di impasti per piastrelle porose. Ceramurgia 1995, 25, 301–314. [Google Scholar]
- Trindade, M.J.; Dias, M.I.; Coroado, J.; Rocha, F. Mineralogical transformations of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Fabbri, B.; Marsigli, M. An approach to the chemistry of pyroxenes formed during firing of Ca-rich silicate ceramics. Clay Miner. 1999, 33, 443–445. [Google Scholar] [CrossRef]
- Rathossi, C.; Pontikes, Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments: Part II. Chemistry of pyrometamorphic minerals and comparison with ancient ceramics. J. Eur. Ceram. Soc. 2010, 30, 1853–1866. [Google Scholar] [CrossRef]
- De Bonis, A.; Cultrone, G.; Grifa, C.; Langella, A.; Morra, V. Clays from the Bay of Naples (Italy): New insight on ancient and traditional ceramics. J. Eur. Ceram. Soc. 2014, 34, 3229–3244. [Google Scholar] [CrossRef]
- Maritan, L.; Mazzoli, C.; Nodari, L.; Russo, U. Second Iron Age grey pottery from Este (northeastern Italy): Study of provenance and technology. Appl. Clay Sci. 2005, 29, 31–44. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Hajjaji, M. A chloritic-illitic clay from Morocco: Temperature–time–transformation and neoformation. Appl. Clay Sci. 2009, 45, 83–89. [Google Scholar] [CrossRef]
- Grapes, R. Pyrometamorphism; Springer: Berlin/Heidelberg, Germany, 2011; p. 365. [Google Scholar]
- Bauluz, B.; Mayayo, M.J.; Yuste, A.; Fernnadez-Nieto, C.; Gonzalez Lopez, J.M. TEM study of mineral transformation in fired carbonated clays: Relevance to brick making. Clay Miner. 2004, 39, 333–344. [Google Scholar] [CrossRef]
- Nodari, L.; Marcuz, E.; Maritan, L.; Mazzoli, C.; Russo, U. Hematite nucleation and growth in the firing of carbonate-rich clay for pottery production. J. Eur. Ceram. Soc. 2007, 27, 4665–4673. [Google Scholar] [CrossRef]
- Maggetti, M.; Heimann, R.B. Bildung und Stabilität von Gehlenit in römischer Feinkeramik. Schweiz. Miner. Petrogr. Mitt. 1979, 59, 413–417. [Google Scholar]
- Hajjaji, M.; Kacim, S. Clay-calcite mixes: Sintering and phase formation. British Ceram. Trans. 2004, 103, 29–32. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, C.; Simon, V.; Hoeck, V.; Gál, Á. Thermal Behaviour of a Carbonatic Clay: A Multi-Analytical Approach. Minerals 2025, 15, 390. https://doi.org/10.3390/min15040390
Ionescu C, Simon V, Hoeck V, Gál Á. Thermal Behaviour of a Carbonatic Clay: A Multi-Analytical Approach. Minerals. 2025; 15(4):390. https://doi.org/10.3390/min15040390
Chicago/Turabian StyleIonescu, Corina, Viorica Simon, Volker Hoeck, and Ágnes Gál. 2025. "Thermal Behaviour of a Carbonatic Clay: A Multi-Analytical Approach" Minerals 15, no. 4: 390. https://doi.org/10.3390/min15040390
APA StyleIonescu, C., Simon, V., Hoeck, V., & Gál, Á. (2025). Thermal Behaviour of a Carbonatic Clay: A Multi-Analytical Approach. Minerals, 15(4), 390. https://doi.org/10.3390/min15040390






