Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Term
2.2. Eligibility Criteria
2.3. Exclusion Criteria
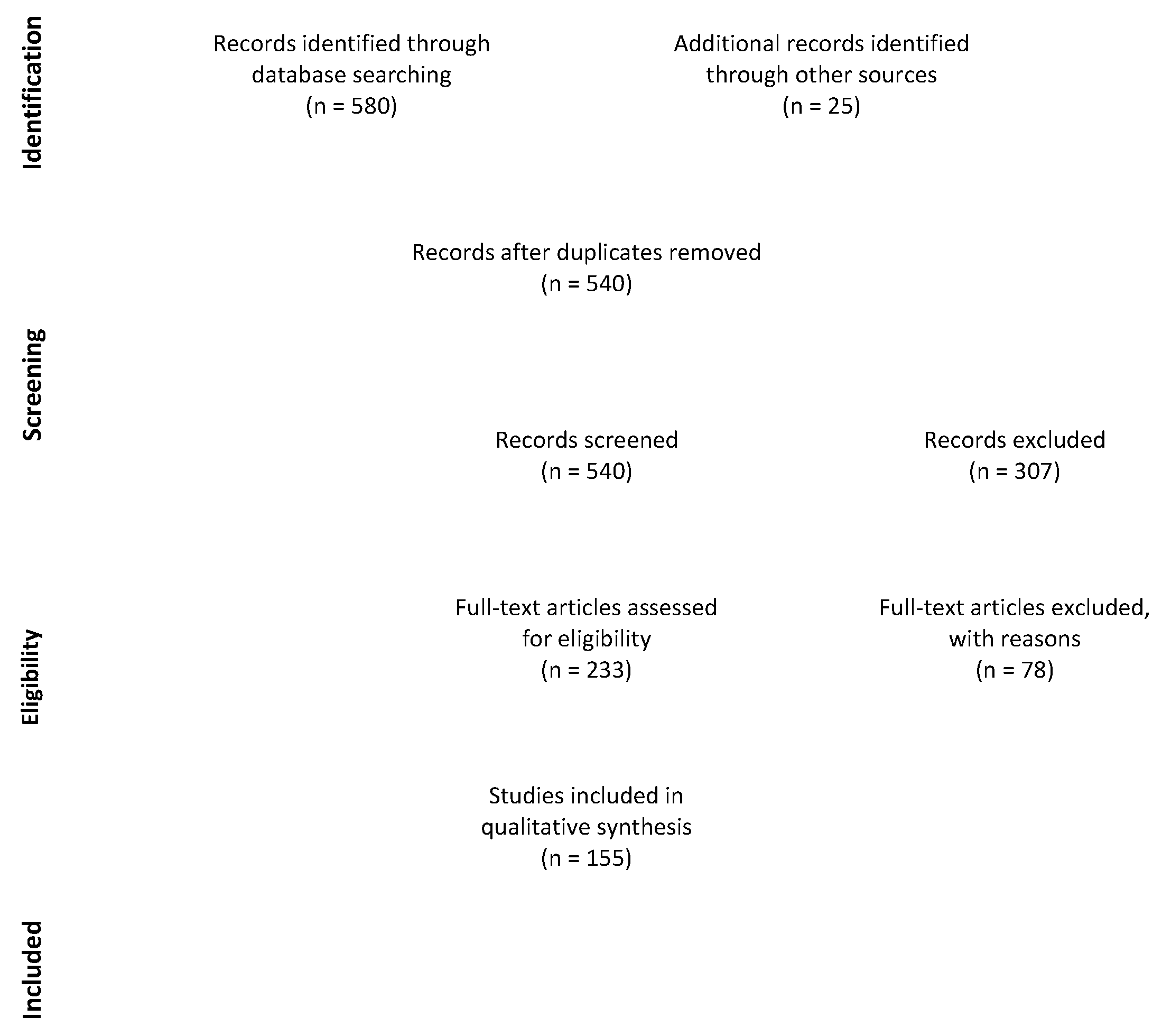
2.4. Study Selection and Data Collection Process
3. Results
3.1. Synthetic Opioids
3.2. Synthetic Cannabinoids
3.3. Stimulants, Psychedelics, and Hallucinogens
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Dignam, G.; Bigham, C. Novel psychoactive substances: A practical approach to dealing with toxicity from legal highs. BJA Educ. 2017, 17, 172–177. [Google Scholar] [CrossRef] [Green Version]
- EMCDDA. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic—An Update from the EU Early Warning System. Available online: https://www.emcdda.europa.eu/system/files/publications/13464/20205648_TD0320796ENN_PDF_rev.pdf (accessed on 1 April 2021).
- Maurer, H.; Brandt, S.D. New Psychoactive Substances, 1st ed.; Springer Nature Switzerland: Cham, Switzerland, 2018. [Google Scholar]
- Baumeister, D.; Tojo, L.M.; Tracy, D.K. Legal highs: Staying on top of the flood of novel psychoactive substances. Ther. Adv. Psychopharmacol. 2015, 5, 97–132. [Google Scholar] [CrossRef] [Green Version]
- EMCDDA. Drug European Drug Report 2020: Trends and Developments. Available online: https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf (accessed on 21 March 2021).
- EMCDDA. New Psychoactive Substances in Europe. An Update from the EU Early Warning System (March 2015). Available online: https://www.emcdda.europa.eu/system/files/publications/65/TD0415135ENN.pdf (accessed on 1 April 2021).
