Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade
Abstract
:1. Introduction
2. Predictive/Prognostic Biomarkers for Selecting CRC for Immunotherapy
2.1. Microsatellite Status
2.2. Tumor Mutational Burden
2.3. Immunoscore
2.4. POLD1/POLE
2.5. PD-1/PD-L1 Expression
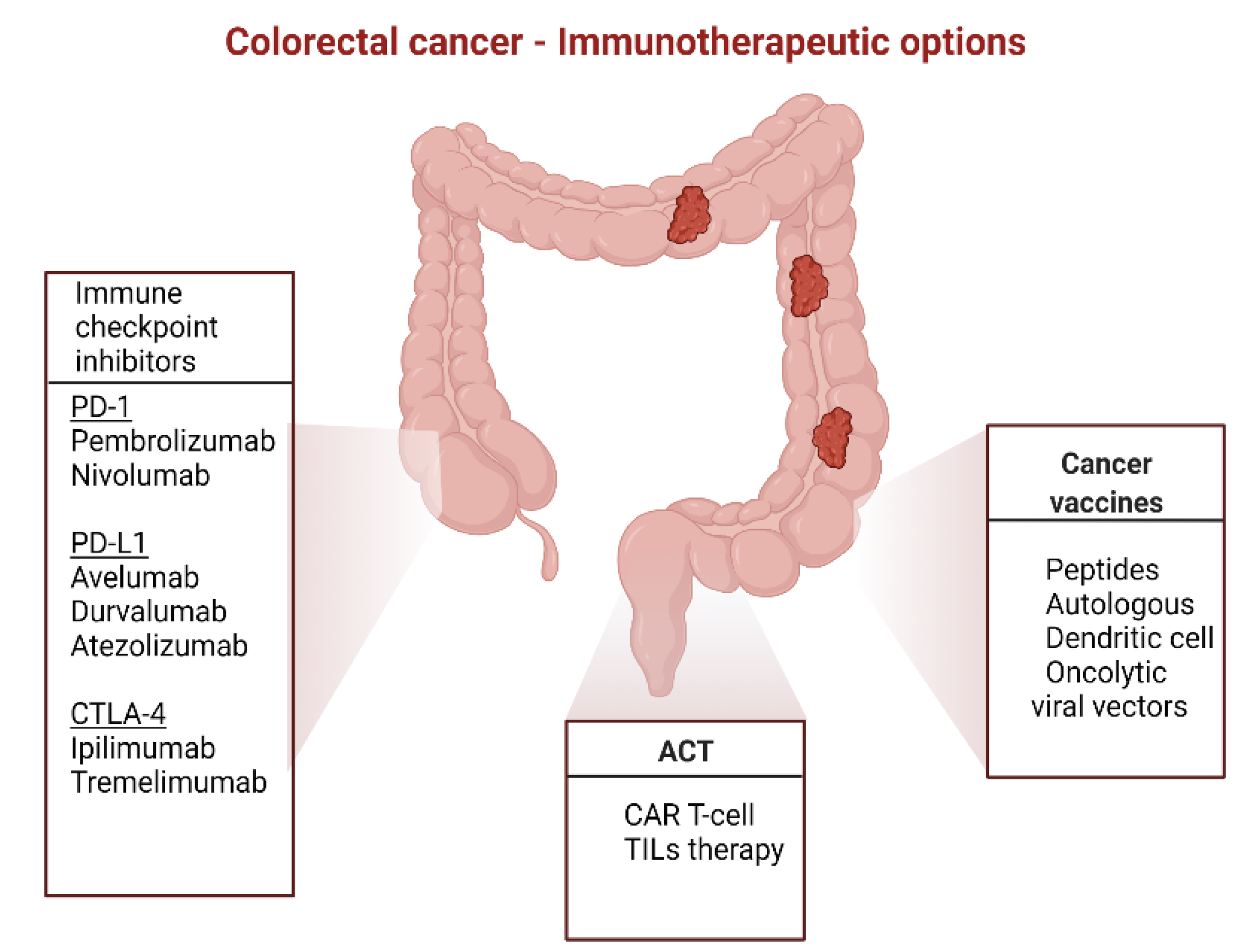
3. Immunotherapy in CRC
3.1. Immunomodulatory mAbs
3.2. Neoadjuvant Setting
3.3. Adoptive Cell Transfer
3.4. Cancer Vaccines
3.5. Highlights on Randomized Clinical Trials
4. Resistance to Immunotherapy
5. The Future of Immunotherapy in CRC
5.1. A New Generation of Immune Checkpoint Inhibitors
5.2. Synergy of Immunotherapy with Other Therapies for MSI-L/pMMR
5.2.1. Immunotherapy with Radiotherapy
5.2.2. Immunotherapy with Chemotherapy
5.2.3. Immunotherapy with Chemotherapy and Targeted Agents
5.2.4. Immunotherapy with MEK Inhibition
5.2.5. Immunotherapy with Colony-Stimulating Factor 1 Receptor
5.2.6. Immunotherapy with Carcinoembryonic Antigen T-Cell Bispecific
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Glossary
| AA | African American |
| AE | adverse events |
| B. Fragilis | Bacillus Fragilis |
| B2M | β2 microglobulin |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| CAR | chimeric antigen receptor |
| CEA | carcinoembryonic antigen |
| CIMP | CpG island methylator phenotype |
| CR | complete response |
| CRC | colorectal cancer |
| AE | adverse events |
| B. Fragilis | Bacillus Fragilis |
| B2M | β2 microglobulin |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| CAR | chimeric antigen receptor |
| CEA | carcinoembryonic antigen |
| CIMP | CpG island methylator phenotype |
| CR | complete response |
| CRC | colorectal cancer |
| CSF | colony-stimulating factor |
| CSF1R | colony-stimulating factor 1 receptor |
| CTLA4 | cytotoxic T lymphocyte-associated antigen 4 |
| DCR | disease control rate |
| DFS | disease-free survival |
| dMMR | mismatch repair |
| F. nucleatum | Fusobacterium Nucleatum |
| HDACi | histone deacetylase inhibitors |
| HER2 | human epidermal growth factor 2 |
| HLA | human leukocyte antigen |
| HMAs | methyltransferase inhibitors |
| ICI | immune checkpoint inhibitors |
| ICOS | inducible T-cell costimulator |
| IFN-γ | interferon-γ |
| IgG4 | immunoglobulin G4 |
| JAK | Janus kinase |
| JAK1 and JAK2 | Janus kinases 1 and 2 |
| KRAS | Kirsten rat sarcoma 2 viral oncogene homolog |
Abbreviations
| mAbs | monoclonal antibodies |
| MAGE | melanoma associated antigen |
| mCRC | metastatic colorectal cancer |
| MDSCc | myeloid-derivated suppressor cells |
| MEK | acronym for MAPK/ERK Kinase-extracellular signal-regulated kinase/extracellular signal-regulated kinase |
| MHC I | major histocompatibility complex I |
| MLH1 | human mutL homolog 1 |
| MLH6 | human mutL homolog 6 |
| MMR | mismatch repair |
| MSH2 | MutS Homolog 2 |
| MSI | microsatellite instability |
| MSI-H | microsatellite instability-high |
| MSI-L | microsatellite instability-low |
| MSS | microsatellite stable |
| NCRs | negative checkpoint regulators |
| NGS | next generation sequencing |
| NK | natural killer |
| NRAS | neuroblastoma RAS viral oncogene homolog |
| NTRK | Neurotrophic tyrosine receptor kinase |
| ORR | overall response rate |
| OS | overall survival |
| PD-1 | programmed cell death-1 |
| PD-L | programmed cell death-ligand 1 |
| PFS | progression free survival |
| PI3K-AKT-mTOR | Phosphoinositide 3-kinases-Protein kinase B-mechanistic target of rapamycin |
| PLGF2 | placental growth factor 2 |
| POLD1 DNA | polymerase delta |
| POLE DNA | polymerase epsilon |
| RAS | rat sarcoma 2 viral oncogene homolog |
| RP2D | recommended phase 2 dose |
| RR | response rate |
| STATs | signal transducer and activator of transcription proteins |
| TAA | tumor associated antigens |
| TCGA | Tumor Cancer Genome Atlas |
| TCR | T-cell receptor |
| TIL | tumor infiltrating lymphocytes |
| TMB | tumor mutational burden |
| TMB-H | tumor mutational burden-high |
| TME | tumor microenvironment |
| TNFRSF | tumor necrosis factor receptor superfamily |
| TNFα | tumor necrosis factor-α |
| TNM | tumor node metastasis |
| TSAs | tumor specific antigens |
| VEGF | vascular endothelial growth factor |
| VISTA V | domain Ig suppressor of T cell activation |
| WHO | world health organization |
References
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal Cancer Genetics, Incidence and Risk Factors: In Search for Targeted Therapies. Cancer Manag. Res. 2020, 12, 9869–9882. [Google Scholar] [CrossRef]
- Sharma, I.; Kim, S.; Sridhar, S.; Basha, R. Colorectal Cancer: An Emphasis on Factors Influencing Racial/Ethnic Disparities. Crit. Rev. Oncog. 2020, 25, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ollberding, N.J.; Nomura, A.M.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Racial/ethnic differences in colorectal cancer risk: The multiethnic cohort study. Int. J. Cancer 2010, 129, 1899–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.; DeSantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA A Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xynos, E.; Gouvas, N.; Triantopoulou, C.; Tekkis, P.; Vini, L.; Tzardi, M.; Boukovinas, I.; Androulakis, N.; Athanasiadis, A.; Christodoulou, C.; et al. Clinical practice guidelines for the surgical management of colon cancer: A consensus statement of the hellenic and cypriotolorectal cancer study group by the HeSMO. Ann. Gastroenterol. 2016, 29, 3–17. [Google Scholar] [PubMed]
