Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide
Abstract
1. Introduction
2. Results
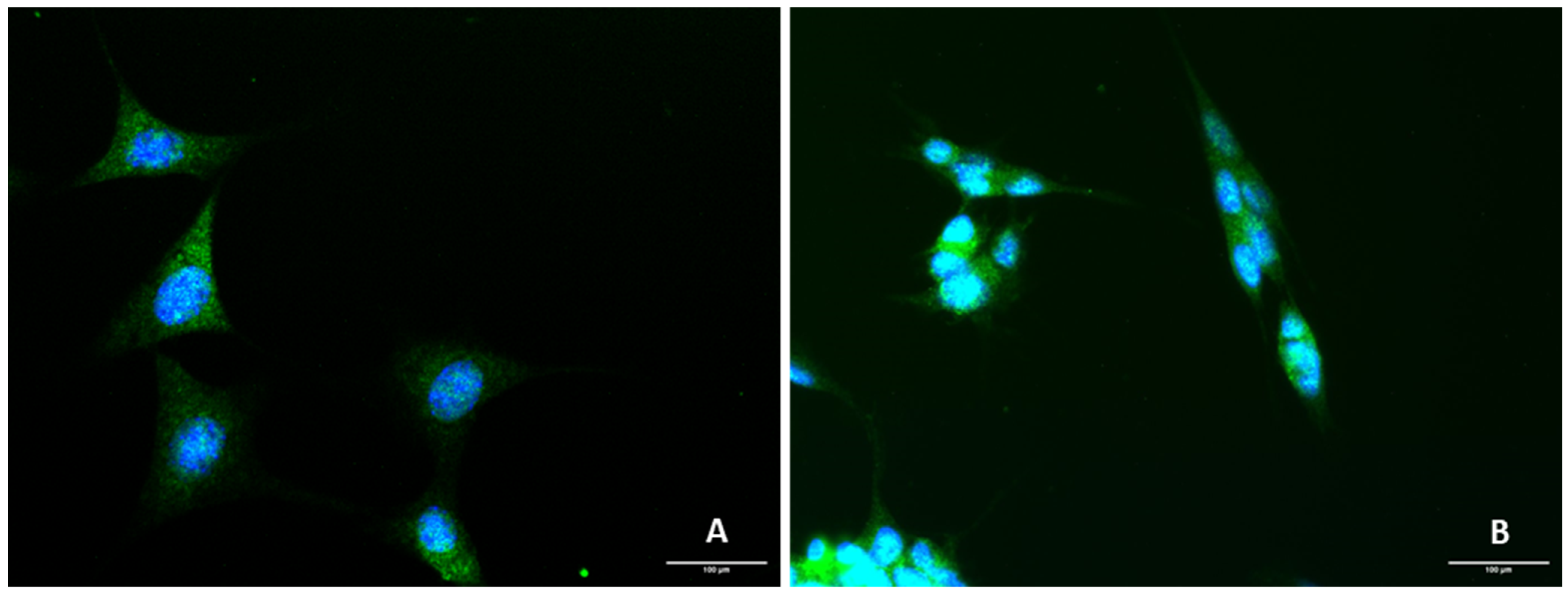
2.1. Presence of 5-HT3 Receptor on SH-SY5Y and HT-22 Cells
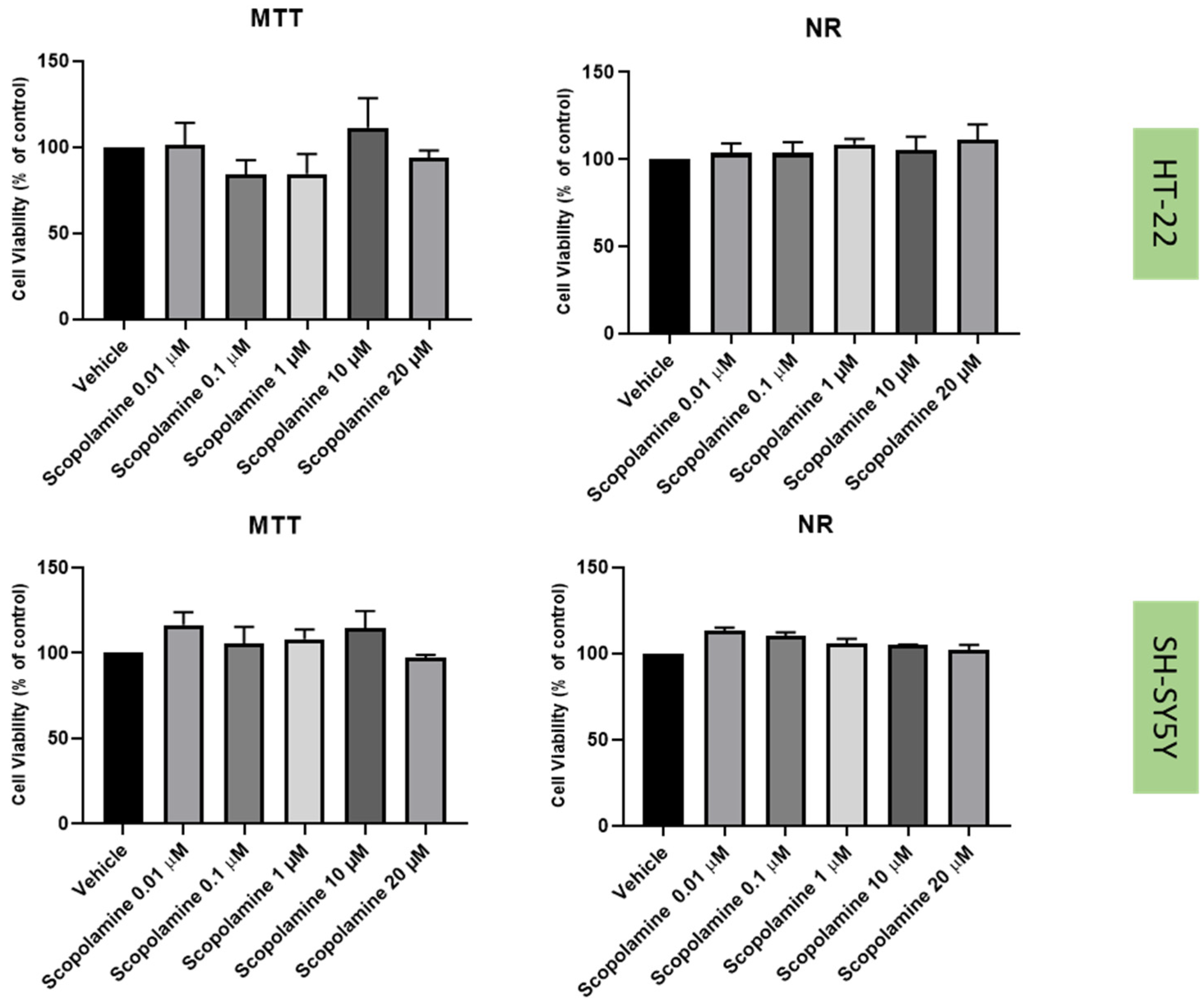
