Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and In Vitro Exploration of Their Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of Extracts
2.2. Preparation and Optimization of Nanoparticles
2.3. AgNPs Characterization
2.4. Antimicrobial Testing
2.5. Antioxidant Activity
3. Results
3.1. Taguchi Design Experiment for Optimization of Reaction Parameters
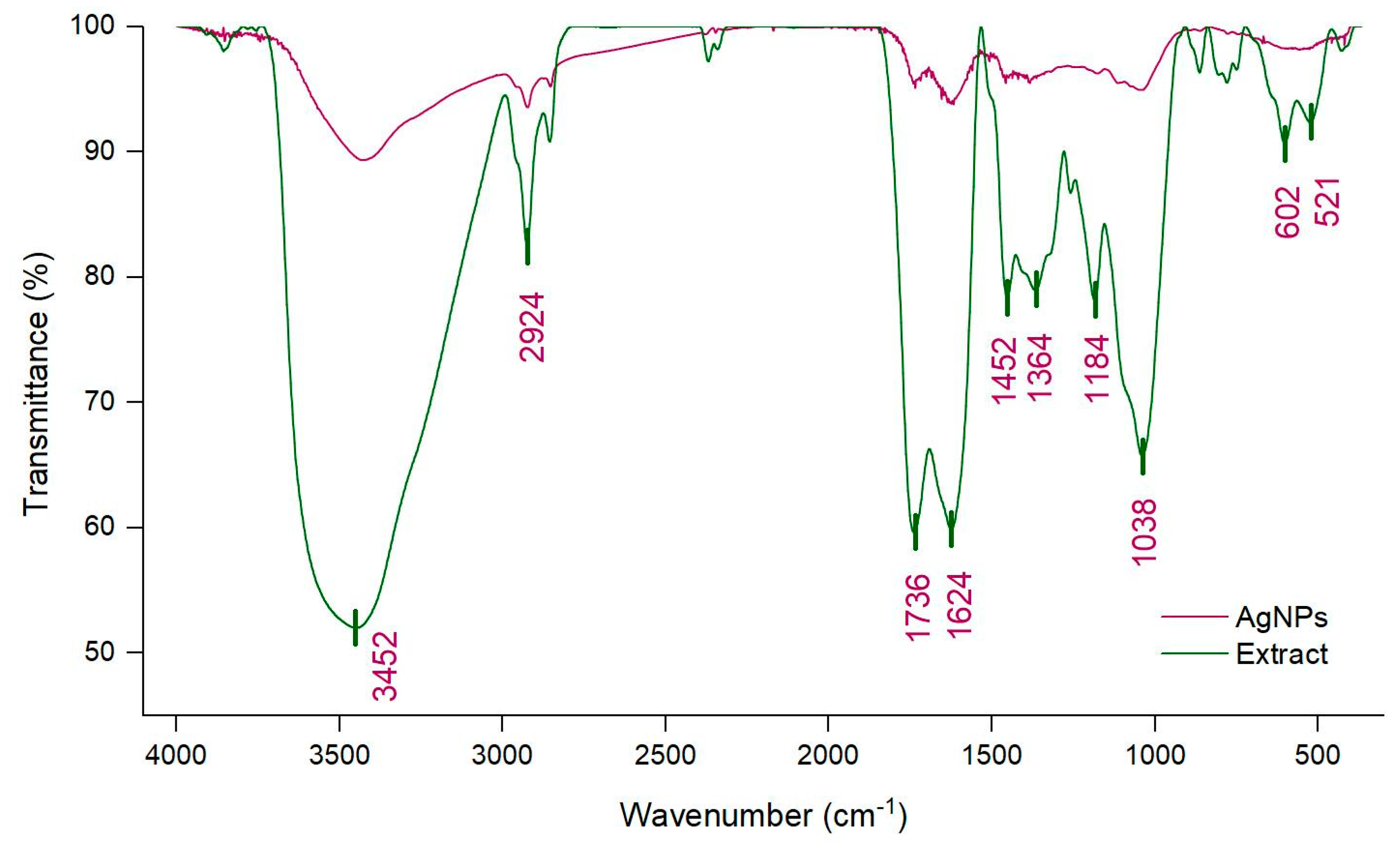
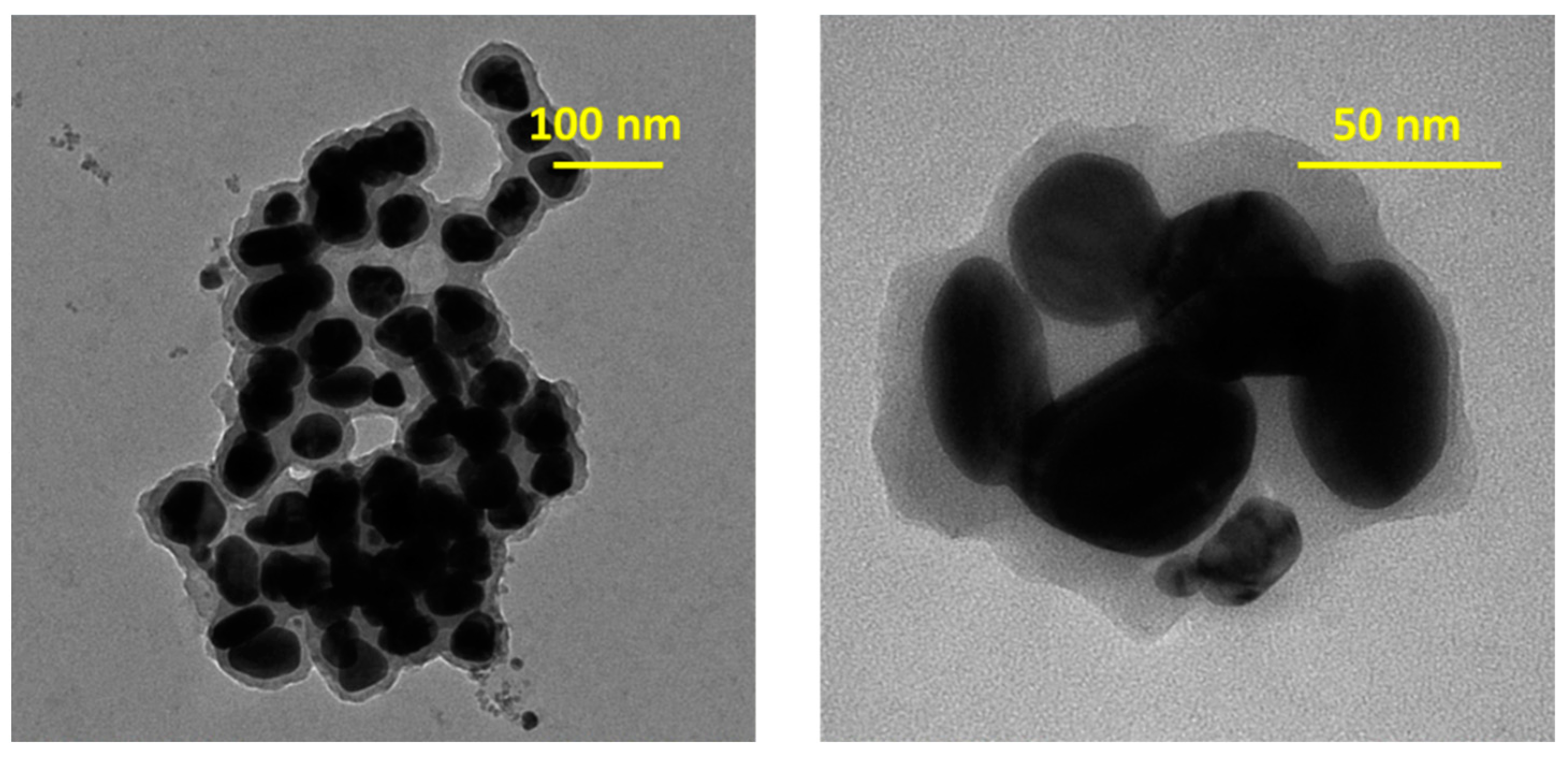
3.2. Physico-Chemical Characterization of AgNPs
3.3. Antimicrobial Activity
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Meaning |
| AAPH | 2,2′-azobis-(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AgNPs | Silver nanoparticles |
| AgNO3 | Silver nitrate |
| FTIR | Fourier transform infrared spectroscopy |
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EDX | Energy dispersive X-ray analysis |
| EC50 | Half maximal effective concentration |
| GAE | Gallic acid equivalents |
| LOX | 15-lipoxygenase assay |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| NA | Avogadro’s number |
| ROS | Reactive oxygen species |
| ESEM | Environmental scanning electron microscope |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| UV-Vis | Ultraviolet-visible |
References
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 751075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzad, F.; Naghib, S.M.; Kouhbanani, M.A.J.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem. 2021, 94, 92–104. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Kumar, V.P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Artichoke (Cynara scolymus L.) mediated rapid analysis of silver nanoparticles and their utilisation on the cancer cell treatments. J. Comput. Theor. Nanosci. 2018, 15, 1818–1829. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Ficai, A.; Oprea, O.; Nicoara, A.I.; Vasile, B.S.; Boanta, L.; Marin, A.; Andronescu, E.; Holban, A.-M. Comparative antimicrobial activity of silver nanoparticles obtained by wet chemical reduction and solvothermal methods. Int. J. Mol. Sci. 2022, 23, 5982. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent advances in green synthesis, characterization, and applications of bioactive metallic nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Sirwi, A.; Ismail, S.H.; Awad, H.H.; Gad, S.S. Hepatoprotective effect of silver nanoparticles at two different particle sizes: Comparative study with and without silymarin. Curr. Issues Mol. Biol. 2022, 44, 2923–2938. [Google Scholar] [CrossRef]