- Bertero, L.; Massa, F.; Metovic, J.; Zanetti, R.; Castellano, I.; Ricardi, U.; Papotti, M.; Cassoni, P. Eighth Edition of the UICC Classification of Malignant Tumours: An overview of the changes in the pathological TNM classification criteria—What has changed and why? Virchows Arch. 2018, 472, 519–531. [Google Scholar] [CrossRef]
- Wells, K.O.; Hawkins, A.T.; Krishnamurthy, D.M.; Dharmarajan, S.; Glasgow, S.C.; Hunt, S.R.; Mutch, M.G.; Wise, P.; Silviera, M.L. Omission of Adjuvant Chemo-therapy Is Associated with Increased Mortality in Patients with T3N0 Colon Cancer with Inadequate Lymph Node Harvest. Dis. Colon Rectum 2017, 15–21. [Google Scholar] [CrossRef]
- Loree, J.M.; Mulder, K.E.; Ghosh, S.; Spratlin, J.L. CAPOX Associated with Toxicities of Higher Grade but Improved Disease-Free Survival When Compared with FOLFOX in the Adjuvant Treatment of Stage III Colon Cancer. Clin. Colorectal Cancer 2014, 13, 172–177. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved Overall Survival with Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yothers, G.; O’Connell, M.J.; Allegra, C.J.; Kuebler, J.P.; Colangelo, L.H.; Petrelli, N.J.; Wolmark, N. Oxaliplatin as Adjuvant Therapy for Colon Cancer: Updated Results of NSABP C-07 Trial, Including Survival and Subset Analyses. J. Clin. Oncol. 2011, 29, 3768–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmoll, H.-J.; Tabernero, J.; Maroun, J.; de Braud, F.G.M.; Price, T.; Van Cutsem, E.; Hill, M.; Hoersch, S.; Rittweger, K.; Haller, D.G. Capecitabine Plus Oxaliplatin Compared with Fluorouracil/Folinic Acid as Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J. Clin. Oncol. 2015, 33, 3733–3740. [Google Scholar] [CrossRef]
- Punt, C.J.A.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2016, 14, 235–246. [Google Scholar] [CrossRef]
- Huiskens, J.; Van Gulik, T.M.; Van Lienden, K.P.; Engelbrecht, M.R.; Meijer, G.A.; Van Grieken, N.C.; Schriek, J.; Keijser, A.; Mol, L.; Molenaar, I.Q.; et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases: The randomized phase III CAIRO5 study of the Dutch Colorectal Cancer Group. J. Clin. Oncol. 2015, 33, TPS3622. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.A.; Asmis, T.R. Overview of Systemic Therapy for Colorectal Cancer. Clin. Colon Rectal Surg. 2009, 22, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Verdaguer, H.; Tabernero, J.; Macarulla, T. Ramucirumab in metastatic colorectal cancer: Evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Syed, Y.Y.; McKeage, K. Aflibercept: A Review in Metastatic Colorectal Cancer. Drugs 2015, 75, 1435–1445. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Larkin, J.; Oya, M.; Thistlethwaite, F.; Martignoni, M.; Nathan, P.; Powles, T.; McDermott, D.; Robbins, P.B.; Chism, D.D.; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018, 19, 451–460. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumransub, N.; Vantanasiri, K.; Prakash, A.; Lou, E. Advances and new frontiers for immunotherapy in colorectal cancer: Setting the stage for neoadjuvant success? Mol. Ther. Oncolytics 2021, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.L. Current microsatellite instability testing in management of colorectal cancer. Clinical Colorectal Cancer. 2021, 20, e12–e20. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, S.; Li, X.; Zhang, D.; Aisa, A.; Yuan, Y. General treatment for metastatic colorectal cancer: From KEYNOTE 177 study. Transl. Oncol. 2021, 14, 101122. [Google Scholar] [CrossRef]
- Kanani, A.; Veen, T.; Søreide, K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br. J. Surg. 2021, 108, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- De la Chapelle, A. Microsatellite Instability. N. Engl. J. Med. 2009, 349, 209–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jascur, T.; Boland, C.R. Structure and function of the components of the human DNA mismatch repair system. Int. J. Cancer 2006, 119, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, F.; Naseem, M.; Lenz, H.J.; Salem, M.E. Microsatellite Instability in Colorectal Cancer: Overview of Its Clinical Signifi-cance and Novel Perspectives. Clin. Adv. Hematol. Oncol. 2018, 16, 735. [Google Scholar] [PubMed]
- Gelsomino, F.; Barbolini, M.; Spallanzani, A.; Pugliese, G.; Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat. Rev. 2016, 51, 19–26. [Google Scholar] [CrossRef] [Green Version]
- McConechy, M.; Talhouk, A.; Li-Chang, H.; Leung, S.; Huntsman, D.; Gilks, C.; McAlpine, J. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol. Oncol. 2015, 137, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef]
- Ashktorab, H.; Smoot, D.T.; Carethers, J.M.; Rahmanian, M.; Kittles, R.; Vosganian, G.; Doura, M.; Nidhiry, E.; Naab, T.; Momen, B.; et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin. Cancer Res. 2003, 9. [Google Scholar]