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef] [Green Version]
- Miliano, C.; Margiani, G.; Fattore, L.; Luca, M.A.D. Sales and advertising channels of new psychoactive substances (NPS): Internet, social networks, and smartphone apps. Brain Sci. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, M.M.; Cannaert, A.; Stove, C.P. In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances. Arch. Toxicol. 2020, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Kopajtic, T.A.; Madras, B.K. Pharmacological Research as a key component in mitigating the opioid overdose crisis. Trends Pharmacol. Sci. 2018, 39, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of fentanyl: An emerging problem to face. Forensic Sci. Int. 2018, 289, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Centola, C.; Giorgetti, R. Fentanyl novel derivative-related deaths. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2605. [Google Scholar] [CrossRef]
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of novel synthetic opioids. J. Addict. Med. 2017, 11, 256–265. [Google Scholar] [CrossRef]
- Solimini, R.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Giorgetti, R. Pharmacotoxicology of non-fentanyl derived new synthetic opioids. Front. Pharmacol. 2018, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Vo, Q.; Mahinthichaichan, P.; Shen, J.; Ellis, C. How mu-opioid receptor recognizes fentanyl. Nat. Commun. 2021, 12, 984. [Google Scholar] [CrossRef]
- Cannaert, A.; Ambach, L.; Blanckaert, P.; Stove, C.P. Activity-based detection and bioanalytical confirmation of a fatal carfentanil intoxication. Front. Pharmacol. 2018, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [Green Version]
- Charbogne, P.; Kieffer, B.L.; Befort, K. 15 Years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 2014, 76, 204–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naji, A.; Ramsingh, D. Oral Transmucosal Fentanyl. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554511/ (accessed on 1 April 2021).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
- Baumann, M.H.; Majumdar, S.; Rouzic, V.L.; Hunkele, A.; Uprety, R.; Huang, X.P.; Xu, J.; Roth, B.L.; Pan, Y.-X.; Pasternak, G.W. Pharmacological characterization of Novel Synthetic Opioids (NSO) found in the recreational drug marketplace. Neuropharmacology 2018, 134, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.; Pichini, S.; Pacifici, R.; Tagliabracci, A.; Busardò, F.P.; Auwärter, V.; Solimini, R. Metabolic pathways and potencies of new fentanyl analogs. Front. Pharmacol. 2019, 10, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.K.; Hales, T.G.; Rao, V.J.; NicDaeid, N.; McKenzie, C. The Search for the “next” euphoric non-fentanil novel synthetic opioids on the illicit drugs market: Current status and horizon scanning. Forensic Toxicol. 2019, 37, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, L.; Vandeputte, M.; Deventer, M.; Wouters, E.; Cannaert, A.; Stove, C.P. Assessment of structure-activity relationships and biased agonism at the mu opioid receptor of novel synthetic opioids using a novel, stable bio-assay platform. Biochem. Pharmacol. 2020, 177, 113910. [Google Scholar] [CrossRef]
- Kolesnikova, T.O.; Shevyrin, V.A.; Eltsov, O.S.; Khatsko, S.L.; Demin, K.A.; Galstyan, D.S.; de Abreu, M.S.; Kalueff, A.V. Psychopharmacological characterization of an emerging drug of abuse, a synthetic opioid U-47700, in adult zebrafish. Brain Res. Bull. 2021, 167, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bilel, S.; Azevedo, N.; Arfè, R.; Tirri, M.; Gregori, A.; Serpelloni, G.; De-Giorgio, F.; Frisoni, P.; Neri, M.; Calò, G.; et al. In Vitro and in vivo pharmacological characterization of the synthetic opioid MT-45. Neuropharmacology 2020, 171, 108110. [Google Scholar] [CrossRef]
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and its analogs: Non-fentanyl synthetic opioids impacting the recreational drug market. Brain Sci. 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 2018, 134, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.; Mallareddy, J.R.; Yoshida, K.; Bustamante, V.; Lee, T.; Krstenansky, J.L.; Zambon, A.C. Synthesis and pharmacological characterization of ethylenediamine synthetic opioids in human Μ-opiate Receptor 1 (OPRM1) expressing cells. Pharmacol Res. Perspect. 2019, 7, e00511. [Google Scholar] [CrossRef] [Green Version]
- Blanckaert, P.; Cannaert, A.; Uytfanghe, K.V.; Hulpia, F.; Deconinck, E.; Calenbergh, S.V.; Stove, C. Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene. Drug Test Anal. 2020, 12, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. AH-7921: The list of new psychoactive opioids is expanded. Forensic Toxicol. 2015, 33, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, T.; Lyutenska, M.; Urban, B.W.; Wittmann, M. Direct effects of morphine but not of fentanyl-type opioids on human 5-HT3A receptors in outside-out patch-clamp studies. Eur. J. Pain 2014, 18, 1165–1172. [Google Scholar] [CrossRef]
- Baldo, B.A. Opioid analgesic drugs and serotonin toxicity (syndrome): Mechanisms, animal models, and links to clinical effects. Arch. Toxicol. 2018, 92, 2457–2473. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.J.H.; Koeppe, R.A.; Shao, X.; Rodnick, M.E.; Sowa, A.R.; Henderson, B.D.; Stauff, J.; Sherman, P.S.; Arteaga, J.; Carlo, D.J.; et al. The effects of intramuscular naloxone dose on Mu receptor displacement of Carfentanil in Rhesus monkeys. Molecules 2020, 25, 1360. [Google Scholar] [CrossRef] [Green Version]