- Tunalier, Z.; Koşar, M.; Küpeli, E.; Çaliş, İ.; Başer, K.H.C. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J. Ethnopharmacol. 2007, 110, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Granica, S.; Kiss, A.K. Lythrum salicaria L.—Underestimated medicinal plant from European traditional medicine. A review. J. Ethnopharmacol. 2015, 170, 226–250. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, T. Comparative Histological, Phytochemical, Microbiological, and Pharmacological Characterization of Some Lythrum salicaria L. Populations; University of Pécs: Pécs, Hungary, 2014. [Google Scholar]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.; Cavero, R.; Calvo, M. Screening of Spanish medicinal plants for antioxidant and antifungal activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Becker, H.; Scher, J.M.; Speakman, J.-B.; Zapp, J. Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia 2005, 76, 580–584. [Google Scholar] [CrossRef]
- Khanavi, M.; Moshteh, M.; Manayi, A.; Reza Shams, M.; Vazirian, M.; Ajani, Y.; Nasser Ost, S. Cytotoxic activity of Lythrum salicaria L. Res. J. Biol. Sci. 2011, 6, 55–57. [Google Scholar] [CrossRef] [Green Version]
- Mohammadalinejhad, S.; Almasi, H.; Esmaiili, M. Simultaneous green synthesis and in-situ impregnation of silver nanoparticles into organic nanofibers by Lythrum salicaria extract: Morphological, thermal, antimicrobial and release properties. Mater. Sci. Eng. C 2019, 105, 110115. [Google Scholar] [CrossRef] [PubMed]
- Srećković, N.Z.; Nedić, Z.P.; Liberti, D.; Monti, D.M.; Mihailović, N.R.; Katanić Stanković, J.S.; Dimitrijević, S.; Mihailović, V.B. Application potential of biogenically synthesized silver nanoparticles using Lythrum salicaria L. extracts as pharmaceuticals and catalysts for organic pollutant degradation. RSC Adv. 2021, 11, 35585–35599. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Phytomediated photo-induced green synthesis of silver nanoparticles using Matricaria chamomilla L. and its catalytic activity against rhodamine B. Biomolecules 2020, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.-L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-functionalized silver nanoparticles derived from conifer bark extracts and evaluation of their antimicrobial and cytogenotoxic effects. Molecules 2022, 27, 217. [Google Scholar] [CrossRef] [PubMed]