- Brim, H.; Mokarram, P.; Naghibalhossaini, F.; Saberi-Firoozi, M.; Al-Mandhari, M.; Al-Mawaly, K.; Al-Mjeni, R.; Al-Sayegh, A.; Raeburn, S.; Lee, E.; et al. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol. Cancer 2008, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Ashktorab, H.; Smoot, D.T.; Farzanmehr, H.; Fidelia-Lambert, M.; Momen, B.; Hylind, L.; Iacosozio-Dononue, C.; Carethers, J.M.; Goel, A.; Boland, C.R.; et al. Clinicopathological features and microsatellite instability (MSI) in colorectal cancers from African Americans. Int. J. Cancer 2005, 116, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.; Ma, J.; Liu, Y.; Liang, J.; Wu, Y.; Yang, X.; Xu, E.; Li, Y.; Xi, Y. Screening of MSI detection loci and their heterogeneity in East Asian colorectal cancer patients. Cancer Med. 2019, 8, 2157–2166. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.S.; Bondy, M.L.; El-Badawy, S.A.; Mokhtar, N.; Eissa, S.; Bayoumy, S.; Seifeldin, I.A.; Houlihan, P.S.; Lukish, J.R.; Watanabe, T.; et al. Contrasting molecular pathology of colorectal carcinoma in Egyptian and Western patients. Br. J. Cancer 2001, 85, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Pandey, V.; Prabhu, J.S.; Payal, K.; Rajan, V.; Deepak, C.; Barde, S.; Jagannath, P.; Borges, A.; Sridhar, T.S. Assessment of microsatellite instability in colorectal carci-noma at an Indian center. Int. J. Colorectal Dis. 2007, 22, 777–872. [Google Scholar] [CrossRef]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current Achievements and Future Perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2019, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- De’angelis, G.L.; Bottarelli, L.; Azzoni, C.; De’angelis, C.; Leandro, N.; Di Mario, G.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Gupta, R.; Sinha, S.; Paul, R.N. The impact of microsatellite stability status in colorectal cancer. Curr. Probl. Cancer 2018, 42, 548–559. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kronbichler, A.; Eisenhut, M.; Hong, S.H.; Van Der Vliet, H.J.; Kang, J.; Shin, J.I.; Gamerith, G. Tumor Mutational Burden and Efficacy of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1798. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Solit, D.; Chan, T.; Kurzrock, R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: A decision centered on empowering patients and their physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and safety of pembrolizumab in pre-viously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galon, J.; Fridman, W.H.; Pagès, F. The Adaptive Immunologic Microenvironment in Colorectal Cancer: A Novel Perspective: Figure 1. Cancer Res. 2007, 67, 1883–1886. [Google Scholar] [CrossRef] [Green Version]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2019, 26, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Walkowska, J.; Kallemose, T.; Jönsson, G.; Jönsson, M.; Andersen, O.; Andersen, M.H.; Svane, I.M.; Langkilde, A.; Nilbert, M.; Therkildsen, C. Immunoprofiles of colorectal cancer from Lynch syndrome. OncoImmunology 2018, 8, e1515612. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Lanzi, A. Immunoscore and its introduction in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 152–161. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Marliot, F.; Ou, F.-S.; Bifulco, C.B.; Lugli, A.; Zlobec, I.; Rau, T.T.; Hartmann, A.; Masucci, G.V.; et al. Validation of the Immunoscore (IM) as a prognostic marker in Stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J. Clin. Oncol. 2016, 34 (Suppl. 15), 3500. [Google Scholar] [CrossRef]
- Kirilovsky, A.; Marliot, F.; El Sissy, C.; Haicheur, N.; Galon, J.; Pagès, F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int. Immunol. 2016, 28, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Marliot, F.; Chen, X.; Kirilovsky, A.; Sbarrato, T.; El Sissy, C.; Batista, L.; Eynde, M.V.D.; Haicheur-Adjouri, N.; Anitei, M.-G.; Musina, A.-M.; et al. Analytical validation of the Immunoscore and its associated prognostic value in patients with colon cancer. J. Immunother. Cancer 2020, 8, e000272. [Google Scholar] [CrossRef]
- Marliot, F.; Lafontaine, L.; Galon, J. Immunoscore assay for the immune classification of solid tumors: Technical aspects, improvements and clinical perspectives. Methods Enzymol. 2019, 636, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Shi, Q.; Hermitte, F.; Heying, E.N.; Benson, A.B.; Gill, S.; Goldberg, R.M.; Kahlenberg, M.S.; Nair, S.; Shields, A.F.; et al. Association of immune markers and Immunoscore with survival of stage III colon carcinoma (CC) patients (pts) treated with adjuvant FOLFOX: NCCTG N0147 (Alliance). J. Clin. Oncol. 2017, 35, 3579. [Google Scholar] [CrossRef]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Jannet, R.B.; Louvet, C.; Mineur, L.; et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann. Oncol. 2020, 31, 921–929. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, P.B.; Dong, Z.; Gusenleitner, D.; Giobbie-Hurder, A.; Severgnini, M.; Zhou, J.; Manos, M.; Eastman, L.M.; Maecker, H.T.; Hodi, F.S. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J. Immunother. Cancer 2018, 6, 18. [Google Scholar] [CrossRef]