2.2. Effect of Mirtazapine, Scopolamine, and Lamotrigine on SH-SY5Y and HT-22 Viability
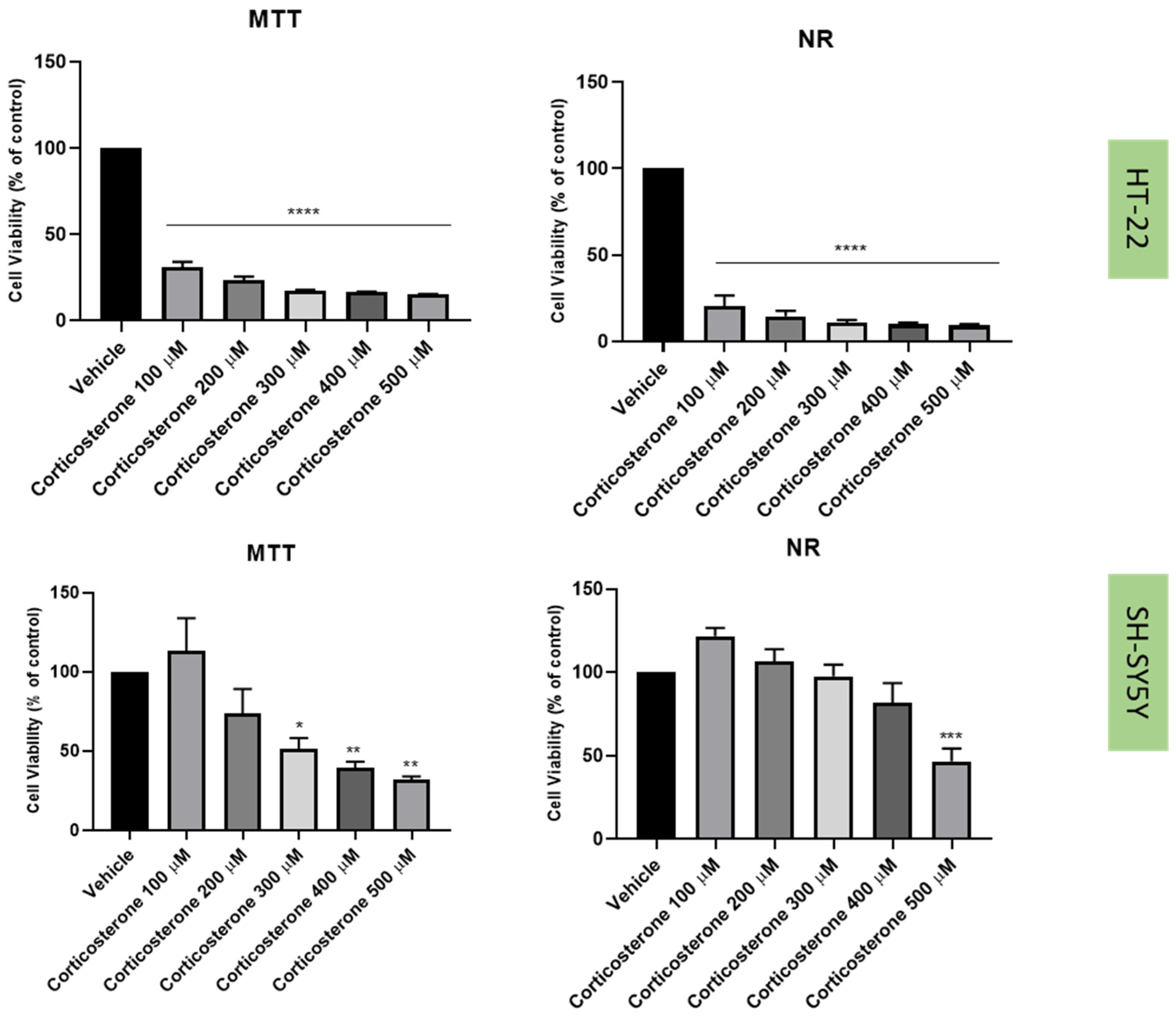
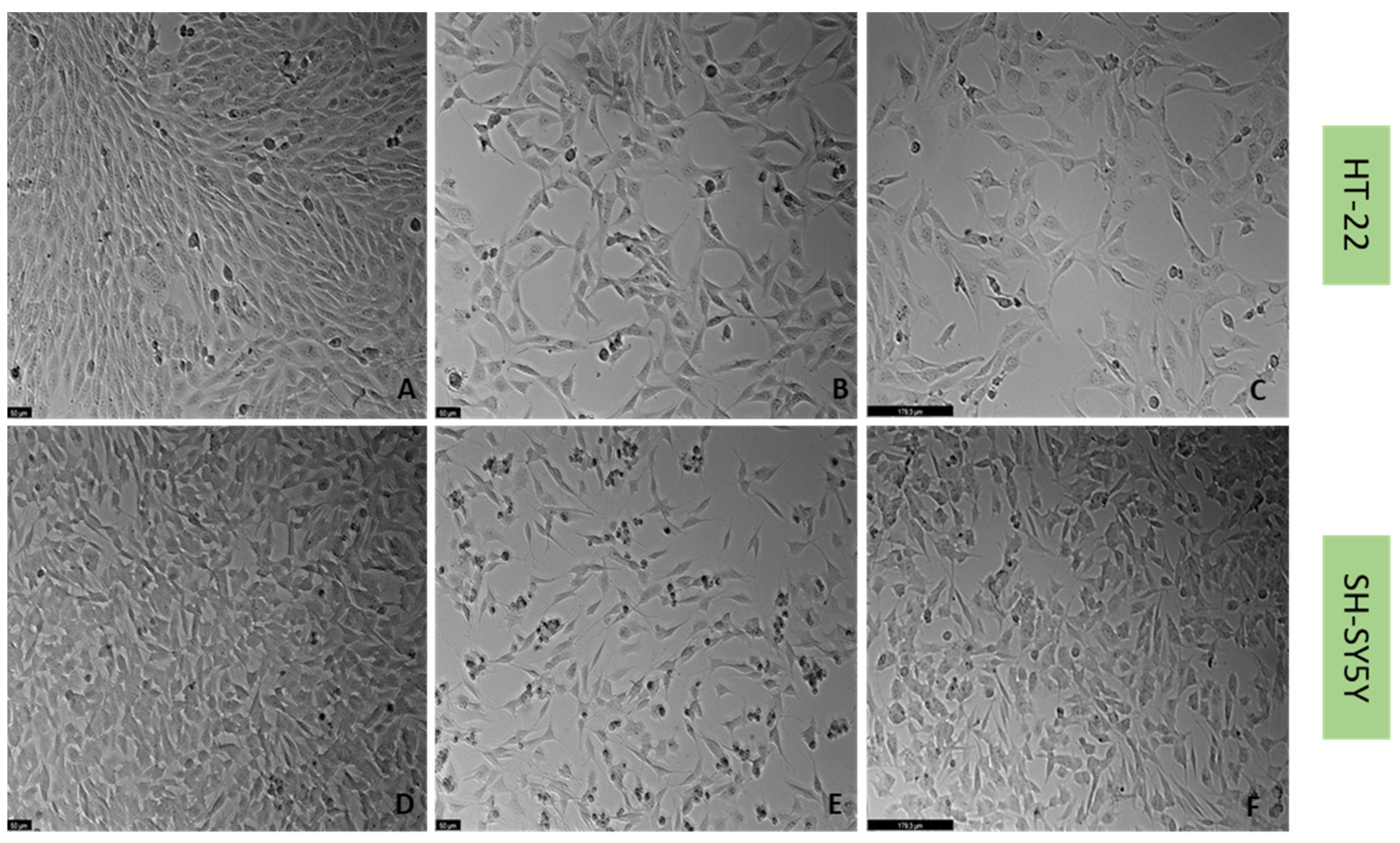
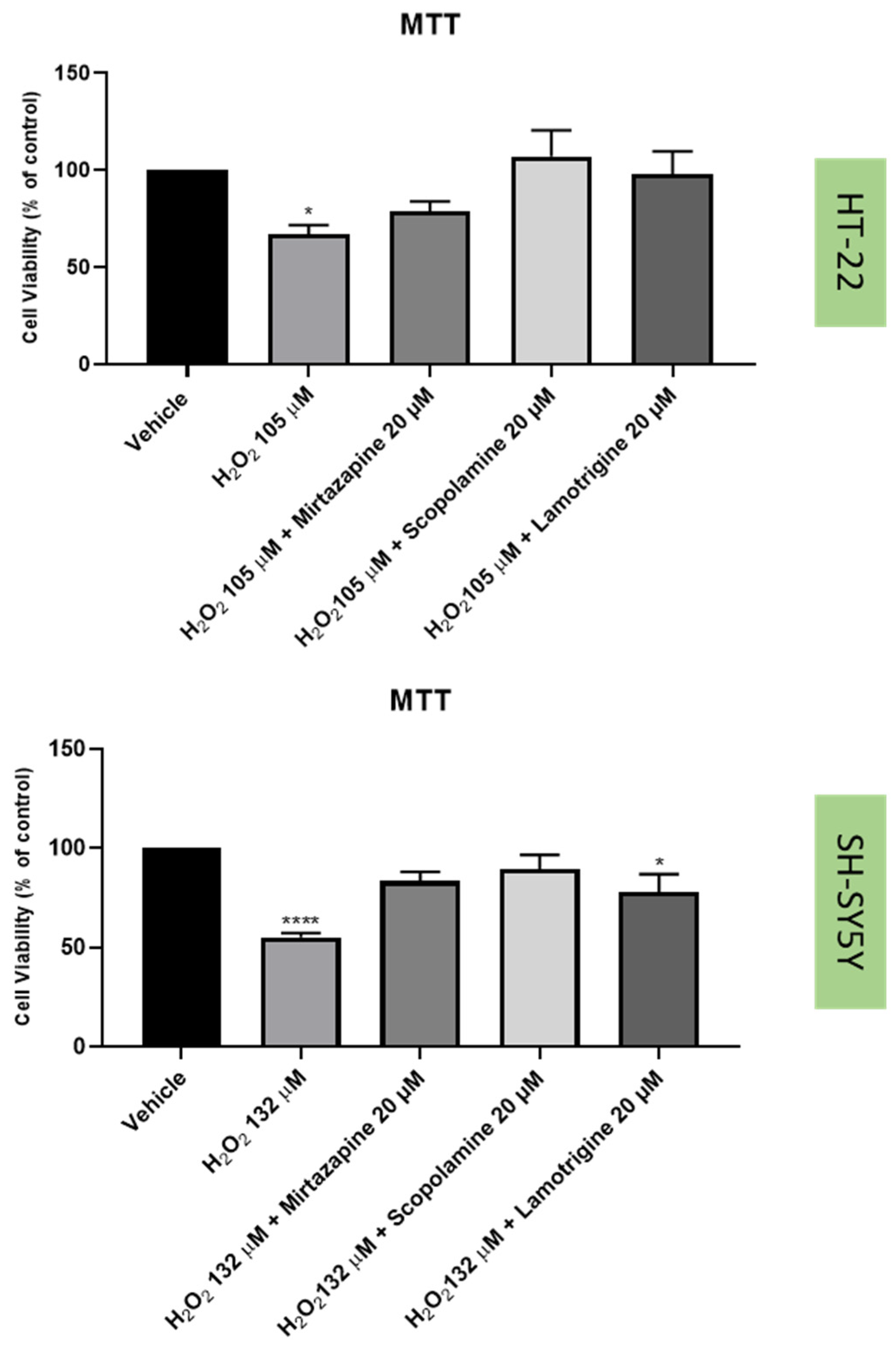