- Gird, C.; Nencu, I.; Popescu, M.; Costea, T.; Duţu, L.; Balaci, T.; Olaru, O. Chemical, antioxidant and toxicity evaluation of rosemary leaves and its dry extract. Farmacia 2017, 65, 978–983. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute. M44-A2: Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline—Second Edition; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2009; ISBN 1562387030. [Google Scholar]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-lipoxygenase from orange peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Barros, L.; Falcão, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Velhal, S.G.; Latpate, R.V.; Kulkarni, S.D.; Jaybhaye, R.G. Taguchi design for parameter optimization of size-controlled synthesis of silver nanoparticles. Int. J. Emerg. Technol. Comput. Appl. Sci. 2015, 12, 144–149. [Google Scholar]
- Kim, S.M.; Park, K.S.; Kim, K.D.; Park, S.D.; Kim, H.T. Optimization of parameters for the synthesis of bimodal Ag nanoparticles by Taguchi method. J. Ind. Eng. Chem. 2009, 15, 894–897. [Google Scholar] [CrossRef]
- Kumari, S.C.; Selvakumar, V.; Padma, P.N.; Anuradha, K. Optimization studies on green synthesis of silver nanoparticles from different plant extracts using Taguchi design. Indian J. Sci. Technol. 2021, 14, 2888–2898. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef] [PubMed]
- Rakib-Uz-Zaman, S.M.; Hoque Apu, E.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.R.; Khan, M.A.; Shuborna, N.S.; Shams, S.M.; et al. Biosynthesis of silver nanoparticles from Cymbopogon citratus leaf extract and evaluation of their antimicrobial properties. Challenges 2022, 13, 18. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of biogenic silver nanoparticles with eco-friendly processes using Ganoderma lucidum extract and evaluation of their theranostic applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Singhal, M.; Chatterjee, S.; Kumar, A.; Syed, A.; Bahkali, A.H.; Gupta, N.; Nimesh, S. Exploring the antibacterial and antibiofilm efficacy of silver nanoparticles biosynthesized using Punica granatum leaves. Molecules 2021, 26, 5762. [Google Scholar] [CrossRef] [PubMed]