- Quezada-Marín, J.I.; Lam, A.K.; Ochiai, A.; Odze, R.D.; Washington, K.M.; Fukayama, M.; Rugge, M.; Klimstra, D.S.; Nagtegaal, I.D.; Tan, P.-H.; et al. Gastrointestinal tissue-based molecular biomarkers: A practical categorisation based on the 2019 World Health Organization classification of epithelial digestive tumours. Histopathology 2020, 77, 340–350. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the “Immunoscore” in the clas-sification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimmer, K.; Beilken, A.; Nustede, R.; Ripperger, T.; Lamottke, B.; Ure, B.; Steinmann, D.; Reineke-Plaass, T.; Lehmann, U.; Zschocke, J.; et al. A novel germline POLE mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Fam. Cancer 2016, 16, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrin, L.; Fanale, D.; Brando, C.; Fiorino, A.; Corsini, L.R.; Sciacchitano, R.; Filorizzo, C.; Dimino, A.; Russo, A.; Bazan, V. POLE, POLD1, and NTHL1: The last but not the least hereditary cancer-predisposing genes. Oncogene 2021, 40, 5893–5901. [Google Scholar] [CrossRef]
- Mur, P.; García-Mulero, S.; del Valle, J.; Magraner-Pardo, L.; Vidal, A.; Pineda, M.; Cinnirella, G.; Martín-Ramos, E.; Pons, T.; López-Doriga, A.; et al. Role of POLE and POLD1 in familial cancer. Genet. Med. 2020, 22, 2089–2100. [Google Scholar] [CrossRef]
- Palles, C.; Cazier, J.-B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; Almeida, E.G.; Salguero, I.; et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013, 45, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitzer, E.; Tomlinson, I. Replicative DNA polymerase mutations in cancer. Curr. Opin. Genet. Dev. 2014, 24, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Domingo, E.; Freeman-Mills, L.; Rayner, E.; Glaire, M.; Briggs, S.; Vermeulen, L.; Fessler, E.; Medema, J.P.; Boot, A.; Morreau, H.; et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 2016, 1, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Fakih, M.; Gong, J.; Wang, C.; Lee, P.P.; Chu, P. Molecular Insights in Patient Care Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation. JNCCN—J. Natl. Compr. Cancer Netw. 2017, 15, 142–147. [Google Scholar]
- Van Gool, I.C.; Eggink, F.A.; Freeman-Mills, L.; Stelloo, E.; Marchi, E.; de Bruyn, M.; Palles, C.; Nout, R.A.; de Kroon, C.D.; Osse, E.M.; et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin. Cancer Res. 2015, 21, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol./Hematol 2016, 100, 88–98. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Hersom, M.; Jørgensen, J.T. Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer. Ther. Drug Monit. 2018, 40, 9–16. [Google Scholar] [CrossRef]
- André, T.; Overman, M.; Lonardi, S.; Aglietta, M.; McDermott, R.; Wong, K.Y.M.; Morse, M.; Hendlisz, A.; Moss, R.A.; Ledeine, J.-M.; et al. Analysis of tumor PD-L1 expression and biomarkers in relation to clinical activity in patients (PTS) with deficient DNA mismatch repair (dmmr)/high microsatellite instability (MSI-h) metastatic colorectal cancer (MCRC) treated with nivolumab (NIVO) + ipilimumab (IPI): Checkmate 142. Ann. Oncol. 2017, 28, v163. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xue, R.; Pan, C. Prognostic and clinicopathological value of PD-L1 in colorectal cancer: A systematic review and me-ta-analysis. OncoTargets Ther. 2019, 12, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, X.; Teng, F.; Kong, L. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huyghe, N.; Baldin, P.; Van Den Eynde, M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol. Rep. 2020, 8, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P. Classification of current anticancer immuno-therapies. Oncotarget 2014, 5, 12472. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 1–26. [Google Scholar] [CrossRef]
- Jain, P.; Jain, C.; Velcheti, V. Role of immune-checkpoint inhibitors in lung cancer. Ther. Adv. Respir. Dis. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.; Benítez-Ribas, D.; Sánchez, A.; Balaguer, F. Current Treatments of Metastatic Colorectal Cancer with Immune Check-point Inhibitors—2020 Update. J. Clin. Med. 2020, 9, 3520. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable Cancer Regression Off-Treatment and Effective Reinduction Therapy with an Anti-PD-1 Antibody. Clin. Cancer Res. 2012, 19, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Khoja, L.; Butler, M.O.; Kang, S.P.; Ebbinghaus, S.; Joshua, A.M. Pembrolizumab. J. Immunother. Cancer 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. In FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (accessed on 4 April 2021).
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Franke, A.J.; Skelton, W.P.; Starr, J.S.; Parekh, H.; Lee, J.J.; Overman, M.J.; Allegra, C.; George, T.J. Immunotherapy for colorectal cancer: A review of current and novel Therapeutic approaches. JNCI J. Natl. Cancer Inst. 2019, 111, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- FDA Grants Nivolumab Accelerated Approval for MSI-H or dMMR Colorectal Cancer | FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-msi-h-or-dmmr-colorectal-cancer (accessed on 15 December 2021).
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Ipilimumab for MSI-H or dMMR Metastatic Colorectal Cancer | FDA [Internet]. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-ipilimumab-msi-h-or-dmmr-metastatic-colorectal-cancer (accessed on 7 August 2021).