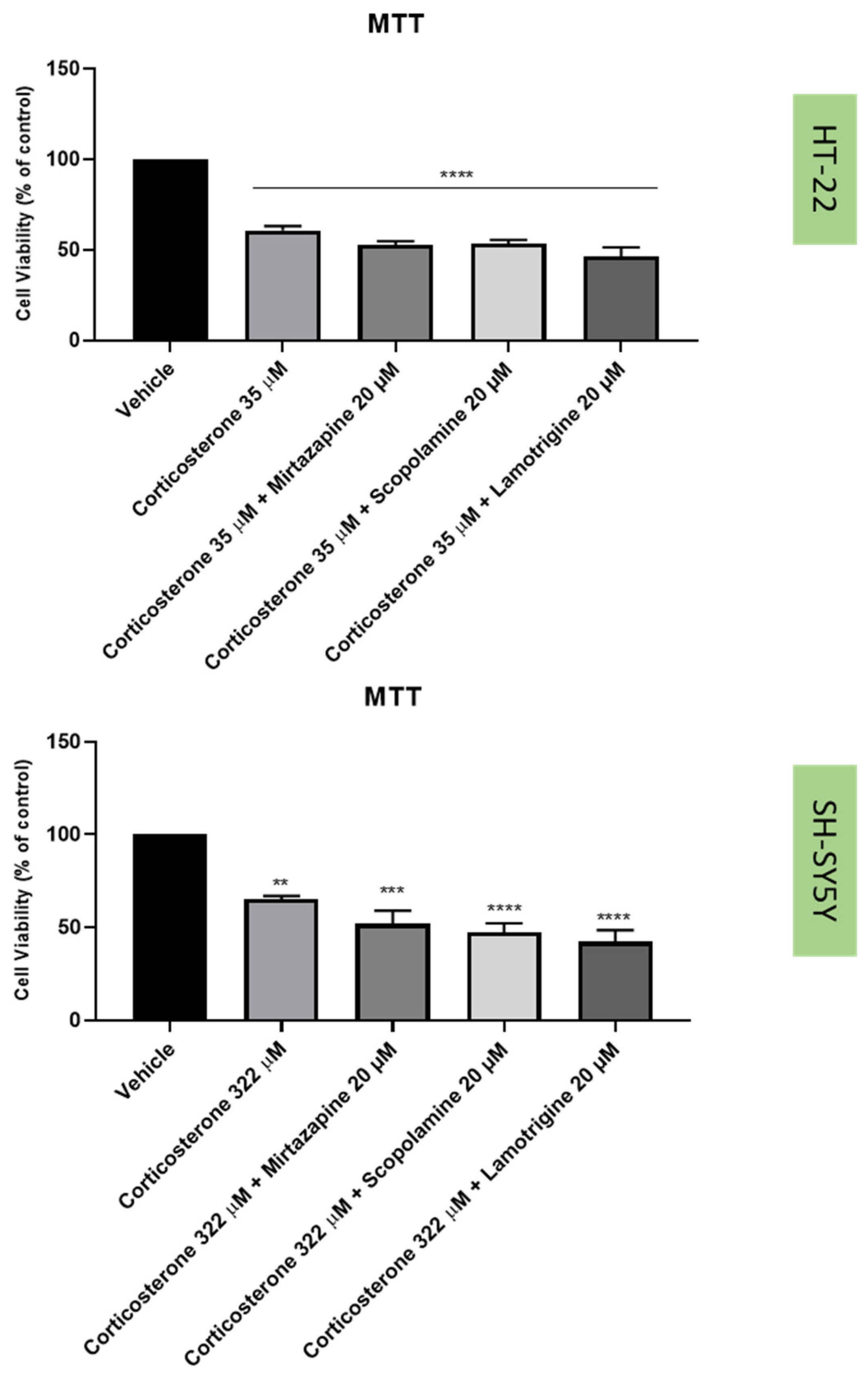
2.3. Effect of Hydrogen Peroxide and Corticosterone on SH-SY5Y and HT-22 Viability
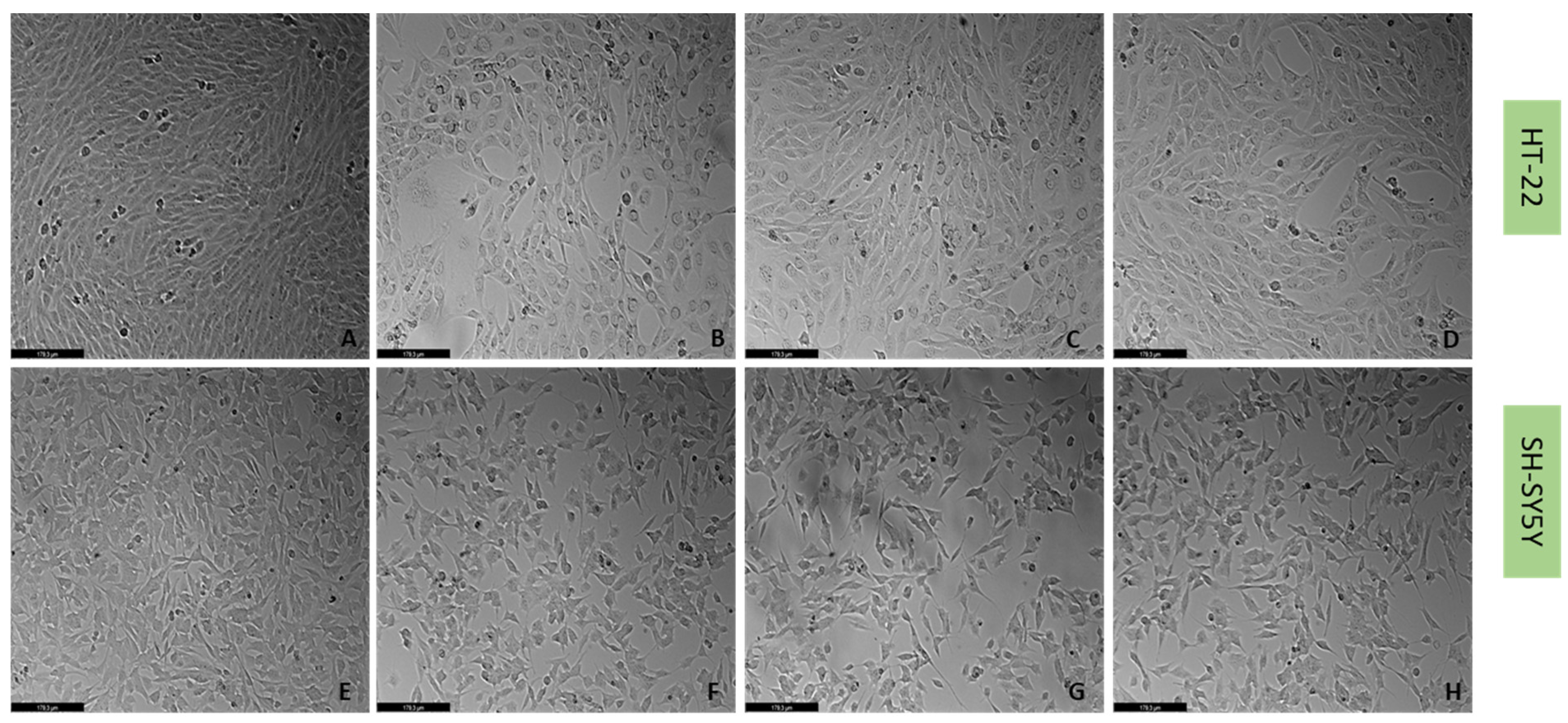
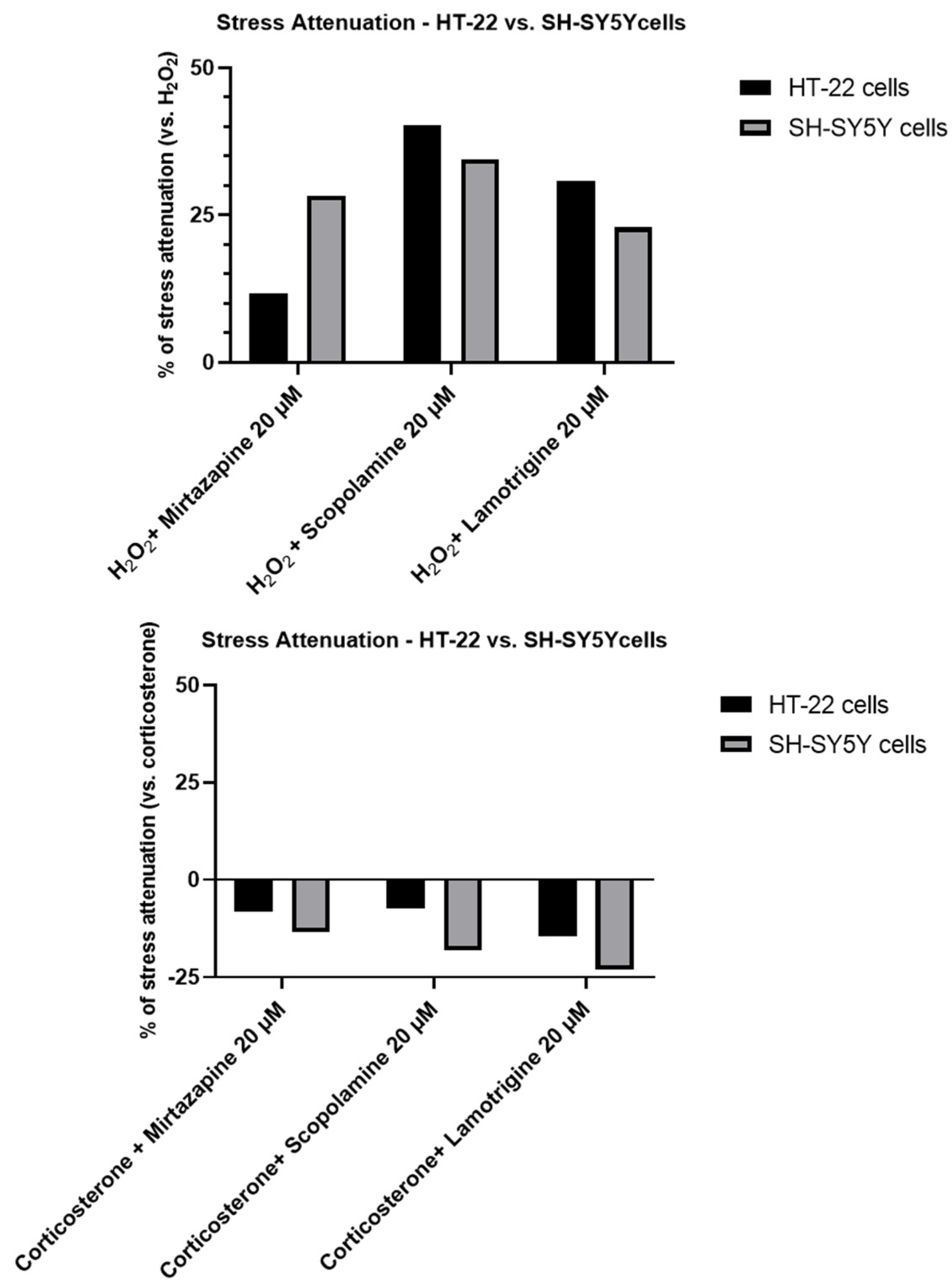