- Mat Yusuf, S.N.A.; Che Mood, C.N.A.; Ahmad, N.H.; Sandai, D.; Lee, C.K.; Lim, V. Optimization of biogenic synthesis of silver nanoparticles from flavonoid-rich Clinacanthus nutans leaf and stem aqueous extracts. R. Soc. Open Sci. 2020, 7, 200065. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Suar, M. Biological effects of green-synthesized metal nanoparticles: A mechanistic view of antibacterial activity and cytotoxicity. In Advanced Nanostructured Materials for Environmental Remediation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 145–171. [Google Scholar]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Nakavuma, J.L.; Maaza, M.; Sackey, J.; Nxumalo, E.; Kirabira, J.B. Green strategy–based synthesis of silver nanoparticles for antibacterial applications. Front. Nanotechnol. 2021, 3, 697303. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Chang, Y.-C.; Chen, H.-H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J. Food Drug Anal. 2018, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Aazam, E.S.; Zaheer, Z. Growth of Ag-nanoparticles in an aqueous solution and their antimicrobial activities against Gram positive, Gram negative bacterial strains and Candida fungus. Bioprocess Biosyst. Eng. 2016, 39, 575–584. [Google Scholar] [CrossRef]
- Franzolin, M.R.; Courrol, D.d.S.; de Souza Barreto, S.; Courrol, L.C. Eugenia uniflora L. silver and gold nanoparticle synthesis, characterization, and evaluation of the photoreduction process in antimicrobial activities. Microorganisms 2022, 10, 999. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.V.; Lathiff, M.K.M.A.; Muthuboopathi, G. Synthesis and characterization of bioinspired silver nanoparticles by aqueous leaf extract of Indigofera cassioides: Evaluation of antimicrobial and cytotoxic activity. J. Nanosci. Technol. 2019, 5, 676–681. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025004. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Hileuskaya, K.; Kraskouski, A.; Kozerozhets, I.; Stepanova, E.; Kuzminski, I.; You, L.; Agabekov, V. Chitosan-capped silver nanoparticles: A comprehensive study of polymer molecular weight effect on the reaction kinetic, physicochemical properties, and synergetic antibacterial potential. SPE Polym. 2022, 3, 77–90. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.M.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Liao, X.; Liu, X.; Li, W.; Huang, R.; Tang, J.; Xu, Q.; Li, X.; Yu, J. Characterization and antimicrobial activity of silver nanoparticles synthesized with the peel extract of mango. Materials 2021, 14, 5878. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Difference between Gram Positive and Gram Negative Cell Wall. Available online: https://www.differencebetween.com/difference-between-gram-positive-and-gram-negative-cell-wall/ (accessed on 3 October 2022).