- Lenz, H.-J.; Lonardi, S.; Zagonel, V.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; Garcia-Alfonso, P.; Neyns, B.; et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: Clinical update. J. Clin. Oncol. 2020, 38, 11. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.Y.; Baek, J.Y.; Cha, Y.J.; Ahn, J.B.; Kim, H.S.; Lee, K.-W.; Kim, J.-W.; Kim, T.-Y.; Chang, W.J.; et al. A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer. Cancer Res. Treat. 2020, 52, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhis, J.; Stein, A.; de Boer, J.P.; Eynde, M.V.D.; Gold, K.A.; Stintzing, S.; Becker, J.C.; Moran, M.; Schroeder, A.; Pennock, G.; et al. Avelumab and cetuximab as a therapeutic combination: An overview of scientific rationale and current clinical trials in cancer. Cancer Treat. Rev. 2021, 97, 102172. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients with Advanced Colorectal Cancer. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ou, F.-S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 2019, 37 (Suppl. S15), e15169. [Google Scholar] [CrossRef]
- Baimas-George, M.; Baker, E.; Kamionek, M.; Salmon, J.S.; Sastry, A.; Levi, D.; Vrochides, D. A Complete Pathological Response to Pem-brolizumab following ex vivo Liver Resection in a Patient with Colorectal Liver Metastases. Chemotherapy 2018, 63, 90–94. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, J.; Deng, Y.; Wang, H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. OncoImmunology 2019, 8, e1663108. [Google Scholar] [CrossRef] [Green Version]
- Chalabi, M.; Fanchi, L.; Berg, J.V.D.; Beets, G.; Lopez-Yurda, M.; Aalbers, A.; Grootscholten, C.; Snaebjornsson, P.; Maas, M.; Mertz, M.; et al. Neoadjuvant ipilimumab plus nivolumab in early stage colon cancer. Ann. Oncol. 2018, 29, viii731. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-F.; Wang, H.Y. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2016, 27, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.C.; Selitsky, S.R.; Chai, S.; Armistead, P.M.; Vincent, B.G.; Serody, J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer 2019, 19, 465–478. [Google Scholar] [CrossRef]
- Richters, M.M.; Xia, H.; Campbell, K.M.; Gillanders, W.E.; Griffith, O.L.; Griffith, M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Fan, J.; Shang, D.; Han, B.; Song, J.; Chen, H.; Yang, J.-M. Adoptive cell transfer: Is it a promising immunotherapy for colorectal cancer? Theranostics. 2018, 8, 5784–5800. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.-C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.-C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Yang, J.C.; Sherry, R.; Hughes, M.S.; Royal, R.; Kammula, U.; Robbins, P.F.; Huang, J.; Citrin, D.E.; Leitman, S.F.; et al. Adoptive Cell Therapy for Patients with Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens. J. Clin. Oncol. 2008, 26, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, C.; Cheng, H.; Huang, S.; Zheng, Y. CAR-T cells for Colorectal Cancer: Target-selection and strategies for improved activity and safety. J. Cancer 2021, 12, 1804–1814. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Met-astatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor–Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Prendergast, G.C. Cancer Vaccines: A Brief Overview. In Vaccine Design; Springer: New York, NY, USA, 2016; Volume 1403, pp. 755–761. [Google Scholar] [CrossRef]
- Geevarghese, S.K.; Geller, D.A.; de Haan, H.A.; Hörer, M.; Knoll, A.E.; Mescheder, A.; Nemunaitis, J.; Reid, T.R.; Sze, D.Y.; Tanabe, K.K.; et al. Phase I/II Study of Oncolytic Herpes Simplex Virus NV1020 in Patients with Extensively Pretreated Refractory Colorectal Cancer Metastatic to the Liver. Hum. Gene Ther. 2010, 21, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kana, S.I.; Essani, K. Immuno-Oncolytic Viruses: Emerging Options in the Treatment of Colorectal Cancer. Mol. Diagn. Ther. 2021, 25, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, M.; Samadi, P.; Pourjafar, M.; Jalali, A. Therapeutic vaccines for colorectal cancer: The progress and future prospect. Int. Immunopharmacol. 2020, 88, 106944. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Niedzwiecki, D.; Marshall, J.L.; Garrett, C.; Chang, D.Z.; Aklilu, M.; Crocenzi, T.S.; Cole, D.J.; Dessureault, S.; Hobeika, A.C.; et al. A Randomized Phase II Study of Immunization with Dendritic Cells Modified with Poxvectors Encoding CEA and MUC1 Compared with the Same Poxvectors Plus GM-CSF for Resected Metastatic Colorectal Cancer. Ann. Surg. 2013, 258, 879–886. [Google Scholar] [CrossRef]
- Bednarczyk, R.A. Addressing HPV vaccine myths: Practical information for healthcare providers. Hum. Vaccines Immunother. 2019, 15, 1628–1638. [Google Scholar] [CrossRef]
- Berry, J.; Vreeland, T.; Trappey, A.; Hale, D.; Peace, K.; Tyler, J.; Walker, A.; Brown, R.; Herbert, G.; Yi, F.; et al. Cancer vaccines in colon and rectal cancer over the last decade: Lessons learned and future directions. Expert Rev. Clin. Immunol. 2016, 13, 235–245. [Google Scholar] [CrossRef]
- Jiang, S.; Good, D.; Wei, M.Q. Vaccinations for Colorectal Cancer: Progress, Strategies, and Novel Adjuvants. Int. J. Mol. Sci. 2019, 20, 3403. [Google Scholar] [CrossRef] [Green Version]
- De Mattos-Arruda, L.; Blanco-Heredia, J.; Aguilar-Gurrieri, C.; Carrillo, J.; Blanco, J. New emerging targets in cancer immuno-therapy: The role of neoantigens. ESMO Open 2020, 4 (Suppl. 3), e000684. [Google Scholar]
- Melero, I.; Gaudernack, G.; Gerritsen, W.R.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Löwer, M.; van de Roemer, N.; de Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C.; et al. Exploiting the Mutanome for Tumor Vaccination. Cancer Res. 2012, 72, 1081–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.; Smith, D.M.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J. Clin. Oncol. 2020, 38, LBA4. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Kloor, M.; Michel, S.; von Knebel Doeberitz, M. Immune evasion of microsatellite unstable colorectal cancers. Int. J. Cancer 2010, 127, 1001–1010. [Google Scholar] [CrossRef]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, B.; Wei, J. Beta2-microglobulin(b2M) in cancer immunotherapies: Biological function, resistance and Remedy. Cancer Letters. 2021, 517, 96–104. [Google Scholar]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [Green Version]
- del Campo, A.B.; Kyte, J.A.; Carretero, J.; Zinchencko, S.; Méndez, R.; González-Aseguinolaza, G.; Ruiz-Cabello, F.; Aamdal, S.; Gaudernack, G.; Garrido, F.; et al. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int. J. Cancer 2014, 134, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Lagos, G.G.; Izar, B.; Rizvi, N.A. Beyond Tumor PD-L1: Emerging Genomic Biomarkers for Checkpoint Inhibitor Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e47–e57. [Google Scholar] [CrossRef] [PubMed]
- Kloor, M.; Becker, C.; Benner, A.; Woerner, S.M.; Gebert, J.; Ferrone, S.; von Knebel Doeberitz, M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005, 65, 6418–6424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer. 2016, 16, 121–126. [Google Scholar] [CrossRef]
- John Rieth, S.S. Mechanisms of Intrinsic Tumor Resistance to Immunotherapy. Int. J. Mol. Sci. 2018, 19, 1393. [Google Scholar]
- Mardis, E.R. Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Ahmadzadeh, M.; Lu, Y.-C.; Gros, A.; Turcotte, S.; Robbins, P.F.; Gartner, J.J.; Zheng, Z.; Li, Y.F.; Ray, S.; et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015, 350, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated with the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. 2019, 37, 286–295. [Google Scholar] [CrossRef]
