The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease
Abstract
:1. Introduction
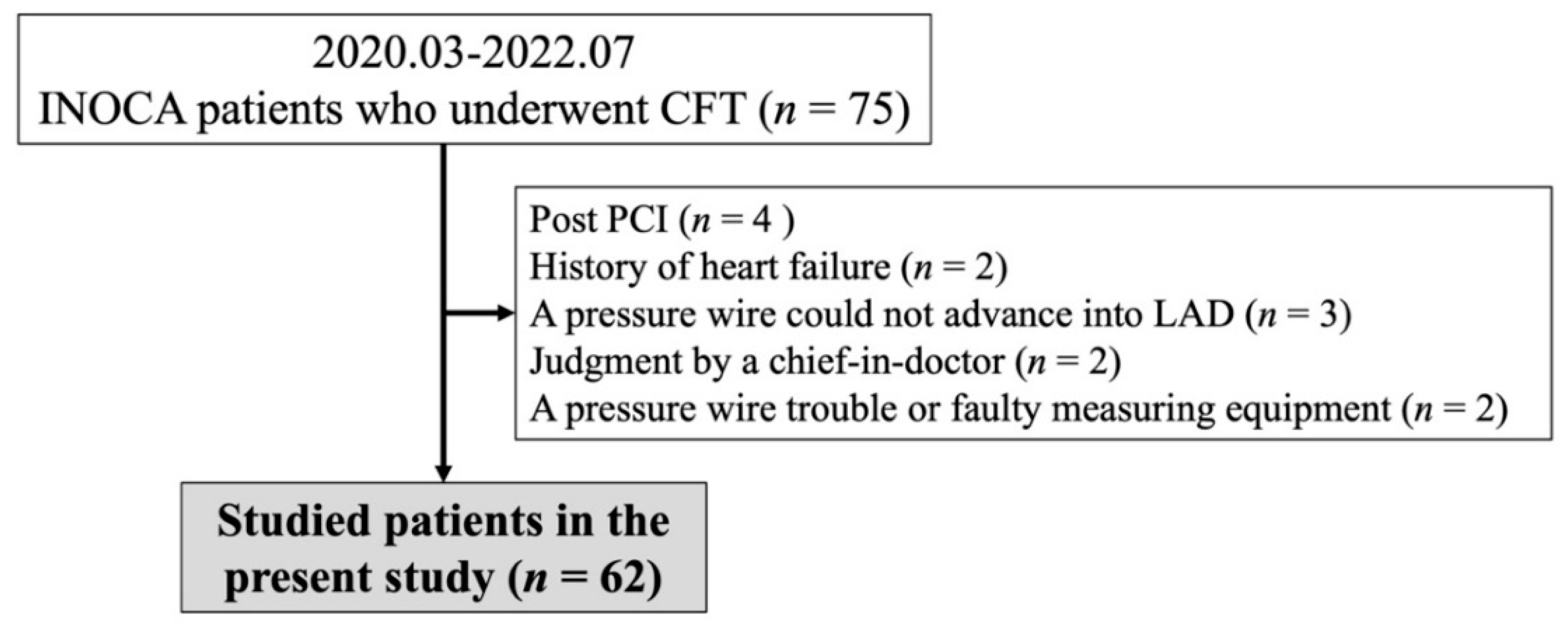
2. Materials and Methods
2.1. Study Population
2.2. CFT
2.3. Definitions of CFT
2.4. Definitions of Clinical Parameters
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics and Clinical Parameters
3.2. VSA-Related Parameters and the Results of CAG and CFT
3.3. Factors Affecting VSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Rasoul-Arzrumly, E.; McDaniel, M.; Mekonnen, G.; Timmins, L.H.; Lutz, J.; Guyton, R.A.; Samady, H. Myocardial bridging: Contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J. Am. Coll. Cardiol. 2014, 63, 2346–2355. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Chen, C.H. Myocardial Bridging: An Up-to-Date Review. J. Invasive Cardiol 2015, 27, 521–528. [Google Scholar]
- Hayashi, T.; Ishikawa, K. Myocardial bridge: Harmless or harmful. Intern. Med. 2004, 43, 1097–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, A.R.; Romanelli, R.; Boucek, R.J. The mural left anterior descending coronary artery, strenuous exercise and sudden death. Circulation 1980, 62, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munakata, K.; Sato, N.; Sasaki, Y.; Yasutake, M.; Kusama, Y.; Takayama, M.; Kishida, H.; Hayakawa, H. Two cases of variant form angina pectoris associated with myocardial bridge—A possible relationship among coronary vasospasm, atherosclerosis and myocardial bridge. Jpn. Circ. J. 1992, 56, 1248–1252. [Google Scholar] [CrossRef] [Green Version]
- Kodama, K.; Morioka, N.; Hara, Y.; Shigematsu, Y.; Hamada, M.; Hiwada, K. Coronary vasospasm at the site of myocardial bridge—Report of two cases. Angiology 1998, 49, 659–663. [Google Scholar] [CrossRef]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Mitsuba, N.; Hata, T.; Nakama, Y.; Kisaka, T.; Kijima, Y. Acute myocardial infarction associated with myocardial bridging in a young adult. Intern. Med. 2004, 43, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Teragawa, H.; Fujii, Y.; Ueda, T.; Murata, D.; Nomura, S. Case of angina pectoris at rest and during effort due to coronary spasm and myocardial bridging. World J. Cardiol. 2015, 7, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Cerit, L.; Duygu, H. Myocardial bridging and sudden death. Int. J. Cardiol. 2017, 229, 11. [Google Scholar] [CrossRef]
- Hostiuc, S.; Rusu, M.C.; Hostiuc, M.; Negoi, R.I.; Negoi, I. Cardiovascular consequences of myocardial bridging: A meta-analysis and meta-regression. Sci. Rep. 2017, 7, 14644. [Google Scholar] [CrossRef] [Green Version]
- Teragawa, H.; Fukuda, Y.; Matsuda, K.; Hirao, H.; Higashi, Y.; Yamagata, T.; Oshima, T.; Matsuura, H.; Chayama, K. Myocardial bridging increases the risk of coronary spasm. Clin. Cardiol. 2003, 26, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Seo, H.S.; Na, J.O.; Suh, S.Y.; Choi, C.U.; Kim, E.J.; Rha, S.W.; Park, C.G.; Oh, D.J. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart 2008, 94, 765–769. [Google Scholar] [CrossRef]
- Im, S.I.; Rha, S.-W.; Choi, B.G.; Choi, S.Y.; Kim, S.W.; Na, J.O.; Choi, C.U.; Lim, H.E.; Kim, J.W.; Kim, E.J.; et al. Angiographic and Clinical Characteristics according to Intracoronary Acetylcholine Dose in Patients with Myocardial Bridge. Cardiology 2013, 125, 250–257. [Google Scholar] [CrossRef]