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Medchemcomm 2018, 9, 212–225. [Google Scholar] [CrossRef]
- Kang, K.-H.; Liou, H.-H.; Hour, M.-J.; Liou, H.-C.; Fu, W.-M. Protection of dopaminergic neurons by 5-lipoxygenase inhibitor. Neuropharmacology 2013, 73, 380–387. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuruki, Y.; Matsumoto, H.; Tsukada, M.; Tsukahara, H.; Takajo, T.; Tsuchida, K.; Anzai, K. Method to improve azo-compound (AAPH)-induced hemolysis of erythrocytes for assessing antioxidant activity of lipophilic compounds. Chem. Pharm. Bull. 2021, 69, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Li, X.; Zhang, T.; Mitani, T.; Yasuda, M.; Nanba, F.; Toda, T.; Yamashita, Y.; Ashida, H. Black soybean seed coat polyphenols prevent AAPH-induced oxidative DNA-damage in HepG2 cells. J. Clin. Biochem. Nutr. 2017, 60, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Yang, K.; Cao, R. Synthesis, radical-scavenging qctivities, and protective effects against AAPH-induced oxidative damage in DNA and erythrocytes of piperine derivatives. J. Chem. 2020, 2020, 9026286. [Google Scholar] [CrossRef]
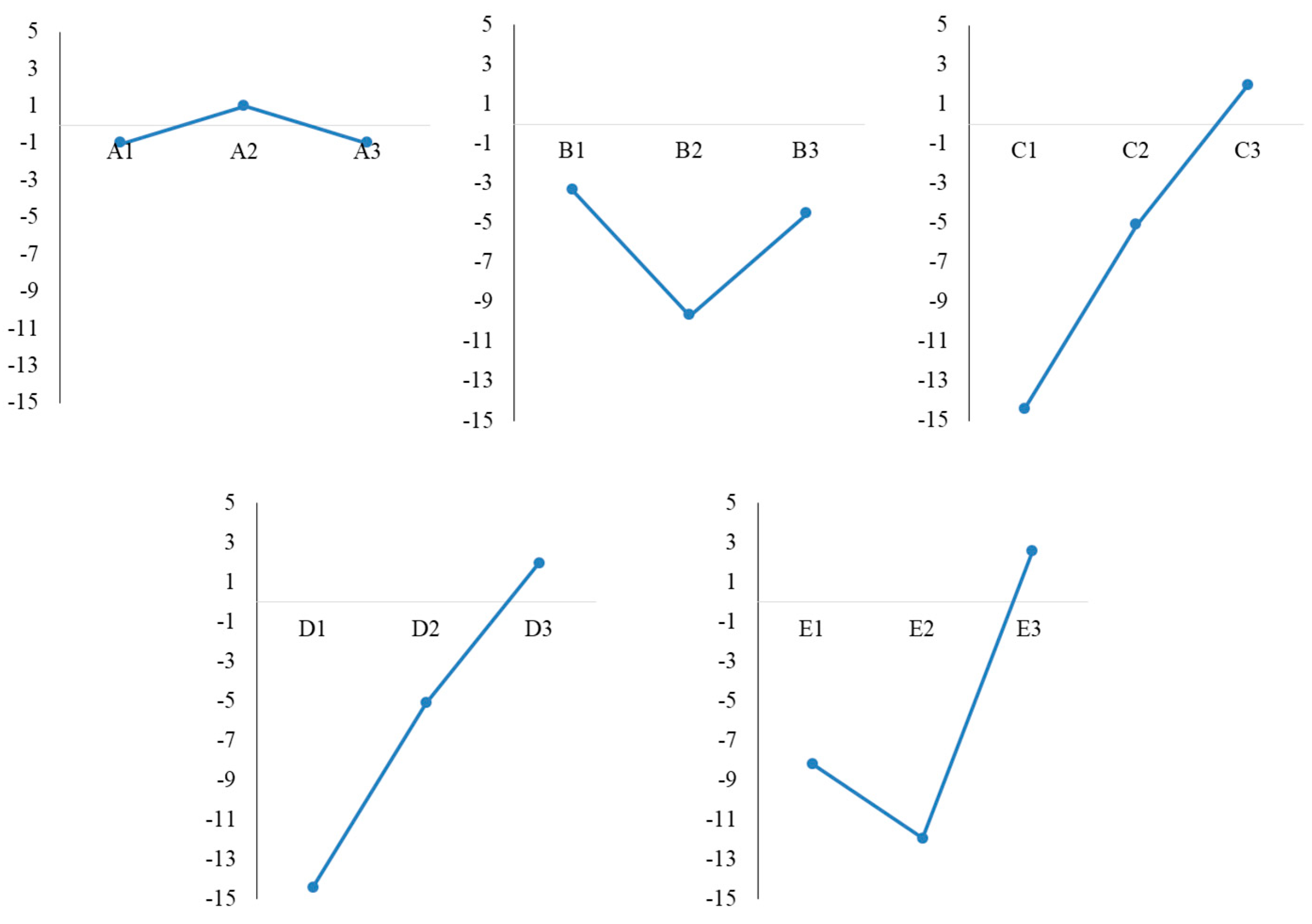



| Factor | Levels | ||
|---|---|---|---|
| 1st | 2nd | 3rd | |
| A AgNO3 concentration M | 1 | 3 | 5 |
| B Extract:AgNO3 ratio | 1:9 | 5:5 | 9:1 |
| C pH | 3 | 5 | 8 |
| D Reaction temperature (℃) | 20 | 40 | 60 |
| E Reaction time (minutes) | 60 | 120 | 180 |
| Level | Factors | Average of Absorbance | S/N Ratio | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| L1 | 1 | 1 | 1 | 1 | 1 | 0.1165 | −18.67 |