- Chesney, J.A.; Mitchell, R.A.; Yaddanapudi, K. Myeloid-derived suppressor cells—A new therapeutic target to overcome resistance to cancer immunotherapy. J. Leukoc. Biol. 2017, 102, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvi-ronment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Gao, X.; Sui, H.; Zhao, S.; Gao, X.; Su, Y.; Qu, P. Immunotherapy Targeting Myeloid-Derived Suppressor Cells (MDSCs) in Tumor Microenvironment. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Kim, K.; Skora, A.D.; Li, Z.; Liu, Q.; Tam, A.J.; Blosser, R.L.; Diaz, L.A., Jr.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11774-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt, V.E.T.; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucker, A.; Zhao, F.; Pieper, N.; Heeke, C.; Maltaner, R.; Stadtler, N.; Real, B.; Bielefeld, N.; Howe, S.; Weide, B.; et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 2017, 8, 15440. [Google Scholar] [CrossRef]
- Albacker, L.A.; Wu, J.; Smith, P.; Warmuth, M.; Stephens, P.J.; Zhu, P.; Yu, L.; Chmielecki, J. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS ONE 2017, 12, e0176181. [Google Scholar] [CrossRef]
- Stelloo, E.; Versluis, M.; Nijman, H.W.; De Bruyn, M.; Plat, A.; Osse, E.M.; Van Dijk, R.H.; Nout, R.A.; Creutzberg, C.; de Bock, G.H.; et al. Microsatellite instability derived JAK1 frameshift mutations are associated with tumor immune evasion in endometrioid endometrial cancer. Oncotarget 2016, 7, 39885–39893. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Ramsay, L.; Ahanfeshar-Adams, M.; Lajoie, M.; Schadendorf, D.; Alain, T.; Watson, I.R. Mutations in the IFNγ-JAK-STAT Pathway Causing Resistance to Immune Checkpoint Inhibitors in Melanoma Increase Sensitivity to Oncolytic Virus Treatment. Clin Cancer Res. 2021, 27, 3432–3442. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaguchi, T.; Goto, Y.; Kido, K.; Mochimaru, H.; Sakurai, T.; Tsukamoto, N.; Kudo-Saito, C.; Fujita, T.; Sumimoto, H.; Kawakami, Y. Immune Suppression and Resistance Mediated by Constitutive Activation of Wnt/β-Catenin Signaling in Human Melanoma Cells. J. Immunol. 2012, 189, 2110–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Jaffee, E.M.; Zaidi, N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Investig. 2018, 128, 3209–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Patterns of TIGIT Expression in Lymphatic Tissue, Inflammation, and Cancer. Dis. Markers 2019, 2019, 5160565. [Google Scholar] [CrossRef]
- Granier, C.; Vinatier, E.; Colin, E.; Mandavit, M.; Dariane, C.; Verkarre, V.; Biard, L.; El Zein, R.; Lesaffre, C.; Galy-Fauroux, I.; et al. Multiplexed Immunofluorescence Analysis and Quantification of Intratumoral PD-1+ Tim-3+ CD8+ T Cells. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1–specific CD8+T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [Green Version]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non–Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.-H.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, L.; Gao, Q.; Yuan, P.; Zhao, P.; Yuan, H.; Fan, H.; Li, T.; Qin, P.; Han, L.; et al. Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer. Oncotarget 2015, 6, 20592–20603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElTanbouly, M.; Croteau, W.; Noelle, R.J.; Lines, J.L. VISTA: A novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 2019, 42, 101308. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Lakhani, N.J.; Johnson, M.L.; Park, H.; Wang, D.; Yap, T.A.; Dowlati, A.; Maki, R.G.; Lynce, F.; Ulahannan, S.V.; et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J. Clin. Oncol. 2019, 37, 2508. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, W.M.D.; Forde, P.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.; Hodi, S.; et al. Abstract CT183: Phase (Ph) I/II study of MBG453± spartalizumab (PDR001) in patients (pts) with advanced malignancies. Cancer Res. 2019, 79, CT183. [Google Scholar]
- Harding, J.J.; Patnaik, A.; Moreno, V.; Stein, M.; Jankowska, A.M.; de Mendizabal, N.V.; Liu, Z.T.; Koneru, M.; Calvo, E. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): Interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J. Clin. Oncol. 2019, 37, 12. [Google Scholar] [CrossRef]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.; Dominguez, D.; Chan, W.-C.; Andrade, J.; Zha, Y.; Wray, B.; Miska, J.; Qin, L.; Cole, L.; Coates, S.; et al. OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity. J. Clin. Investig. 2020, 130, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Jia, Y.P.; Hao, Y.; Peng, J.R.; Qian, Z.Y. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Fukunaga, K.; Ishihara, A.; Larsson, H.M.; Potin, L.; Hosseinchi, P.; Galliverti, G.; Swartz, M.A.; Hubbell, J.A. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018, 9, 2237. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvi-ronment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Rousseau, B.; Vidal, J.; Colle, R.; Diaz, L.A.; André, T. Immune Checkpoint Inhibition in Colorectal Cancer: Microsatellite Instability and Beyond. Target. Oncol. 2019, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, M.H. Advances in Treatment of Colorectal Cancer. Am. Surg. 2005, 71, 892–900. [Google Scholar] [CrossRef]
- Thomas, J.; Leal, A.; Overman, M.J. Clinical Development of Immunotherapy for Deficient Mismatch Repair Colorectal Cancer. Clin. Colorectal Cancer 2020, 19, 73–81. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Floudas, C.S.; Brar, G.; Mabry-Hrones, D.; Duffy, A.G.; Wood, B.; Levy, E.; Krishnasamy, V.; Fioravanti, S.; Bonilla, C.M.; Walker, M.; et al. A pilot study of AMP-224, a PD-L2 Fc fusion protein, in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2016, 34, 560. [Google Scholar]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Jiang, T.; Xiao, Y.; Wang, Q.; Zeng, Z.; Cai, P.; Zhao, Y.; Zhao, Z.; Wu, D.; Lin, H.; et al. Good tumor response to chemoradioimmunotherapy in dMMR/MSI-H Advanced Colorectal Cancer: A Case Series. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Matos, A.I.; Carreira, B.; Peres, C.; Moura, L.I.F.; Conniot, J.; Fourniols, T.; Scomparin, A.; Martínez-Barriocanal, Á.; Arango, D.; Conde, J.P.; et al. Nanotechnology is an important strategy for combi-national innovative chemo-immunotherapies against colorectal cancer. J. Control. Release 2019, 307, 108–138. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahda, S.; Noonan, A.M.; Bekaii-Saab, T.S.; O’Neil, B.H.; Sehdev, A.; Shaib, W.L.; Helft, P.R.; Loehrer, P.J.; Tong, Y.; Liu, Z.; et al. A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer. J. Clin. Oncol. 2017, 35, 3541. [Google Scholar] [CrossRef]
- Germano, G.; Lamba, S.E.; Rospo, G.; Barault, L.; Magrì, A.; Maione, F.; Russo, M.; Crisafulli, G.; Bartolini, A.; Lerda, G.; et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017, 552, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Fiano, V.; Trevisan, M.; Trevisan, E.; Senetta, R.; Castiglione, A.; Sacerdote, C.; Gillio-Tos, A.; De Marco, L.; Grasso, C.; Magistrello, M.; et al. MGMT promoter methylation in plasma of glioma patients receiving temozolomide. J. Neuro-Oncol. 2014, 117, 347–357. [Google Scholar] [CrossRef] [Green Version]
- NIVOLUMAB Plus IPILIMUMAB and TEMOZOLOMIDE in Microsatellite Stable, MGMT Silenced Metastatic Colorectal Cancer [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT03832621 (accessed on 10 September 2021).