- Saito, Y.; Kitahara, H.; Shoji, T.; Tokimasa, S.; Nakayama, T.; Sugimoto, K.; Fujimoto, Y.; Kobayashi, Y. Relation between severity of myocardial bridge and vasospasm. Int. J. Cardiol. 2017, 248, 34–38. [Google Scholar] [CrossRef]
- Nam, P.; Choi, B.G.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; Jang, W.Y.; Kim, W.; Choi, J.Y.; Park, E.J.; et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis 2018, 270, 8–12. [Google Scholar] [CrossRef]
- Teragawa, H.; Oshita, C.; Ueda, T. The Myocardial Bridge: Potential Influences on the Coronary Artery Vasculature. Clin. Med. Insights Cardiol. 2019, 13, 1179546819846493. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Hibi, K.; Ogino, Y.; Maejima, N.; Kikuchi, S.; Kirigaya, H.; Kirigaya, J.; Sato, R.; Nakahashi, H.; Minamimoto, Y.; et al. Impact of Myocardial Bridge on Life-Threatening Ventricular Arrhythmia in Patients With Implantable Cardioverter Defibrillator. J. Am. Heart Assoc. 2020, 9, e017455. [Google Scholar] [CrossRef]
- Arai, R.; Kano, H.; Suzuki, S.; Semba, H.; Arita, T.; Yagi, N.; Otsuka, T.; Matsuno, S.; Matsuhama, M.; Kato, Y.; et al. Myocardial bridging is an independent predictor of positive spasm provocation testing by intracoronary ergonovine injections: A retrospective observational study. Heart Vessels 2019, 35, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Pargaonkar, V.S.; Kimura, T.; Kameda, R.; Tanaka, S.; Yamada, R.; Schwartz, J.G.; Perl, L.; Rogers, I.S.; Honda, Y.; Fitzgerald, P.; et al. Invasive assessment of myocardial bridging in patients with angina and no obstructive coronary artery disease. EuroIntervention 2021, 16, 1070–1078. [Google Scholar] [CrossRef]
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’Amario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients With Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535. [Google Scholar] [CrossRef]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.; Escaned, J. Myocardial bridge as a cause of persistent post percutaneous coronary intervention angina identified with exercise intracoronary physiology. Eur. Heart J. 2017, 38, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, S.; Hayashida, A.; Hirohata, A.; Sakaguchi, T. Intraoperative coronary angiography and fractional flow reserve measurement with dobutamine infusion in supra-arterial myotomy for a myocardial bridge: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab268. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Teragawa, H.; Oshita, C.; Orita, Y. Clinical significance of prolonged chest pain in vasospastic angina. World J. Cardiol. 2020, 12, 450–459. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaikita, K.; Yamamoto, E.; Jinnouchi, H.; Tsujita, K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc. Interv. Ther. 2021, 36, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Oshita, C.; Uchimura, Y.; Akazawa, R.; Orita, Y. Coronary Microvascular Vasodilatory Function: Related Clinical Features and Differences According to the Different Coronary Arteries and Types of Coronary Spasm. J. Clin. Med. 2021, 11, 130. [Google Scholar] [CrossRef]
