Evaluating Ocular Response in the Retina and Optic Nerve Head after Single and Fractionated High-Energy Protons
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Irradiation with High Energy Protons
2.2. Eye and Retina Preparation
2.3. Terminal Deoxynucleotidyl Transferase Dutp Nick End Labeling (TUNEL) Assay
2.4. Immunostaining for 4-Hydroxynonenal (4-HNE)
2.5. Immunohistochemistry for Aquaporin-4 (AQP-4) and Vascular Double-Labeling
2.6. Immunostaining Assays for Platelet Endothelial Cell Adhesion Molecule (PECAM-1) and Zonula Occludens-1 (ZO-1)
2.7. Quantification of Immunostaining
2.8. Statistical Analysis
3. Results
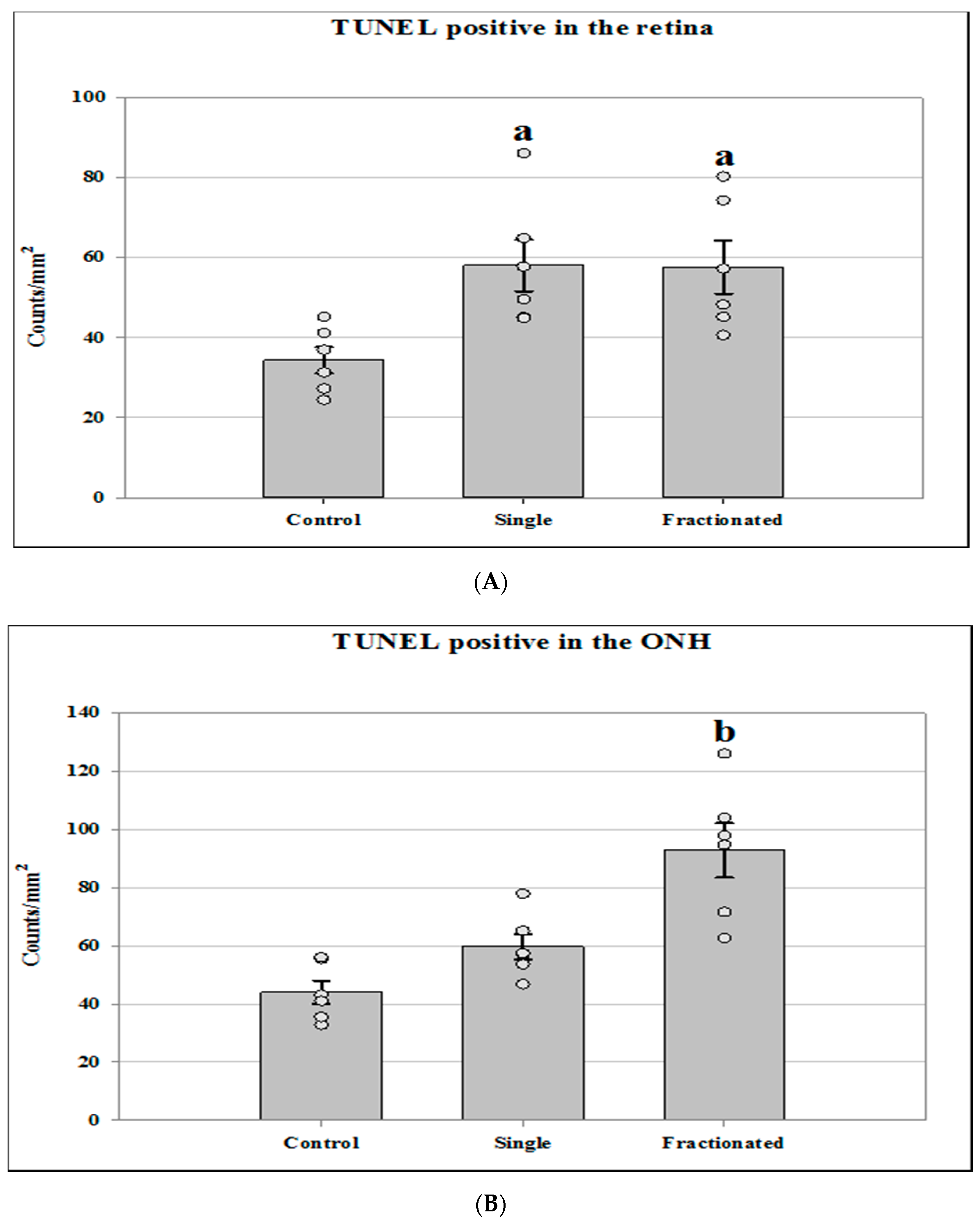
3.1. Apoptotic Damage in the Retina
3.2. Oxidative Damage Biomarker Using 4-HNE
3.3. Alteration of BRB Integrity Using Water Channel Protein AQP4, Adhesion Molecule PECAM-1, and Tight Junction Protein ZO-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. Managing Space Radiation Risk in the New Era of Space Exploration; National Academies Press: Washington, DC, USA, 2008. [Google Scholar] [CrossRef]
- Mao, X.W.; Boerma, M.; Rodriguez, D.; Campbell-Beachler, M.; Jones, T.; Stanbouly, S.; Sridharan, V.; Nishiyama, N.C.; Wroe, A.; Nelson, G.A. Combined Effects of Low-Dose Proton Radiation and Simulated Microgravity on the Mouse Retina and the Hematopoietic System. Radiat. Res. 2018, 192, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.W.; Green, L.M.; Mekonnen, T.; Lindsey, N.; Gridley, D.S. Gene expression analysis of oxidative stress and apoptosis in proton-irradiated rat retina. In Vivo 2010, 24, 425–430. [Google Scholar]
- Mao, X.W.; Boerma, M.; Rodriguez, D.; Campbell-Beachler, M.; Jones, T.; Stanbouly, S.; Sridharan, V.; Wroe, A.; Nelson, G.A. Acute Effect of Low-Dose Space Radiation on Mouse Retina and Retinal Endothelial Cells. Radiat. Res. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Davis, J.G.; Wan, X.S.; Ware, J.H.; Kennedy, A.R. Dietary Supplements Reduce the Cataractogenic Potential of Proton and HZE-Particle Radiation in Mice. Radiat. Res. 2010, 173, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. NPJ Microgravity 2020, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stenger, M.B.; Laurie, S.S.; Sadda, S.R.; Sadun, A.A.; Macias, B.R.; Huang, A.S. Focus on the Optic Nerve Head in Spaceflight-Associated Neuro-ocular Syndrome. Ophthalmology 2019, 126, 1604–1606. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef]
- Slater, J.D. Development and operation of the Loma Linda University Medical Center proton facility. Technol. Cancer Res. Treat. 2007, 6, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.; Møller, A. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548, 463–471. [Google Scholar] [CrossRef]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Yang, Y.; Sharma, R.; Sharma, A.; Awasthi, S.; Awasthi, Y.C. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 2003, 50, 319–336. [Google Scholar] [CrossRef]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef]