- Rickli, A.; Liakoni, E.; Hoener, M.C.; Liechti, M.E. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: Link to clinical reports of serotonin syndrome. Brit. J. Pharmacol. 2018, 175, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Barann, M.; Stamer, U.M.; Lyutenska, M.; Stüber, F.; Bönisch, H.; Urban, B. Effects of opioids on human serotonin transporters. Naunyn Schmiedeberg’s Arch. Pharmacol. 2015, 388, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Torralva, R.; Eshleman, A.J.; Swanson, T.L.; Schmachtenberg, J.L.; Schutzer, W.E.; Bloom, S.H.; Wolfrum, K.M.; Reed, J.F.; Janowsky, A. Fentanyl but not morphine interacts with non-opioid recombinant human neurotransmitter receptors and transporters. J. Pharmacol. Exp. Ther. 2020, 374, 376–391. [Google Scholar] [CrossRef]
- Berry-Cabán, C.S.; Kleinschmidt, P.E.; Rao, D.S.; Jenkins, J. Synthetic cannabinoid and cathinone use among US soldiers. US Army Med. Dep. J. 2012, 19–24. [Google Scholar]
- Every-Palmer, S. Synthetic Cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depen. 2011, 117, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.; Marusich, J.; Huffman, J.W.; Balster, R.L.; Thomas, B. Hijacking of basic research: The case of synthetic Cannabinoids. Methods Rep. RTi. Press 2011, 2011, 17971. [Google Scholar] [CrossRef]
- Sachdev, S.; Vemuri, K.; Banister, S.D.; Longworth, M.; Kassiou, M.; Santiago, M.; Makriyannis, A.; Connor, M. In vitro determination of the efficacy of illicit synthetic cannabinoids at CB1 receptors. Brit. J. Pharmacol. 2019, 176, 4653–4665. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.; Cannaert, A.; Linnet, K.; Stove, C.P. Application of an activity-based receptor bioassay to investigate the in vitro activity of selected indole- and indazole-3-carboxamide-based synthetic Cannabinoids at CB1 and CB2 receptors. Drug Test Anal. 2019, 11, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Gamage, T.F.; Farquhar, C.E.; Lefever, T.W.; Marusich, J.A.; Kevin, R.C.; McGregor, I.S.; Wiley, J.L.; Thomas, B.F. Molecular and behavioral pharmacological characterization of abused synthetic cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J. Pharmacol. Exp. Ther. 2018, 365, 437–446. [Google Scholar] [CrossRef]
- Angerer, V.; Mogler, L.; Steitz, J.; Bisel, P.; Hess, C.; Schoeder, C.T.; Müller, C.E.; Huppertz, L.M.; Westphal, F.; Schäper, J.; et al. Structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE. Drug Test Anal. 2018, 10, 597–603. [Google Scholar] [CrossRef]
- Ford, B.M.; Tai, S.; Fantegrossi, W.E.; Prather, P.L. Synthetic pot: Not your grandfather’s marijuana. Trends Pharmacol. Sci. 2017, 38, 257–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, S.; Fantegrossi, W.E. Neuropharmacology of New Psychoactive Substances (NPS), the science behind the headlines. Curr. Top. Behav. Neurosci. 2016, 249–262. [Google Scholar] [CrossRef]
- Cannaert, A.; Storme, J.; Franz, F.; Auwärter, V.; Stove, C.P. Detection and activity profiling of synthetic Cannabinoids and their metabolites with a newly developed bioassay. Anal. Chem. 2016, 88, 11476–11485. [Google Scholar] [CrossRef] [Green Version]
- Wiley, J.L.; Lefever, T.W.; Marusich, J.A.; Grabenauer, M.; Moore, K.N.; Huffman, J.W.; Thomas, B.F. Evaluation of first generation synthetic Cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology 2016, 110, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Hess, C.; Schoeder, C.T.; Pillaiyar, T.; Madea, B.; Müller, C.E. Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol. 2016, 34, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Brunt, T.M.; Bossong, M.G. The neuropharmacology of cannabinoid receptor ligands in central signaling pathways. Eur. J. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Andersen, H.K. Molecular pharmacology of synthetic cannabinoids: Delineating CB1 receptor-mediated cell signaling. Int. J. Mol. Sci. 2020, 21, 6115. [Google Scholar] [CrossRef]
- Spanagel, R. Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Potential mechanisms underlying the deleterious effects of synthetic Cannabinoids found in spice/K2 products. Brain Sci. 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Chen, J.; Harrington, A.; Vinod, K.Y.; Hegde, M.L.; Hegde, V.L. Cannabinoid exposure during pregnancy and its impact on immune function. Cell. Mol. Life Sci. 2019, 76, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Canazza, I.; Ossato, A.; Trapella, C.; Fantinati, A.; Luca, M.A.D.; Margiani, G.; Vincenzi, F.; Rimondo, C.; Rosa, F.D.; Gregori, A.; et al. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology 2016, 233, 3685–3709. [Google Scholar] [CrossRef]
- Wilson, C.D.; Tai, S.; Ewing, L.; Crane, J.; Lockhart, T.; Yarbrough, A.L.; Fujiwara, R.; Radominska-Pandya, A.; Fantegrossi, W.E. Convulsant effects of abused synthetic cannabinoids JWH-018 and 5F-AB-PINACA are mediated by agonist actions at CB1 receptors in mice. J. Pharmacol. Exp. Ther. 2018, 368, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.W.; Gramling, B.R.; Justinova, Z.; Thorndike, E.B.; Baumann, M.H. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depen. 2017, 179, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Grim, T.W.; Morales, A.J.; Gonek, M.M.; Wiley, J.L.; Thomas, B.F.; Endres, G.W.; Sim-Selley, L.J.; Selley, D.E.; Negus, S.S.; Lichtman, A.H. Stratification of Cannabinoid 1 Receptor (CB1R) agonist efficacy: Manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J. Pharmacol. Exp. Ther. 2016, 359, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Grim, T.W.; Wiebelhaus, J.M.; Morales, A.J.; Negus, S.S.; Lichtman, A.H. Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depen. 2015, 150, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Worob, A.; Wenthur, C. DARK classics in chemical neuroscience: Synthetic Cannabinoids (Spice/K2). ACS Chem. Neurosci. 2019, 11, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Deyama, S.; Domoto, M.; Zhang, T.; Sasase, H.; Fukao, A.; Esaki, H.; Hinoi, E.; Kaneko, S.; Kaneda, K. Effects of the synthetic Cannabinoid 5F-AMB on anxiety and recognition memory in mice. Psychopharmacology 2019, 236, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.; Moran, J. Distinct pharmacology and metabolism of K2 synthetic Cannabinoids compared to Δ9-THC: Mechanism underlying greater toxicity? Life Sci. 2014, 97, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Domoto, M.; Sasase, H.; Wada, S.; Ito, S.; Deyama, S.; Hinoi, E.; Kaneko, S.; Kaneda, K. The synthetic Cannabinoid 5F-AMB changes the balance between excitation and inhibition of layer V pyramidal neurons in the mouse medial prefrontal cortex. Psychopharmacology 2018, 235, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Forster, M.J. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology 2016, 233, 1901–1910. [Google Scholar] [CrossRef] [Green Version]
- Kevin, R.C.; Anderson, L.; McGregor, I.S.; Boyd, R.; Manning, J.J.; Glass, M.; Connor, M.; Banister, S.D. CUMYL-4CN-BINACA is an efficacious and potent pro-convulsant synthetic cannabinoid receptor agonist. Front. Pharmacol. 2019, 10, 595. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Zielińska, A.; Souto, E.B.; Wielgus, K. Cannabidiol in neurological and neoplastic diseases: Latest developments on the molecular mechanism of action. Int. J. Mol. Sci. 2021, 22, 4294. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.; Simola, N.; Piras, G.; Caria, F.; Onaivi, E.S.; Luca, M.A.D. Neurochemical and behavioral characterization after acute and repeated exposure to novel synthetic cannabinoid agonist 5-MDMB-PICA. Brain Sci. 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Carmo, H.; Carvalho, F.; Silva, J.P. Synthetic Cannabinoids and their impact on neurodevelopmental processes. Addict. Biol. 2020, 25, e12824. [Google Scholar] [CrossRef] [PubMed]
- Canazza, I.; Ossato, A.; Vincenzi, F.; Gregori, A.; Rosa, F.D.; Nigro, F.; Rimessi, A.; Pinton, P.; Varani, K.; Borea, P.A.; et al. Pharmaco-toxicological effects of the novel third-generation fluorinate synthetic cannabinoids, 5F-ADBINACA, AB-FUBINACA, and STS-135 in mice. In vitro and in vivo studies. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruba, L.; McMahon, L.R. Apparent affinity estimates and reversal of the effects of synthetic Cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by Rimonabant in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2017, 362, 278–286. [Google Scholar] [CrossRef]
- Almada, M.; Costa, L.; Fonseca, B.M.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. The synthetic cannabinoid WIN-55,212 induced-apoptosis in cytotrophoblasts cells by a mechanism dependent on CB1 receptor. Toxicology 2017, 385, 67–73. [Google Scholar] [CrossRef]
- Tomiyama, K.; Funada, M. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: The involvement of Cannabinoid CB1 receptors and apoptotic cell death. Toxicol. Appl. Pharm. 2014, 274, 17–23. [Google Scholar] [CrossRef]
- Tomiyama, K.; Funada, M. Synthetic Cannabinoid CP-55,940 induces apoptosis in a human skeletal muscle model via regulation of CB1 receptors and l-Type Ca2+ channels. Arch. Toxicol. 2021, 95, 617–630. [Google Scholar] [CrossRef]
- Koller, V.J.; Zlabinger, G.J.; Auwärter, V.; Fuchs, S.; Knasmueller, S. Toxicological profiles of selected synthetic Cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB1. Arch. Toxicol. 2013, 87, 1287–1297. [Google Scholar] [CrossRef]
- Koller, V.J.; Auwärter, V.; Grummt, T.; Moosmann, B.; Mišík, M.; Knasmüller, S. Investigation of the in vitro toxicological properties of the synthetic cannabimimetic drug CP-47,497-C8. Toxicol. Appl. Pharm. 2014, 277, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Funada, M.; Takebayashi-Ohsawa, M.; Tomiyama, K. Synthetic Cannabinoids enhanced ethanol-induced motor impairments through reduction of central glutamate neurotransmission. Toxicol. Appl. Pharm. 2020, 408, 115283. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cowan, A.; Inan, S.; Geller, E.B.; Meissler, J.J.; Rawls, S.M.; Tallarida, R.J.; Tallarida, C.S.; Watson, M.N.; Adler, M.W.; et al. Opioid-sparing effects of Cannabinoids on morphine analgesia: Participation of CB1 and CB2 receptors. Brit. J. Pharmacol. 2019, 176, 3378–3389. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological evaluation of new constituents of “spice”: Synthetic Cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banister, S.D.; Olson, A.; Winchester, M.; Stuart, J.; Edington, A.R.; Kevin, R.C.; Longworth, M.; Herrera, M.; Connor, M.; McGregor, I.S.; et al. The chemistry and pharmacology of synthetic Cannabinoid SDB-006 and its regioisomeric fluorinated and methoxylated analogs. Drug Test Anal. 2018, 10, 1099–1109. [Google Scholar] [CrossRef]
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.C.; Glass, M.; McGregor, I.S.; Connor, M.; et al. Pharmacology of valinate and tert-leucinate synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254. [Google Scholar] [CrossRef]