| L2 | 1 | 2 | 2 | 2 | 2 | 0.2727 | −11.28 |
| L3 | 1 | 3 | 3 | 3 | 3 | 0.9084 | −0.83 |
| L4 | 2 | 1 | 2 | 2 | 3 | 1.5108 | 3.58 |
| L5 | 2 | 2 | 3 | 3 | 1 | 1.2177 | 1.71 |
| L6 | 2 | 3 | 1 | 1 | 2 | 0.5525 | −5.15 |
| L7 | 3 | 1 | 3 | 3 | 3 | 1.7779 | 4.99 |
| L8 | 3 | 2 | 1 | 1 | 2 | 0.1066 | −19.44 |
| L9 | 3 | 3 | 2 | 2 | 1 | 0.4146 | −7.64 |
| S/N Ratio Average | |||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |
| A | −10.26 | 0.04 | −7.36 |
| B | −3.36 | −9.67 | −4.54 |
| C | −14.42 | −5.11 | 1.95 |
| D | −14.42 | −5.11 | 1.95 |
| E | −8.20 | −11.95 | 2.58 |
| Sample/Standard | Diameter of Inhibition Zones (mm) ± SD | ||
|---|---|---|---|
| S. aureus ATCC 25923 | P. aeruginosa ATCC 27853 | C. albicans ATCC 90028 | |
| Extract | 12.00 ± 0.00 | NA * | 19.06 ± 0.05 |
| AgNPs | 14.00 ± 0.00 | NA * | 21.00 ± 0.00 |
| Ciprofloxacin | 30.00 ± 0.00 | 30.33 ± 0.57 | NT ** |
| Fluconazole | NT ** | NT ** | 29.00 ± 0.00 |
| Samples | S. aureus ATCC 25923 | C. albicans ATCC 90028 | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | |
| Extract | 0.62 | 1.25 | 0.15 | 1.25 |
| AgNPs | 0.31 | 0.62 | 0.03 | 0.31 |
| Sample | 0.1562 mg/mL | 0.3125 mg/mL | 0.625 mg/mL | 1.25 mg/mL | 2.5 mg/mL | 5 mg/mL | EC50 (µg/mL Final Solution) |
| Extract | 8.87 ± 0.55 | 12.22 ± 0.53 | 18.88 ± 0.30 | 29.38 ± 1.04 | 45.43 ± 1.38 | 72.30 ± 1.75 | 46.90 ± 1.74 a |
| AgNPs | 31.07 ± 0.58 | 41.29 ± 0.69 | 51.48 ± 0.44 | 63.93 ± 1.66 | 74.55 ± 1.36 | 98.09 ± 0.20 | 9.40 ± 0.30 b |