2.4. Effect of Mirtazapine, Scopolamine, and Lamotrigine in Combination with Hydrogen Peroxide and Corticosterone on Cellular Viability
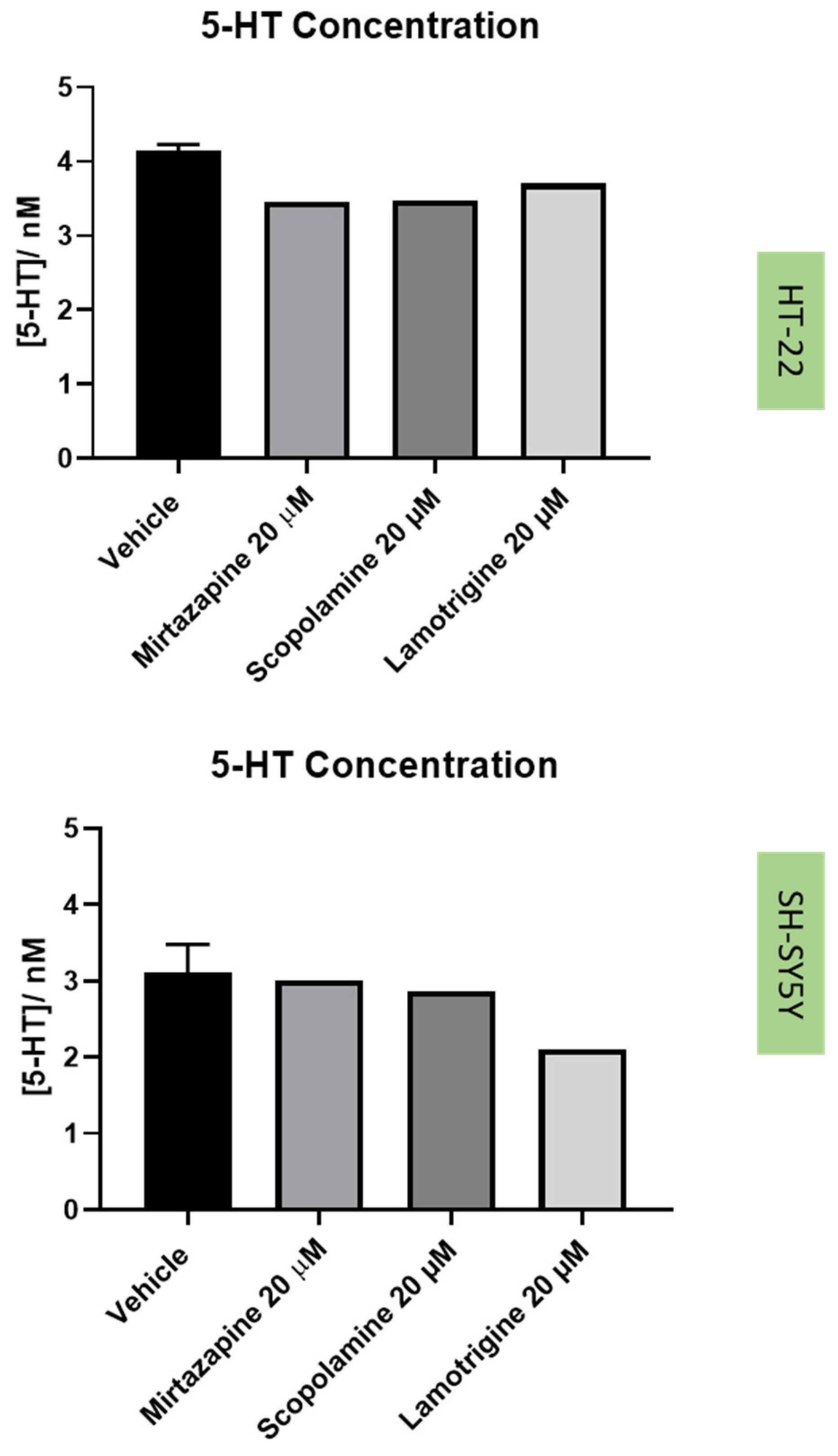
2.5. Effect of the Drugs and Drug Combinations in