- Haschimi, B.; Giorgetti, A.; Mogler, L.; Nagy, T.Z.; Kramer, S.; Halter, S.; Boros, S.; Dobos, A.; Hidvégi, E.; Auwärter, V. The novel psychoactive substance Cumyl-CH-MEGACLONE: Human phase-i metabolism, basic pharmacological characterization and comparison to other synthetic cannabinoid receptor agonists with a γ-Carboline-1-one core. J. Anal. Toxicol. 2020, 45, 277–290. [Google Scholar] [CrossRef]
- Banister, S.D.; Adams, A.; Kevin, R.C.; Macdonald, C.; Glass, M.; Boyd, R.; Connor, M.; McGregor, I.S.; Havel, C.M.; Bright, S.J.; et al. Synthesis and pharmacology of new psychoactive substance 5F-CUMYL-P7AICA, a scaffold-hopping analog of synthetic cannabinoid receptor agonists 5F-CUMYL-PICA and 5F-CUMYL-PINACA. Drug Test Anal. 2019, 11, 279–291. [Google Scholar] [CrossRef]
- Vigolo, A.; Ossato, A.; Trapella, C.; Vincenzi, F.; Rimondo, C.; Seri, C.; Varani, K.; Serpelloni, G.; Marti, M. Novel halogenated derivates of JWH-018: Behavioral and binding studies in mice. Neuropharmacology 2015, 95, 68–82. [Google Scholar] [CrossRef]
- Ametovski, A.; Macdonald, C.; Manning, J.J.; Haneef, S.A.S.; Santiago, M.; Martin, L.; Sparkes, E.; Reckers, A.; Gerona, R.R.; Connor, M.; et al. Exploring stereochemical and conformational requirements at cannabinoid receptors for synthetic cannabinoids related to SDB-006, 5F-SDB-006, CUMYL-PICA, and 5F-CUMYL-PICA. ACS Chem. Neurosci. 2020, 11, 3672–3682. [Google Scholar] [CrossRef]
- Doi, T.; Tagami, T.; Takeda, A.; Asada, A.; Sawabe, Y. Evaluation of carboxamide-type synthetic Cannabinoids as CB1/CB2 receptor agonists: Difference between the enantiomers. Forensic Toxicol. 2018, 36, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Giorgetti, A.; Busardò, F.P.; Tittarelli, R.; Auwärter, V.; Giorgetti, R. Post-mortem toxicology: A systematic review of death cases involving synthetic cannabinoid receptor agonists. Front. Psychiatry 2020, 11, 464. [Google Scholar] [CrossRef]
- Yun, J.; Yoon, K.S.; Lee, T.-H.; Lee, H.; Gu, S.M.; Song, Y.J.; Cha, H.J.; Han, K.M.; Seo, H.; Shin, J.; et al. Synthetic Cannabinoid, JWH-030, induces QT prolongation through HERG channel inhibition. Toxicol. Res. 2016, 5, 1663–1671. [Google Scholar] [CrossRef] [Green Version]
- López-Dyck, E.; Andrade-Urzúa, F.; Elizalde, A.; Ferrer-Villada, T.; Dagnino-Acosta, A.; Huerta, M.; Osuna-Calleros, Z.; Rangel-Sandoval, C.; Sánchez-Pastor, E. ACPA and JWH-133 modulate the vascular tone of superior mesenteric arteries through cannabinoid receptors, BKCa channels, and nitric oxide dependent mechanisms. Pharmacol. Rep. 2017, 69, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Maggo, S.; Ashton, J.C. Effect of cannabinoid receptor agonists on isolated rat atria. J. Cardiovasc. Pharm. 2018, 72, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, H.M.; Yetkin, E.; Ozturk, S. Synthetic cannabinoids and cardiac arrhythmia risk: Review of the literature. Cardiovasc. Toxicol. 2019, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pinson, A.; Yarbrough, A.L.; Bush, J.M.; Cabanlong, C.V.; Shoeib, A.; Jackson, B.K.; Fukuda, S.; Gogoi, J.; Fantegrossi, W.E.; McCain, K.; et al. Metabolism, CB1 cannabinoid receptor binding and in vivo activity of synthetic cannabinoid 5f-akb48: Implications for toxicity. Pharmacol. Biochem. Behav. 2020, 195, 172949. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.D.; Ford, B.M.; Franks, L.N.; Wilson, C.D.; Yarbrough, A.L.; Fujiwara, R.; Su, M.K.; Fernandez, D.; James, L.P.; Moran, J.H.; et al. Atypical pharmacodynamic properties and metabolic profile of the abused synthetic cannabinoid AB-PINACA: Potential contribution to pronounced adverse effects relative to Δ9-THC. Front. Pharmacol. 2018, 9, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longworth, M.; Connor, M.; Banister, S.D.; Kassiou, M. Synthesis and pharmacological profiling of the metabolites of synthetic cannabinoid drugs APICA, STS-135, ADB-PINACA, and 5F-ADB-PINACA. ACS Chem. Neurosci. 2017, 8, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, M.; Brents, L.K.; Franks, L.N.; Moran, J.H.; Prather, P.L. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol. Appl. Pharm. 2013, 269, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Couceiro, J.; Bandarra, S.; Sultan, H.; Bell, S.; Constantino, S.; Quintas, A. Toxicological Impact of JWH-018 and its phase I metabolite N-(3-Hydroxypentyl) on human cell lines. Forensic Sci. Int. 2016, 264, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Goonawardena, A.V.; Pertwee, R.; Hampson, R.E.; Platt, B.; Riedel, G. WIN55,212-2 induced deficits in spatial learning are mediated by cholinergic hypofunction. Behav. Brain Res. 2010, 208, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossato, A.; Uccelli, L.; Bilel, S.; Canazza, I.; Domenico, G.D.; Pasquali, M.; Pupillo, G.; Luca, M.A.D.; Boschi, A.; Vincenzi, F.; et al. Psychostimulant effect of the synthetic cannabinoid JWH-018 and AKB48: Behavioral, neurochemical, and dopamine transporter scan imaging studies in mice. Front. Psychiatry 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Irie, T.; Kikura-Hanajiri, R.; Usami, M.; Uchiyama, N.; Goda, Y.; Sekino, Y. MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar purkinje cells via activation of presynaptic CB1 receptors. Neuropharmacology 2015, 95, 479–491. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Naline, E.; Buenestado, A.; Faisy, C.; Alvarez, J.; Salvator, H.; Abrial, C.; Advenier, C.; Zemoura, L.; Devillier, P. Cannabinoids inhibit cholinergic contraction in human airways through prejunctional CB1 receptors. Brit. J. Pharmacol. 2014, 171, 2767–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantegrossi, W.E.; Wilson, C.D.; Berquist, M.D. Pro-psychotic effects of synthetic cannabinoids: Interactions with central dopamine, serotonin, and glutamate systems. Drug Metab. Rev. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hroudová, J.; Fišar, Z. Cannabinoid-induced changes in the activity of electron transport chain complexes of brain mitochondria. J. Mol. Neurosci. 2015, 56, 926–931. [Google Scholar] [CrossRef]