- Teragawa, H.; Oshita, C.; Ueda, T. History of gastroesophageal reflux disease in patients with suspected coronary artery disease. Heart Vessel. 2019, 34, 1631–1638. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Kaikita, K.; Nakayama, N.; Horio, E.; Yoshimura, H.; Ono, T.; Ohba, K.; Tsujita, K.; Kojima, S.; Tayama, S.; et al. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: Analysis of a single-center study over 20 years. J. Am. Heart Assoc. 2013, 2, e000227. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B.; Lutas, E.M.; Casale, P.N.; Kligfield, P.; Eisenberg, R.R.; Hammond, I.W.; Miller, D.H.; Reis, G.; Alderman, M.H.; Laragh, J.H. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J. Am. Coll. Cardiol. 1984, 4, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Asuwa, N.; Masuda, S.; Ishikawa, Y.; Kiguchi, H.; Shimada, K. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: An ultrastructural study. Mod. Pathol. 1991, 4, 424–431. [Google Scholar] [PubMed]
- Ge, J.; Jeremias, A.; Rupp, A.; Abels, M.; Baumgart, D.; Liu, F.; Haude, M.; Gorge, G.; von Birgelen, C.; Sack, S.; et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur. Heart J. 1999, 20, 1707–1716. [Google Scholar] [CrossRef]
- Hongo, Y.; Tada, H.; Ito, K.; Yasumura, Y.; Miyatake, K.; Yamagishi, M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: Study by quantitative coronary angiography and intravascular ultrasound. Am. Heart J. 1999, 138, 345–350. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Akasaka, Y.; Suzuki, K.; Fujiwara, M.; Ogawa, T.; Yamazaki, K.; Niino, H.; Tanaka, M.; Ogata, K.; Morinaga, S.; et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 2009, 120, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Kitahara, H.; Mastuoka, T.; Mori, N.; Tateishi, K.; Fujimoto, Y.; Kobayashi, Y. Influence of myocardial bridge on atherosclerotic plaque distribution and characteristics evaluated by near-infrared spectroscopy intravascular ultrasound. Heart Vessel. 2022, 37, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Abe, J.I.; Min, W.; Surapisitchat, J.; Yan, C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann. N. Y. Acad. Sci. 2001, 947, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, K.; Matsumoto, Y.; Wang, H.; Piao, Z.; Ohyama, K.; Uzuka, H.; Hao, K.; Tsuburaya, R.; Takahashi, J.; Ito, K.; et al. Absence of adventitial vasa vasorum formation at the coronary segment with myocardial bridge—An optical coherence tomography study. Int. J. Cardiol. 2018, 250, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients With Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Shiode, N.; Kato, M.; Teragawa, H.; Yamada, T.; Hirao, H.; Nomura, K.; Sasaki, N.; Yamagata, T.; Matsuura, H.; Kajiyama, G. Vasomotility and nitric oxide bioactivity of the bridging segments of the left anterior descending coronary artery. Am. J. Cardiol. 1998, 81, 341–343. [Google Scholar] [CrossRef]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Ong, P.; Sechtem, U.; Beltrame, J.; Camici, P.G.; Crea, F.; Kaski, J.C.; Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc. Interv. 2020, 13, 1847–1864. [Google Scholar] [CrossRef]
- Hostiuc, S.; Negoi, I.; Rusu, M.C.; Hostiuc, M. Myocardial Bridging: A Meta-Analysis of Prevalence. J. Forensic. Sci. 2018, 63, 1176–1185. [Google Scholar] [CrossRef] [PubMed]

| MB (−) Group | MB (+) Group | p Value | ||
|---|---|---|---|---|
| No (%) | 47 (76) | 15 (24) | ||