Extracellular 5-HT Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Treatments
4.3. Cell Culture
4.4. Immunofluorescence
4.5. Cell Viability Assays
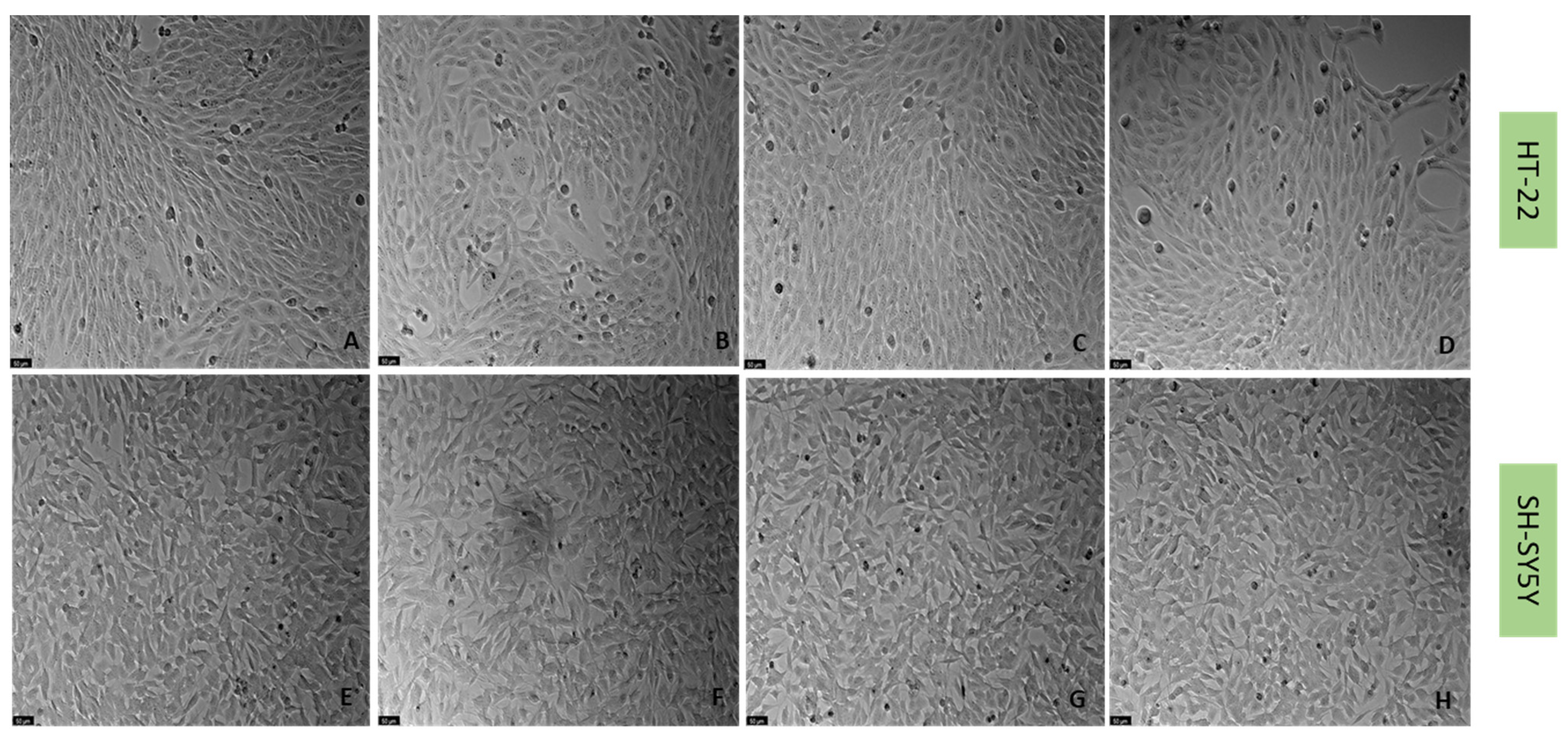
4.6. Cell Morphology Assessment
4.7. HPLC Analysis
4.8. Statistical and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Depressão|SNS24. Available online: https://www.sns24.gov.pt/tema/saude-mental/depressao/#sec-16 (accessed on 13 September 2022).