- National Cancer Institute. Hyperfractionated radiation therapy. In NCI Dictionary of Cancer Terms; 2018. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/hyperfractionated-radiation-therapy (accessed on 12 May 2021).
- Archambeau, J.O.; Mao, X.W.; McMillan, P.J.; Gouloumet, V.L.; Oeinck, S.C.; Grove, R.; Yonemoto, L.T.; Slater, J.D.; Slater, J.M. Dose response of rat retinal microvessels to proton dose schedules used clinically: A pilot study. Int. J. Radiat. Oncol. 2000, 48, 1155–1166. [Google Scholar] [CrossRef]
- Lett, J.T.; Williams, G.R. Effects of Linear Energy Transfer on the Formation and Fate of Radiation Damage to the Photoreceptor Cell Complement of the Rabbit Retina: Implications for the Projected Manned Mission to Mars. In Biological Effects and Physics of Solar and Galactic Cosmic Radiation; NATO ASI Series (Series A: Life Sciences); Horneck, G., Stassinopoulos, E.G., Eds.; Springer: Boston, MA, USA, 1993. [Google Scholar]
- Keng, P.C.; Bergtold, D.S.; Lett, J.T. Effects of Heavy Ions on Rabbit Tissues: Analysis of Low Levels of DNA Damage in Retinal Photoreceptor Cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1983, 43, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, M.; Dewispelaere, R.; Relvas, L.; Elmaleh, V.; Caspers, L.; Bruyns, C.; Willermain, F. Characterization of retinal expression of vascular cell adhesion molecule (VCAM-1) during experimental autoimmune uveitis. Exp. Eye Res. 2012, 101, 27–35. [Google Scholar] [CrossRef]
- Norata, G.D.; Grigore, L.; Raselli, S.; Seccomandi, P.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A. Triglyceride-rich lipoproteins from hypertriglyceridemic subjects induce a pro-inflammatory response in the endothelium: Molecular mechanisms and gene expression studies. J. Mol. Cell. Cardiol. 2006, 40, 484–494. [Google Scholar] [CrossRef]
- Bixel, M.G.; Li, H.; Petri, B.; Khandoga, A.G.; Khandoga, A.; Zarbock, A.; Wolburg-Buchholz, K.; Wolburg, H.; Sorokin, L.; Zeuschner, D.; et al. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 2010, 116, 1172–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, X.; Zhou, Y.; Qiu, S.; Wu, Y.; Xie, M.; Zhu, G.; Liang, S.; Li, H.; Zhou, D.; et al. Metformin reverses the drug resistance of cisplatin in irradiated CNE-1 human nasopharyngeal carcinoma cells through PECAM-1 mediated MRPs down-regulation. Int. J. Med. Sci. 2020, 17, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-M.; Chuang, M.-J.; Liu, J.-H.; Liu, X.-Q.; Ho, L.-K.; Pan, W.H.; Zhang, X.-M.; Liu, C.-M.; Tsai, S.-K.; Kong, C.-W.; et al. Baicalein Protects against Retinal Ischemia by Antioxidation, Antiapoptosis, Downregulation of HIF-1α, VEGF, and MMP-9 and Upregulation of HO-1. J. Ocul. Pharmacol. Ther. 2013, 29, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Muller, W.A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003, 24, 326–333. [Google Scholar] [CrossRef]