- Petrocellis, L.D.; Marzo, V.D. Non-CB1, Non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: Focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharm. 2010, 5, 103–121. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Yano, H.; Adhikari, P.; Naing, S.; Hoffman, A.F.; Baumann, M.H.; Lupica, C.R.; Shi, L. Positive allosteric modulation of the 5-HT 1A receptor by indole-based synthetic cannabinoids abused by humans. ACS Chem. Neurosci. 2020, 11, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Baumann, M.H. Repeated exposure to the “spice” cannabinoid JWH-018 induces tolerance and enhances responsiveness to 5-HT1A receptor stimulation in male rats. Front. Psychiatry 2018, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Yang, R.; Wang, X.; Liu, H.; Zou, L.; Hu, X.; Wu, J.; Zou, A.; Liu, L. Inhibition of 5-HT3 receptors-activated currents by cannabinoids in rat trigeminal ganglion neurons. J. Huazhong Univ. Sci. Technol. Med Sci. 2012, 32, 265–271. [Google Scholar] [CrossRef]
- Linsen, F.; Koning, R.P.J.; Laar, M.; Niesink, R.J.M.; Koeter, M.W.; Brunt, T.M. 4-Fluoroamphetamine in The Netherlands: More than a one-night stand. Addiction 2015, 110, 1138–1143. [Google Scholar] [CrossRef]
- Nelson, M.E.; Bryant, S.M.; Aks, S.E. Emerging drugs of abuse. Emerg. Med. Clin. N. Am. 2014, 32, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.L.; Cunha, K.F.; Lanaro, R.; Cunha, R.L.; Walther, D.; Baumann, M.H. Analytical quantification, intoxication case series, and pharmacological mechanism of action for N-ethylnorpentylone (N-ethylpentylone or Ephylone). Drug Test Anal. 2019, 11, 461–471. [Google Scholar] [CrossRef]
- Karch, S. Cathinone neurotoxicity (“The “3Ms”). Curr. Neuropharmacol. 2015, 13, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath Salts and Synthetic Cathinones: An Emerging Designer Drug Phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitte, H.H.; Freissmuth, M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci. 2015, 36, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, S.J.; Gregg, R.A.; Tran, F.H.; Mo, L.; Weltin, E.; Barker, D.J.; Gentile, T.A.; Watterson, L.R.; Rawls, S.M.; Muschamp, J.W. Comparing rewarding and reinforcing properties between ‘Bath Salt’ 3,4-methylenedioxypyrovalerone (MDPV) and Cocaine using ultrasonic vocalizations in rats. Addict. Biol. 2018, 23, 102–110. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Bilel, S.; Ossato, A.; Tirri, M.; Arfè, R.; Foti, F.; Serpelloni, G.; Frisoni, P.; Neri, M.; Marti, M. Acute and repeated administration of MDPV increases aggressive behavior in mice: Forensic implications. Int J. Leg. Med. 2019, 133, 1797–1808. [Google Scholar] [CrossRef]
- Altun, B.; Çok, İ. Psychoactive bath salts and neurotoxicity risk. Turk. J. Pharm Sci. 2020, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 2014, 88, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Liechti, M. Novel psychoactive substances (designer drugs): Overview and pharmacology of modulators of monoamine signaling. Swiss Med. Wkly. 2015, 145, w14043. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of synthetic cathinones. Handb. Exp. Pharmacol. 2018, 113–142. [Google Scholar] [CrossRef]
- Aarde, S.M.; Taffe, M.A. Predicting the abuse liability of entactogen-class, new and emerging psychoactive substances via preclinical models of drug self-administration. Curr. Top. Behav. Neurosci. 2017, 145–164. [Google Scholar] [CrossRef]
- Gannon, B.M.; Galindo, K.I.; Mesmin, M.P.; Sulima, A.; Rice, K.C.; Collins, G.T. Relative reinforcing effects of second-generation synthetic Cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 2018, 134, 28–35. [Google Scholar] [CrossRef]
- Javadi-Paydar, M.; Nguyen, J.D.; Vandewater, S.A.; Dickerson, T.J.; Taffe, M.A. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology 2018, 134, 57–64. [Google Scholar] [CrossRef]
- Wee, S.; Anderson, K.G.; Baumann, M.H.; Rothman, R.B.; Blough, B.E.; Woolverton, W.L. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J. Pharmacol. Exp. Ther. 2005, 313, 848–854. [Google Scholar] [CrossRef]
- Wee, S.; Woolverton, W.L. Self-Administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol. Biochem. Behav. 2006, 84, 337–343. [Google Scholar] [CrossRef]
- Suyama, J.A.; Banks, M.L.; Negus, S.S. Effects of repeated treatment with methcathinone, mephedrone, and fenfluramine on intracranial self-stimulation in rats. Psychopharmacology 2019, 236, 1057–1066. [Google Scholar] [CrossRef]
- Rickli, A.; Kolaczynska, K.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of the aminorex analogs 4-MAR, 4,4′-DMAR, and 3,4-DMAR. Neurotoxicology 2019, 72, 95–100. [Google Scholar] [CrossRef]
- Zona, L.C.; Grecco, G.G.; Sprague, J.E. Cooling down the bath salts: Carvedilol attenuation of methylone and mephedrone mediated hyperthermia. Toxicol. Lett. 2016, 263, 11–15. [Google Scholar] [CrossRef]
- Silva, B.; Fernandes, C.; de Pinho, P.G.; Remião, F. Chiral resolution and enantioselectivity of synthetic cathinones: A brief review. J. Anal. Toxicol. 2017, 42, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological characterization of designer cathinones in vitro. Brit. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef]