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nuggerud-Galeas, S.; Sáez-Benito Suescun, L.; Berenguer Torrijo, N.; Sáez-Benito Suescun, A.; Aguilar-Latorre, A.; Magallón Botaya, R.; Oliván Blázquez, B. Analysis of depressive episodes, their recurrence and pharmacologic treatment in primary care patients: A retrospective descriptive study. PLoS ONE 2020, 15, e0233454. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D. Cell-Based Systems of Depression: An Overview. In Herbal Medicine in Depression; Springer International Publishing: Cham, Switzerland, 2016; pp. 75–117. ISBN 9783319140216. [Google Scholar]
- Liu, L.; Zheng, J.; Huang, X.F.; Zhu, X.; Ding, S.M.; Ke, H.M.; O’Donnell, J.M.; Zhang, H.T.; Song, G.Q.; Xu, Y. The neuroprotective and antidepressant-like effects of Hcyb1, a novel selective PDE2 inhibitor. CNS Neurosci. Ther. 2018, 24, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, W.; Feng, Y.; Liu, J.; Chen, H.; Wang, D.; Zhao, R. Melatonin alleviates hippocampal GR inhibition and depression-like behavior induced by constant light exposure in mice. Ecotoxicol. Environ. Saf. 2021, 228, 112979. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Guo, L.; Wang, S.; Deng, X.; Ma, S. Modulation of LPA1 receptor-mediated neuronal apoptosis by Saikosaponin-d: A target involved in depression. Neuropharmacology 2019, 155, 150–161. [Google Scholar] [CrossRef]
- Fu, X.; Jiao, J.; Qin, T.; Yu, J.; Fu, Q.; Deng, X.; Ma, S.; Ma, Z. A New Perspective on Ameliorating Depression-Like Behaviors: Suppressing Neuroinflammation by Upregulating PGC-1α. Neurotox. Res. 2021, 39, 872–885. [Google Scholar] [CrossRef]
- Yu, Z.; Kong, D.; Liang, Y.; Zhao, X.; Du, G. Protective effects of VMY-2-95 on corticosterone-induced injuries in mice and cellular models. Acta Pharm. Sin. B 2021, 11, 1903–1913. [Google Scholar] [CrossRef]
- Yang, G.; Li, J.; Cai, Y.; Yang, Z.; Li, R.; Fu, W. Glycyrrhizic Acid Alleviates 6-Hydroxydopamine and Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells through Modulating Autophagy. Neurochem. Res. 2018, 43, 1914–1926. [Google Scholar] [CrossRef]
- Lieberknecht, V.; Engel, D.; Rodrigues, A.L.S.; Gabilan, N.H. Neuroprotective effects of mirtazapine and imipramine and their effect in pro- and anti-apoptotic gene expression in human neuroblastoma cells. Pharmacol. Rep. 2020, 72, 563–570. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.A.; Zunszain, P.A.; Anacker, C.; Musaelyan, K.; Pariante, C.M. Glucocorticoids and Inflammation: A Double-Headed Sword in Depression? Inflamm. Psychiatry 2013, 28, 127–143. [Google Scholar] [CrossRef]
- Rajkumar, R.; Mahesh, R. The auspicious role of the 5-HT3 receptor in depression: A probable neuronal target? J. Psychopharmacol. 2010, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lummis, S.C.R. 5-HT3 Receptors. Curr. Pharm. Des. 2006, 12, 3615. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Oosterhof, C.; Ebert, B.; Sanchez, C.; Haddjeri, N. Role of 5-HT3 Receptors in the Antidepressant Response. Pharmaceuticals 2011, 4, 603. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Kurhe, Y.; Thangaraj, D.; Prabhakar, V.; Kanade, P. Antidepressant-like effects of a novel 5-HT3 receptor antagonist 6z in acute and chronic murine models of depression. Acta Pharmacol. Sin. 2014, 35, 1493–1503. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Radhakrishnan, M.; Borah, M. Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: An investigation in behaviour-based rodent models. Behav. Pharmacol. 2008, 19, 29–40. [Google Scholar] [CrossRef]
- Correia, A.S.; Fraga, S.; Teixeira, J.P.; Vale, N. Cell Model of Depression: Reduction of Cell Stress with Mirtazapine. Int. J. Mol. Sci. 2022, 23, 4942. [Google Scholar] [CrossRef]
- Xu, L.J.; Liu, A.L.; Du, G.H. Scopolamine. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2022; pp. 319–324. [Google Scholar] [CrossRef]
- Renner, U.D.; Oertel, R.; Kirch, W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther. Drug Monit. 2005, 27, 655–665. [Google Scholar] [CrossRef]
- Lochner, M.; Thompson, A.J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology 2016, 108, 220. [Google Scholar] [CrossRef] [PubMed]
- Ebada, M.E. Drug repurposing may generate novel approaches to treating depression. J. Pharm. Pharmacol. 2017, 69, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Schober, D.A.; Nikolayev, A.; Tolstikov, V.V.; Anderson, W.H.; Higgs, R.E.; Kuo, M.-S.; Laksmanan, A.; Catlow, J.T.; Li, X.; et al. Further Evaluation of Mechanisms Associated with the Antidepressantlike Signature of Scopolamine in Mice. CNS Neurol. Disord.-Drug Targets 2017, 16, 492–500. [Google Scholar] [CrossRef]
- Drevets, W.C.; Furey, M.L. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: A randomized, placebo-controlled clinical trial. Biol. Psychiatry 2010, 67, 432–438. [Google Scholar] [CrossRef]
- Furey, M.L.; Khanna, A.; Hoffman, E.M.; Drevets, W.C. Scopolamine Produces Larger Antidepressant and Antianxiety Effects in Women Than in Men. Neuropsychopharmacol. 2010, 35, 2479–2488. [Google Scholar] [CrossRef]