- Pembrolizumab in MMR-Proficient Metastatic Colorectal Cancer Pharmacologically Primed to Trigger Hypermutation Status. Available online: https://clinicaltrials.gov/ct2/show/NCT03519412 (accessed on 10 September 2021).
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A.; Hadjinicolaou, A.V.; Barsouk, A. Immunotherapies and Targeted Therapies in the Treatment of Metastatic Colorectal Cancer. Med. Sci. 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.S.; Hurwitz, H. Combinations of Bevacizumab with Cancer Immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Wallin, J.; Pishvaian, M.J.; Hernandez, G.; Yadav, M.; Jhunjhunwala, S.; Delamarre, L.; He, X.; Powderly, J.; Lieu, C.; Eckhardt, S.G.; et al. Clinical activity and immune correlates from a phase Ib study evaluating atezolizumab (anti-PDL1) in combination with FOLFOX and bevacizumab (anti-VEGF) in meta-static colorectal carcinoma. Cancer Res. 2016, 2651. [Google Scholar]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and An-ti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.C.; Kim, T.; Goh, B.C.; Wallin, J.; Oh, D.Y.; Han, S.; Lee, C.; Hellmann, M.D.; Desai, J.; Lewin, J.; et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J. Clin. Oncol. 2016, 34, 3502. [Google Scholar] [CrossRef]
- Sun, H.-L.; Zhou, X.; Xue, Y.-F.; Wang, K.; Shen, Y.-F.; Mao, J.-J.; Guo, H.-F.; Miao, Z.-N. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J. Gastroenterol. 2012, 18, 3303–3309. [Google Scholar] [CrossRef]
- Arihara, F.; Mizukoshi, E.; Kitahara, M.; Takata, Y.; Arai, K.; Yamashita, T.; Nakamoto, Y.; Kaneko, S. Increase in CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013, 62, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, K.R.; Amaria, R.N.; Ramirez, O.; Callihan, E.B.; Gao, D.; Borakove, M.; Manthey, E.; Borges, V.F.; McCarter, M.D. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol. Immunother. 2013, 62, 1711–1722. [Google Scholar] [CrossRef] [Green Version]
- Cassier, P.A.; Garin, G.; Eberst, L.; Delord, J.-P.; Chabaud, S.; Terret, C.; Montane, L.; Bidaux, A.-S.; Laurent, S.; Jaubert, L.; et al. MEDIPLEX: A phase 1 study of durvalumab (D) combined with pexidartinib (P) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC). J. Clin. Oncol. 2019, 37, 2579. [Google Scholar] [CrossRef]
- Tabernero, J.; Melero, I.; Ros, W.; Argiles, G.; Marabelle, A.; Rodriguez-Ruiz, M.E.; Albanell, J.; Calvo, E.; Moreno, V.; Cleary, J.M.; et al. Phase Ia and Ib studies of the novel carci-noembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017, 35, 3002. [Google Scholar] [CrossRef]

| Study Name | Status | Phase | Study Population | Treatment | Endpoint | Purpose |
|---|---|---|---|---|---|---|
| NCT02982694 | Recruiting | II | Advanced chemotherapy resistant MSI-like CRC | Atezolizumab + bevacizumab | ORR | To determine the anti-tumor effect of atezolizumab in combination with bevacizumab in chemotherapy-resistant MSI-H/dMMR CRC |
| NCT02997228 | Recruiting | III | MSI-H/dMMR mCRC | Atezolizumab vs. atezolizumab + bevacizumab + FOLFOX | PFS | To compare mFOLFOX6/bevacizumab/atezolizumab with atezolizumab alone as the first-line treatment in MSI-H/dMMR mCRC |
| NCT04014530 | Recruiting | I-II | dMMR and pMMR mCRC and dMMR endometrial carcinoma | Pembrolizumab + Ataluren | AE and maximum tolerable dose of Ataluren AE of the combination ORR | Efficacy of pembrolizumab in combination with Alaturen in pMMR/dMMR mCRC and dMMR metastatic endometrial carcinoma |
| NCT03638297 | Recruiting | II | MSI-H/dMMR CRC | Pembrolizumab + COX inhibitor (aspirin) | RR | Safety and efficacy of pembrolizumab in combination with COX inhibitor in MSI-H/dMMR or high TMB CRC |
| NCT04001101 | Recruiting | II | MSI-H/dMMRmetastatic solid tumors | Pembrolizumab + RT (metastatic site) vs. pembrolizumab | ORR | To determine if the ORR is improved by the addition of radiotherapy to pembrolizumab in MSI-H/dMMR metastatic solid tumors, compared to pembrolizumab alone |
| NCT04730544 | Recruiting | II | MSI-H/dMMR mCRC | Nivolumab + ipilimumab | AE PFS | To determine the safety and efficacy of two combination regiments of nivolumab + opilimumab in MSI-H/dMMR mCRC |
| NCT04008030 | Recruiting | III | MSI-H/dMMR mCRC | Nivolumab vs. nivolumab + ipilimumab Nivolumab + ipilimumab vs. chemotherapy | PFS | To compare the clinical benefit of nivolumab alone, nivolumab + ipilimumab or investigator’s choice chemotherapy in MSI-H/dMMR mCRC |
| NCT03104439 | Recruiting | II | MSI-H/dMMR CRC, MMS CRC, pancreatic cancer | Nivolumab + ipilimumab + RT | DCR | To evaluate the combination of nivolumab, ipilimumab, and radiation therapy in MSS/MSI-H/dMMR CRC and pancreatic cancer |
| NCT02060188 | Active, not recruiting | II | Recurrent or metastatic MSI-H and non-MSI-H CRC | Nivolumab Nivolumab + ipilimumab Nivolumab + ipiliumab + cobimetinib Nivolumab + BMS-986016 Nivolumab + daratumumab | ORR | To evaluate nivolumab alone or in combination with other anti-cancer molecules in recurrent or metastatic MSI-H or non-MSI-H CRC |
| NCT03186326 | Recruiting | II | MSI-H/dMMR mCRC | Avelumab | PFS | Tolerance and effectiveness of Avelumab, compared to the second line standard chemotherapy for MSI-H/dMMR mCRC |
| NCT03475953 | Recruiting | I-II | Advanced or metastatic solid tumors, including MSI-H/dMMR CRC | Avelumab + regorafenib | RP2D for regorafenib ORR PFS | To evaluate efficacy and safety of regorafenib in combination with avelumab in advanced/metastatic solid tumors |
| NCT03435107 | Active, not recruiting | II | MSI-H/dMMR or POLE mutated mCRC | Durvalumab | ORR | To investigate durvalumab in previously treated MSI-H/dMMR or POLE mutated mCRC |
| NCT02983578 | Active, not recruiting | II | Advanced pancreatic cancer NSCLC dMMR CRC | Danvatirsen+durvalumab | AEs, SAEs | To evaluate danvatirsen and durvalumab in patients with advanced pancreatic cancer, NSCLC, and dMMR CRC refractory to standard therapy |