- Partilla, J.S.; Dempsey, A.G.; Nagpal, A.S.; Blough, B.E.; Baumann, M.H.; Rothman, R.B. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 2006, 319, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolder, P.C.; Perna, E.B.S.F.; Mason, N.L.; Hutten, N.R.P.W.; Toennes, S.W.; Theunissen, E.L.; Ramaekers, J.G.; Kuypers, K.P.C. Independent elevation of peripheral oxytocin concentrations and reduction in cognitive empathy during 4-fluoroamphetamine intoxication. Hum. Psychopharmacol. Clin. Exp. 2018, 33, e2680. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Baumann, M.H.; Partilla, J.S.; Kavanagh, P.V.; Power, J.D.; Talbot, B.; Twamley, B.; Mahony, O.; O’Brien, J.; Elliott, S.P.; et al. Characterization of a novel and potentially lethal designer drug (±)-cis-para-methyl-4-methylaminorex (4,4’-DMAR, or ‘Serotoni’). Drug Test Anal. 2014, 6, 684–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, G.; Morris, N.; Kavanagh, P.V.; Power, J.D.; Dowling, G.; Twamley, B.; O’Brien, J.; Talbot, B.; Walther, D.; Partilla, J.S.; et al. Synthesis, characterization and monoamine transporter activity of the new psychoactive substance mexedrone and Its N-methoxy Positional Isomer, N-methoxymephedrone. Drug Test Anal. 2017, 9, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Davidson, C.; Raby, C.A.R.; Barrese, V.; Ramsey, J. In vitro neurochemical assessment of methylphenidate and its “legal high” analogs 3,4-CTMP and ethylphenidate in rat nucleus accumbens and bed nucleus of the stria terminalis. Front. Psychiatry 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, H.M.; Shi, Q.; Gruszecka-Kowalik, E.; Schweri, M.M. Synthesis and pharmacology of potential cocaine antagonists. 2. structure−activity relationship studies of aromatic ring-substituted methylphenidate analogs. J. Med. Chem. 1996, 39, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Luethi, D.; Hoener, M.C.; Liechti, M.E. Effects of the new psychoactive substances diclofensine, diphenidine, and methoxphenidine on monoaminergic systems. Eur. J. Pharmacol. 2018, 819, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Dybdal-Hargreaves, N.F.; Holder, N.D.; Ottoson, P.E.; Sweeney, M.D.; Williams, T. Mephedrone: Public health risk, mechanisms of action, and behavioral effects. Eur. J. Pharmacol. 2013, 714, 32–40. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niello, M.; Cintulova, D.; Hellsberg, E.; Jäntsch, K.; Holy, M.; Ayatollahi, L.H.; Cozzi, N.V.; Freissmuth, M.; Sandtner, W.; Ecker, G.F.; et al. Para-trifluoromethyl-methcathinone is an allosteric modulator of the serotonin transporter. Neuropharmacology 2019, 161, 107615. [Google Scholar] [CrossRef]
- Shokry, I.M.; Sinha, V.; Silva, G.D.; Park, S.; Callanan, J.J.; Tao, R. Comparison of Electroencephalogram (EEG) response to MDPV versus the hallucinogenic drugs MK-801 and ketamine in rats. Exp. Neurol. 2019, 313, 26–36. [Google Scholar] [CrossRef]
- Schiavi, S.; Melancia, F.; Carbone, E.; Buzzelli, V.; Manduca, A.; Peinado, P.J.; Zwergel, C.; Mai, A.; Campolongo, P.; Vanderschuren, L.J.M.J.; et al. Detrimental effects of the ‘bath salt’ methylenedioxypyrovalerone on social play behavior in male rats. Neuropsychopharmacology 2020, 45, 2012–2019. [Google Scholar] [CrossRef]
- Marusich, J.A.; Antonazzo, K.R.; Wiley, J.L.; Blough, B.E.; Partilla, J.S.; Baumann, M.H. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 2014, 87, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, J.; Kuczyńska, K.; Zawilska, J.B. Four Synthetic Cathinones: 3-Chloromethcathinone, 4-chloromethcathinone, 4-fluoro-α-pyrrolidinopentiophenone, and 4-methoxy-α-pyrrolidinopentiophenone produce changes in the spontaneous locomotor activity and motor performance in mice with varied profiles. Neurotox. Res. 2020, 38, 536–551. [Google Scholar] [CrossRef]
- Dolan, S.B.; Chen, Z.; Huang, R.; Gatch, M.B. “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology 2018, 133, 171–180. [Google Scholar] [CrossRef]
- Valente, M.J.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; de Pinho, P.G.; Carvalho, M. Neurotoxicity of β-keto amphetamines: Deathly mechanisms elicited by methylone and MDPV in human dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Young, R. Neurobiology of 3,4-Methylenedioxypyrovalerone (MDPV) and α-Pyrrolidinovalerophenone (α-PVP). Brain Res. Bull. 2016, 126, 111–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huskinson, S.L.; Naylor, J.E.; Townsend, E.A.; Rowlett, J.K.; Blough, B.E.; Freeman, K.B. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 2017, 234, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Canal, C.E. Structure–activity relationship study of psychostimulant synthetic cathinones reveals nanomolar antagonist potency of α-pyrrolidinohexiophenone at human muscarinic M 2 receptors. ACS Chem. Neurosci. 2020, 11, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Blough, B.E.; Murnane, K.S.; Canal, C.E. The synthetic cathinone psychostimulant A-PPP antagonizes serotonin 5-HT2A receptors: In vitro and in vivo evidence. Drug Test Anal. 2019, 11, 990–998. [Google Scholar] [CrossRef]
- Rickli, A.; Kopf, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of novel psychoactive benzofurans. Br. J. Pharmacol. 2015, 172, 3412–3425. [Google Scholar] [CrossRef]