- Das, A.; Rangasamy, S.; McGuire, P.G. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight Junctions and Cell Polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS ONE 2013, 8, e61794. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.W.; Nishiyama, N.C.; Byrum, S.D.; Stanbouly, S.; Jones, T.; Drew, A.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Zawieja, D.; et al. Characterization of mouse ocular response to a 35-day spaceflight mission: Evidence of blood-retinal barrier disruption and ocular adaptations. Sci. Rep. 2019, 9, 8215. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Tone, S.O.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2019, 117, 573–583. [Google Scholar] [CrossRef]
- Huang, A.S.; Stenger, M.B.; Macias, B.R. Gravitational Influence on Intraocular Pressure: Implications for Spaceflight and Disease. J. Glaucoma 2019, 28, 756–764. [Google Scholar] [CrossRef]
- Macias, B.R.; Ferguson, C.R.; Patel, N.; Gibson, C.; Samuels, B.C.; Laurie, S.S.; Lee, S.M.C.; Ploutz-Snyder, R.; Kramer, L.; Mader, T.H.; et al. Changes in the Optic Nerve Head and Choroid Over 1 Year of Spaceflight. JAMA Ophthalmol. 2021, 139, 663. [Google Scholar] [CrossRef]
- Marshall-Goebel, K.; Macias, B.R.; Kramer, L.A.; Hasan, K.M.; Ferguson, C.; Patel, N.; Ploutz-Snyder, R.J.; Lee, S.M.C.; Ebert, D.; Sargsyan, A.; et al. Association of Structural Changes in the Brain and Retina after Long-Duration Spaceflight. JAMA Ophthalmol. 2021, 139, 781. [Google Scholar] [CrossRef]
- Hofman, P.; Hoyng, P.; VanderWerf, F.; Vrensen, G.F.; Schlingemann, R.O. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Investig. Ophthalmol. Vis. Sci. 2001, 42, 895–901. [Google Scholar]
- Winneberger, J.; Schöls, S.; Lessmann, K.; Rández-Garbayo, J.; Bauer, A.T.; Yusuf, A.M.; Hermann, D.M.; Gunzer, M.; Schneider, S.W.; Fiehler, J.; et al. Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke. Brain Behav. Immun. 2020, 93, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; De Winne, F.; Stern, C.; De Deyn, P.P. Dilated Prelaminar Paravascular Spaces as a Possible Mechanism for Optic Disc Edema in Astronauts. Aerosp. Med. Hum. Perform. 2018, 89, 1089–1091. [Google Scholar] [CrossRef] [PubMed]





| Biomarkers | Retina | ONH |
|---|---|---|
| TUNEL | Single ↑ ≈ fractionated ↑ | Single ↔ < fractionated ↑ |
| 4-HNE | Single ↑ > fractionated ↑ | Single ↑ > fractioned ↑ |
| AQP-4 | Single ↑ > fractioned ↑ | Single ↔ ≈ fractioned ↔ |
| PECAM-1 | Single ↑ ≈ fractionated ↑ | Single ↑ ≈ fractionated ↑ |
| ZO-1 | Single ↓ ≈ fractionated ↓ | Single ↔ ≈ fractioned ↔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.-W.; Stanbouly, S.; Jones, T.; Nelson, G. Evaluating Ocular Response in the Retina and Optic Nerve Head after Single and Fractionated High-Energy Protons. Life 2021, 11, 849. https://doi.org/10.3390/life11080849
Mao X-W, Stanbouly S, Jones T, Nelson G. Evaluating Ocular Response in the Retina and Optic Nerve Head after Single and Fractionated High-Energy Protons. Life. 2021; 11(8):849. https://doi.org/10.3390/life11080849
Chicago/Turabian StyleMao, Xiao-Wen, Seta Stanbouly, Tamako Jones, and Gregory Nelson. 2021. "Evaluating Ocular Response in the Retina and Optic Nerve Head after Single and Fractionated High-Energy Protons" Life 11, no. 8: 849. https://doi.org/10.3390/life11080849