| Age (years) | 63 ± 14 | 71 ± 15 | 0.06 | |
| Male/Female | 17/30 | 9/6 | 0.1 | |
| Body mass index | 24.1 ± 4.4 | 24.4 ± 3.7 | 0.83 | |
| Coronary risk factors (%) | ||||
| Current smoker | 7 (15) | 1 (7) | 0.41 | |
| Hypertension | 30 (64) | 8 (53) | 0.47 | |
| Dyslipidaemia | 25 (53) | 5 (33) | 0.18 | |
| Diabetes mellitus | 7 (15) | 1 (7) | 0.41 | |
| Family history of CAD (%) | 7 (15) | 3 (20) | 0.64 | |
| CKD (%) | 11 (23) | 2 (13) | 0.4 | |
| Taking statins (%) | 23 (49) | 4 (27) | 0.13 | |
| MB (−) Group | MB (+) Group | p Value | ||
|---|---|---|---|---|
| Blood chemical parameters | ||||
| Total cholesterol (mg/dL) | 192 ± 33 | 176 ± 29 | 0.08 | |
| Triglyceride (mg/dL) | 118 ± 71 | 95 ± 27 | 0.22 | |
| HDL-cholesterol (mg/dL) | 61 ± 15 | 60 ± 13 | 0.9 | |
| LDL-cholesterol (mg/dL) | 109 ± 29 | 97 ± 28 | 0.18 | |
| Fasting blood glucose (mg/dL) | 103 ± 17 | 105 ± 13 | 0.72 | |
| Haemoglobin A1c (%) | 6.0 ± 0.6 | 5.9 ± 0.3 | 0.7 | |
| C-reactive protein (mg/dL) | 0.06 (0.03, 0.10) | 0.03 (0.02, 0.09) | 0.33 | |
| eGFR (mL/min/1.73 m2) | 69.8 ± 13.1 | 6.8.3 ± 15.4 | 0.71 | |
| NT proBNP (pg/mL) | 71 (36, 150) | 131 (64, 214) | 0.17 | |
| Echographic parameters | ||||
| LVEF (%) | 66 ± 6 | 65 ± 7 | 0.4 | |
| LVMI (g/m2) | 83 ± 21 | 79 ± 20 | 0.48 | |
| FMD (%) | 4.2 ± 2.5 | 3.5 ± 2.0 | 0.33 | |
| NMD (%) | 14.5 ± 7.0 | 13.5 ± 3.1 | 0.59 | |
| MB (−) Group | MB (+) Group | p Value | ||
|---|---|---|---|---|
| Chest symptoms | ||||
| At rest/exertion/both | 43/0/4 | 12/1/2 | 0.17 | |
| CAG | ||||
| Atherosclerosis of both coronary arteries (%) | 23 (49) | 7 (47) | 0.88 | |
| Atherosclerosis on LAD (%) | 14 (30) | 7 (47) | 0.23 | |
| Length of MB (mm) | 15.4 ± 5.1 | |||
| % Squeezing of MB segments | 40.1 ± 14.1 | |||
| CFT | ||||
| Presence of coronary spasm (%) | 26 (55) | 13 (87) | 0.03 | |
| Presence of coronary spasm in LAD (%) | 25 (53) | 13 (87) | 0.02 | |
| Types of coronary spasm (diffuse/focal/MVS/no-spasms) | 14/11/10/12 | 5/8/2/0 | 0.06 | |
| Presence of multi-vessel spasms (%, n) | 13 (52, n = 25) | 7 (58, n = 12) | 0.72 | |
| The dose of ACh at spasm provocation (Low/moderate/high, n) | 2/19/4 (n = 25) | 2/7/4 (n = 13) | 0.38 | |
| Pd/Pa at baseline | 0.96 ± 0.02 | 0.96 ± 0.02 | 0.57 | |
| FFR | 0.92 ± 0.05 | 0.92 ± 0.03 | 0.88 | |
| CFR | 2.8 ± 1.1 | 2.7 ± 1.4 | 0.88 | |
| IMR | 30.0 ± 17.3 | 26.9 ± 11.0 | 0.52 | |
| Presence of CMD | 28 (60) | 8 (53) | 0.67 | |
| Factors | Odds Ratio | p Value |
|---|---|---|
| Presence of MB | 5.15 | 0.02 |
| Male gender | 2.73 | 0.09 |
| %FMD | 1.31 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teragawa, H.; Oshita, C.; Uchimura, Y. The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease. Life 2022, 12, 1560. https://doi.org/10.3390/life12101560
Teragawa H, Oshita C, Uchimura Y. The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease. Life. 2022; 12(10):1560. https://doi.org/10.3390/life12101560
Chicago/Turabian StyleTeragawa, Hiroki, Chikage Oshita, and Yuko Uchimura. 2022. "The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease" Life 12, no. 10: 1560. https://doi.org/10.3390/life12101560
APA StyleTeragawa, H., Oshita, C., & Uchimura, Y. (2022). The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease. Life, 12(10), 1560. https://doi.org/10.3390/life12101560