- Dawson, P.; Opacka-Juffry, J.; Moffatt, J.D.; Daniju, Y.; Dutta, N.; Ramsey, J.; Davidson, C. The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2014, 48, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Gibbons, S.; Treble, R.; Setola, V.; Huang, X.-P.; Roth, B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013, 700, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marusich, J.A.; Antonazzo, K.R.; Blough, B.E.; Brandt, S.D.; Kavanagh, P.V.; Partilla, J.S.; Baumann, M.H. The new psychoactive substances 5-(2-Aminopropyl)Indole (5-IT) and 6-(2-Aminopropyl)Indole (6-IT) interact with monoamine transporters in brain tissue. Neuropharmacology 2016, 101, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, T.; Brandt, S.D. 5-(2-Aminopropyl)Indole (5-IT): A psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO). Drug Test Anal. 2014, 6, 607–613. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cai, W.T.; Lee, Y.H.; Park, K.T.; Lee, Y.S.; Kim, J.-H. The expression of methiopropamine-induced locomotor sensitization requires dopamine D2, but not D1, receptor activation in the rat. Behav. Brain Res. 2016, 311, 403–407. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Recent Advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulie, C.B.M.; Jensen, A.A.; Halberstadt, A.L.; Kristensen, J.L. DARK classics in chemical neuroscience: NBOMes. ACS Chem. Neurosci. 2019, 11, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.-H.; Kim, S.-E.; Lee, B.-R.; Ko, Y.-H.; Seo, J.-Y.; Kim, S.-K.; Ma, S.-X.; Kim, Y.-J.; Jeong, Y.; Pham, D.T.; et al. 25C-NBF, a new psychoactive substance, has addictive and neurotoxic potential in rodents. Arch. Toxicol. 2020, 94, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; You, K.Y.; Kim, M.; Lee, H.J.; Yoo, S.Y.; Lee, K.W.; Lee, Y.S.; et al. 25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential. Addict. Biol. 2020, 25, e12850. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Hur, K.-H.; Ko, Y.-H.; Kim, K.; Lee, B.-R.; Kim, Y.-J.; Kim, S.-K.; Kim, S.-E.; Lee, Y.-S.; Kim, H.-C.; et al. A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents. Brain Res. Bull. 2019, 152, 19–26. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; Kim, M.; Lee, H.J.; Ryu, H.W.; Lee, Y.S.; Kim, H.J.; Cheong, J.H. Two newly-emerging substituted phenethylamines MAL and BOD induce differential psychopharmacological effects in rodents. J. Psychopharmacol. 2020, 34, 1056–1067. [Google Scholar] [CrossRef]
- Kang, H.; Park, P.; Bortolotto, Z.A.; Brandt, S.D.; Colestock, T.; Wallach, J.; Collingridge, G.L.; Lodge, D. Ephenidine: A new psychoactive agent with ketamine-like NMDA receptor antagonist properties. Neuropharmacology 2017, 112, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Zanda, M.T.; Fadda, P.; Chiamulera, C.; Fratta, W.; Fattore, L. Methoxetamine, a novel psychoactive substance with serious adverse pharmacological effects. Behav. Pharmacol. 2016, 27, 489–496. [Google Scholar] [CrossRef]
- Kronstrand, R.; Guerrieri, D.; Vikingsson, S.; Wohlfarth, A.; Gréen, H. Fatal poisonings associated with new psychoactive substances. Handb. Exp. Pharmacol. 2018, 1–47. [Google Scholar] [CrossRef]
- Kraemer, M.; Boehmer, A.; Madea, B.; Maas, A. Death cases involving certain new psychoactive substances: A review of the literature. Forensic Sci. Int. 2019, 298, 186–267. [Google Scholar] [CrossRef]
- Helander, A.; Bäckberg, M.; Beck, O. Drug trends and harm related to New Psychoactive Substances (NPS) in Sweden from 2010 to 2016: Experiences from the STRIDA Project. PLoS ONE 2020, 15, e0232038. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. High-Risk Drug Use and New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/system/files/publications/4540/TD0217575ENN.pdf (accessed on 1 April 2021).
- EMCDDA. Guidance Note 2. EMCDDA Technical Report. EMCDDA Operating Guidelines for the Risk Assessment of New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/system/files/publications/13482/downloads/Guidance%20Note%202.%20EMCDDA%20technical%20report.pdf (accessed on 1 April 2021).
- Wood, D.M.; Dargan, P.I. Understanding how data triangulation identifies acute toxicity of novel psychoactive drugs. J. Med. Toxicol. 2012, 8, 300–303. [Google Scholar] [CrossRef] [Green Version]
- UNODC. The Challenge of New Psychoactive Substances. Available online: https://www.unodc.org/documents/scientific/NPS_Report.pdf (accessed on 1 April 2021).
- Boscolo-Berto, R.; Viel, G.; Cecchi, R.; Terranova, C.; Vogliardi, S.; Bajanowski, T.; Ferrara, S.D. Journals publishing bio-medicolegal research in Europe. Int. J. Leg. Med. 2012, 126, 129–137. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgetti, A.; Pascali, J.P.; Fais, P.; Pelletti, G.; Gabbin, A.; Franchetti, G.; Cecchetto, G.; Viel, G. Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications. Life 2021, 11, 440. https://doi.org/10.3390/life11050440
Giorgetti A, Pascali JP, Fais P, Pelletti G, Gabbin A, Franchetti G, Cecchetto G, Viel G. Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications. Life. 2021; 11(5):440. https://doi.org/10.3390/life11050440
Chicago/Turabian StyleGiorgetti, Arianna, Jennifer P. Pascali, Paolo Fais, Guido Pelletti, Andrea Gabbin, Giorgia Franchetti, Giovanni Cecchetto, and Guido Viel. 2021. "Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications" Life 11, no. 5: 440. https://doi.org/10.3390/life11050440
APA StyleGiorgetti, A., Pascali, J. P., Fais, P., Pelletti, G., Gabbin, A., Franchetti, G., Cecchetto, G., & Viel, G. (2021). Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications. Life, 11(5), 440. https://doi.org/10.3390/life11050440