| Gallic acid | 17.23 ± 1.69 | 28.15 ± 2.32 | 47.23 ± 1.58 | 61.15 ± 1.80 | 72.14 ± 2.81 | 89.25 ± 1.74 | 11.98 ± 0.97 a |
| Sample | 0.1562 mg/mL | 0.3125 mg/mL | 0.625 mg/mL | 1.25 mg/mL | 2.5 mg/mL | 5 mg/mL | EC50 (µg/mL Final Solution) |
|---|---|---|---|---|---|---|---|
| Extract | 4.09 ± 0.18 | 7.92 ± 0.39 | 14.01 ± 0.56 | 21.67 ± 0.84 | 30.56 ± 1.04 | 38.26 ± 0.95 | - |
| AgNPs | 11.05 ± 0.31 | 16.58 ± 0.46 | 23.59 ± 0.63 | 35.51 ± 0.42 | 46.98 ± 0.97 | 57.45 ± 0.89 | 213.94 ± 13.4 a |
| Gallic acid | 26.48 ± 0.21 | 37.19 ± 0.14 | 49.63 ± 0.17 | 60.04 ± 0.01 | 73.51 ± 0.06 | 82.01 ± 0.01 | 44.83 ± 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corciovă, A.; Mircea, C.; Burlec, A.F.; Fifere, A.; Moleavin, I.T.; Sarghi, A.; Tuchiluș, C.; Ivănescu, B.; Macovei, I. Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and In Vitro Exploration of Their Biological Activities. Life 2022, 12, 1643. https://doi.org/10.3390/life12101643
Corciovă A, Mircea C, Burlec AF, Fifere A, Moleavin IT, Sarghi A, Tuchiluș C, Ivănescu B, Macovei I. Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and In Vitro Exploration of Their Biological Activities. Life. 2022; 12(10):1643. https://doi.org/10.3390/life12101643
Chicago/Turabian StyleCorciovă, Andreia, Cornelia Mircea, Ana Flavia Burlec, Adrian Fifere, Ioana Turin Moleavin, Alexandra Sarghi, Cristina Tuchiluș, Bianca Ivănescu, and Irina Macovei. 2022. "Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and In Vitro Exploration of Their Biological Activities" Life 12, no. 10: 1643. https://doi.org/10.3390/life12101643