- Kim, K.J.; Jeun, S.H.; Sung, K.W. Lamotrigine, an antiepileptic drug, inhibits 5-HT 3 receptor currents in NCB-20 neuroblastoma cells. Korean J. Physiol. Pharmacol. 2017, 21, 169–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abelaira, H.M.; Réus, G.Z.; Ribeiro, K.F.; Zappellini, G.; Ferreira, G.K.; Gomes, L.M.; Carvalho-Silva, M.; Luciano, T.F.; Marques, S.O.; Streck, E.L.; et al. Effects of acute and chronic treatment elicited by lamotrigine on behavior, energy metabolism, neurotrophins and signaling cascades in rats. Neurochem. Int. 2011, 59, 1163–1174. [Google Scholar] [CrossRef]
- Betchel, N.T.; Fariba, K.A.; Saadabadi, A. Lamotrigine. In The Essence of Analgesia and Analgesics; Cambridge University Press: Cambridge, UK, 2022; pp. 306–309. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Urashima, K.; Sakai, S.; Morimoto, Y.; Kanegae, S.; Kinoshita, H.; Imamura, A.; Ozawa, H. The effectiveness of lamotrigine for persistent depressive disorder: A case report. Neuropsychopharmacol. Rep. 2022, 42, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Veronese, N.; Zaninotto, L.; Van Der Loos, M.L.M.; Gao, K.; Schaffer, A.; Reis, C.; Normann, C.; Anghelescu, I.G.; Correll, C.U. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: A comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectr. 2016, 21, 403–418. [Google Scholar] [CrossRef]
- Goh, K.K.; Chen, C.H.; Chiu, Y.H.; Lu, M.L. Lamotrigine augmentation in treatment-resistant unipolar depression: A comprehensive meta-analysis of efficacy and safety. J. Psychopharmacol. 2019, 33, 700–713. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Antidepressants in Alzheimer’s Disease: A Focus on the Role of Mirtazapine. Pharmaceuticals 2021, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, P.; Rao, W.; Dai, S.; Zhang, L.; Ma, W.; Pu, J.; Yu, Y.; Wang, J.; Fei, Z. Homer1a attenuates hydrogen peroxide-induced oxidative damage in HT-22 cells through AMPK-dependent autophagy. Front. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Lee, H.; Park, H.; Jeon, J.W.; Cho, W.-K.; Ma, J.Y. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complement. Altern. Med. 2015, 15, 171. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Montilla, P.; Padillo, F.J.; Bujalance, I.; Muñoz, M.C.; Muntané, J.; Túnez, I. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol. Exp. (Wars) 2006, 66, 1–6. [Google Scholar] [PubMed]
- Wilkinson, L.O.; Auerbach, S.B.; Jacobs, B.L. Extracellular serotonin levels change with behavioral state but not with pyrogen-induced hyperthermia. J. Neurosci. 1991, 11, 2732–2741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramos-Hryb, A.B.; Platt, N.; Freitas, A.E.; Heinrich, I.A.; López, M.G.; Leal, R.B.; Kaster, M.P.; Rodrigues, A.L.S. Protective Effects of Ursolic Acid Against Cytotoxicity Induced by Corticosterone: Role of Protein Kinases. Neurochem. Res. 2019, 44, 2843–2855. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Wang, X.-Q.; Liu, M.-F.; Ye, B.-J. Meta-analysis of association between TPH2 single nucleotide poiymorphism and depression. Neurosci. Biobehav. Rev. 2022, 134, 104517. [Google Scholar] [CrossRef]
- Ma, R.D.; Zhou, G.J.; Qu, M.; Yi, J.H.; Tang, Y.L.; Yang, X.Y.; Nie, Y.X.; Gu, H.F. Corticosterone induces neurotoxicity in PC12 cells via disrupting autophagy flux mediated by AMPK/mTOR signaling. CNS Neurosci. Ther. 2020, 26, 167–176. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Kurhe, Y. Effect of a novel 5-HT3 receptor antagonist 4i, in corticosterone-induced depression-like behavior and oxidative stress in mice. Steroids 2015, 96, 95–102. [Google Scholar] [CrossRef]
- Joëls, M.; Karst, H.; Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol. 2018, 223, e13066. [Google Scholar] [CrossRef] [PubMed]
- HT-22 Mouse Hippocampal Neuronal Cell Line|SCC129. Available online: https://www.merckmillipore.com/PT/en/product/HT-22-Mouse-Hippocampal-Neuronal-Cell-Line,MM_NF-SCC129?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 31 May 2022).
- SH-SY5Y|ATCC. Available online: https://www.atcc.org/products/crl-2266 (accessed on 31 May 2022).
- John Jayakumar, J.A.K.; Panicker, M.M.; Basu, B. Serotonin 2A (5-HT2A) receptor affects cell–matrix adhesion and the formation and maintenance of stress fibers in HEK293 cells. Sci. Rep. 2020, 10, 21675. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Duarte, D.; Silva, I.; Reguengo, H.; Oliveira, J.C.; Vale, N. Serotonin after β-Adrenoreceptors’ Exposition: New Approaches for Personalized Data in Breast Cancer Cells. J. Pers. Med. 2021, 11, 954. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Silva, I.; Oliveira, J.C.; Reguengo, H.; Vale, N. Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide. Life 2022, 12, 1645. https://doi.org/10.3390/life12101645
Correia AS, Silva I, Oliveira JC, Reguengo H, Vale N. Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide. Life. 2022; 12(10):1645. https://doi.org/10.3390/life12101645
Chicago/Turabian StyleCorreia, Ana Salomé, Isabel Silva, José Carlos Oliveira, Henrique Reguengo, and Nuno Vale. 2022. "Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide" Life 12, no. 10: 1645. https://doi.org/10.3390/life12101645
APA StyleCorreia, A. S., Silva, I., Oliveira, J. C., Reguengo, H., & Vale, N. (2022). Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide. Life, 12(10), 1645. https://doi.org/10.3390/life12101645