| Study Name | Phase | Study Population | Treatment | Primary Endpoint | Results | Purpose |
|---|---|---|---|---|---|---|
| NCT02460198 | II | Previously treated LA unresectable or mCRC MSI-H/dMMR | Cohort A: pembrolizumab after ≥2 prior lines of therapy Cohort B: pembrolizumab after ≥1 prior line of therapy | ORR | OR = 33%/33% | To determine the efficacy of pembrolizumab monotherapy in previously treated LA unresectable or mCRC MSI-H/dMMR patients |
| NCT01876511 | II | MSI tumors (Cohort A: MSI + CRC; Cohort B: MSI − CRC; Cohort C: MSI + non-CRC) | Pembrolizumab | irPFS (A,B), irORR (A,B), irPFS (C), ORR (A,C), PFS (A,C) | IrORR A = 40%, irPFS A = 78%; irORR B = 0%, irPFS B = 11%, Median PFS A = not reached; Median OS A = not reached; Median PFS B = 2.2 months; Median OS B = 5 months; irORR C = 71%, irPFS = 67% | To determine the anti-tumoral activity of pembrolizumab in MSI/MSS cohorts |
| NCT02178722 | I/II | Selected cancers (including MSI-H CRC) | Pembrolizumab + epacadosat | I: TEAE; II: ORR | Acceptable safety profile ORR CRC = N/A | To assess the safety, tolerability, and efficacy of combination therapy pembrolizumab + epacadosat in patients with certain cancers. |
| NCT02335918 | I II | Advanced refractory solid tumors (including CRC) | Varlilumab + nivolumab | I: TEAE II: ORR | Acceptable safety profile PR = 5% CRC SD = 17% CRC | To determine the clinical benefit, safety, and tolerability of combination therapy between varlilumumab + nivolumab in certain advanced refractory solid tumors. |
| NCT02227667 | II | Advanced MSI-H CRC | Durvalumab | ORR | 22% | To determine the effects of durvalumab therapy in advanced MSI-H CRC patients. |
| NCT02777710 | I | Metastatic/ advanced CRC and PaC | Durvalumab + pexidartinib | 1.DLT 2.ORR | Acceptable safety profile ORR (2 m) = 21% | To evaluate the safety and activity of durvalumab combined with pexidartinib in patients with metastatic/advanced pancreatic or CRC |
| Study Name | Phase | Study Population | Treatment | Primary Endpoint | Results | Purpose |
|---|---|---|---|---|---|---|
| NCT02981524 | II | Advanced pMMR CRC | Pembrolizumab+ cyclophosphamide+ Colon cancer vaccine | ORR | No OR with DCR = 18% | To assess the efficacy (as measured by RECIST criteria) of therapy with CY/GVAX in combination with pembrolizumab in patients with advanced pMMR CRC |
| NCT03274804 | I | Refractory MSS/ pMMR mCRC | Pembrolizumab + Maraviroc | Feasibility rate of the combined therapy | FR = 94.7% | To determine the feasibility rate of combination therapy between pembrolizumab and maraviroc in previously treated subjects who have refractory MSS/pMMR mCRC |
| NCT02860546 | II | MSS CRC | Nivolumab + tipiracil hydrochloride | irORR | No tumor response | To evaluate the efficacy of nivolumab + tipiracil hydrochloride in patients with MSS refractory mCRC |
| NCT03258398 | II | MSS CRC | Avelumab + tomivosertib vs. tomivosertib | Part 1: DLT during the first treatment cycle Part 2: ORR | Part 1: Acceptable safety profile for combination therapy Part 2: N/A | To evaluate the safety, tolerability, and anti-tumor activity of tomivosertib with or without avelumab in MSS CRC patients |
| NCT02811497 | II | Advanced solid tumors (including MSS CRC) | Azacitidine + durvalumab | ORR | No OR with DCR = 7.1 and median PFS = 1.9 m and OS = 5 m | To assess the antitumor activity of azacitidine in combination with druvalumab in advanced solid tumors |
| NCT03005002 | I | MSS mCRC (Liver) | Durvalumab + tremelimumab following radioembolization (RE) with SIR-spheres | Safety and hepatic response rate | Safety of RE followed by D + T Lack of clinical response | To determine the safety and the hepatic response rate of durvalumab+tremelimuma following RE in MSS CRC that has spread to the liver |
| NCT02876224 | Ib | Non MSI-H mCRC | Cobimetinib + Bevacizumab + atezolizumab | TEAE | Acceptable safety profile and manageable AEs | To assess the safety, tolerability, and pharmacokinetics of oral cobimetinib with IV atezolizumab and bevacizumab in previously treated mCRC with non-MSI-H |
| NCT02260440 | II | Chemo-refractory MSS mCRC | Pembrolizumab + azacitidine | ORR | OR = 3% | To evaluate the anti-tumor activity, safety, and tolerability of pembrolizumab in combination with azacitidine in subjects with chemo-refractory MSS mCRC |
| NCT03168139 | I/II | MSS mCRC mPaC | Olaptesed pegol vs. olaptesed pegol + pembrolizumab | Pharmaco-dynamics Safety and tolerability | Induction of immune response and acceptable safety profile | To explore safety, tolerability, and efficacy of olaptesed monotherapy or in combination with pembrolizumab in patients with MSS mCRC and pancreatic cancer |
| NCT02788279 | III | mCRC | Atezolizumab (A) vs. atezolizumab (A)+ cobimetinib (C) vs. regorafenib (R) | OS | OS (A) = 7.10 m OS (A + C) = 8.87 m OS (R) = 8.51 m | To compare regorafenib to cobimetinib + atezolizumab and atezolizumab monotherapy in the setting of mCRC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorzo, A.; Galos, D.; Volovat, S.R.; Lungulescu, C.V.; Burz, C.; Sur, D. Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life 2022, 12, 229. https://doi.org/10.3390/life12020229
Gorzo A, Galos D, Volovat SR, Lungulescu CV, Burz C, Sur D. Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life. 2022; 12(2):229. https://doi.org/10.3390/life12020229
Chicago/Turabian StyleGorzo, Alecsandra, Diana Galos, Simona Ruxandra Volovat, Cristian Virgil Lungulescu, Claudia Burz, and Daniel Sur. 2022. "Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade" Life 12, no. 2: 229. https://doi.org/10.3390/life12020229
APA StyleGorzo, A., Galos, D., Volovat, S. R., Lungulescu, C. V., Burz, C., & Sur, D. (2022). Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life, 12(2), 229. https://doi.org/10.3390/life12020229






