Abstract
(1) Background: Although invasive fungal infections are a major cause of neonatal morbidity and mortality, data on the incidence and outcomes of localized abscesses in solid organs due to fungal infections are scarce. The aim of this study was to consolidate evidence and enhance our understanding on neonatal liver abscesses due to invasive fungal infections. (2) Methods: An electronic search of the PubMed and Scopus databases was conducted, considering studies that evaluated fungal liver abscesses in the neonatal population. Data on the epidemiology, clinical course, treatment, and outcome of these infections were integrated in our study. (3) Results: Overall, 10 studies were included presenting data on 19 cases of neonatal fungal liver abscesses. Candida spp. were the most common causative pathogens (94.7%). Premature neonates constituted the majority of cases (93%), while umbilical venous catheter placement, broad spectrum antibiotics, and prolonged parenteral nutrition administration were identified as other common predisposing factors. Diagnosis was established primarily by abdominal ultrasonography. Medical therapy with antifungal agents was the mainstay of treatment, with Amphotericin B being the most common agent (47%). Abscess drainage was required in four cases (21%). Eradication of the infection was achieved in the majority of cases (80%). (4) Conclusions: Even though fungal liver abscess is a rare entity in the neonatal population, clinicians should keep it in mind in small, premature infants who fail to respond to conventional treatment for sepsis, particularly if an indwelling catheter is in situ. A high index of suspicion is necessary in order to achieve a timely diagnosis and the initiation of the appropriate treatment.
1. Introduction
Invasive fungal infections (IFI) are a major cause of neonatal morbidity and mortality, affecting mainly preterm and very low birth weight (VLBW) neonates (<1500 g). Candida species are responsible for the vast majority of IFI in this age population, with a reported incidence of 5–10 cases per 100,000 live-born infants [1]. Other fungal species such as Malassezia, Aspergillus, and Zygomycetes contribute to a lesser degree to the overall disease burden [2].
A host of unique predisposing factors such as immature immune function and host epithelial protection, hospitalization in an intensive care setting, and frequent invasive procedures render neonates particularly vulnerable to infectious insults. The incidence of invasive candidiasis (IC) is inversely related to the gestational age and birth weight (BW), rising from 0.06% in hospitalized neonates with a BW > 1500 g to 2–5% in VLBW infants [3]. The spectrum of clinical manifestations of Candida infection in neonates includes bloodstream and urinary tract infections, meningitis, endophthalmitis, endocarditis, osteomyelitis, and the localized abscesses of solid organs.
Neonatal liver abscess (LA) is a rare but life-threatening entity, associated with a high mortality rate. Since 1930, less than 150 cases have been reported in the literature [4,5,6,7]. The increased survival rate of extremely premature babies due to advances in neonatal care, and the wide use of bedside imaging modalities such as portable ultrasound, have led to an increased rate in the reporting of this entity over the last few decades. The most common pathogens accounting for neonatal LA are Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Klebsiella spp., and Pseudomonas spp. [8]. However, data on the incidence, clinical course, and outcome of fungal liver abscess in the neonatal population are scarce.
The aim of this systematic review was to consolidate evidence and enhance our understanding on neonatal fungal liver abscesses regarding the microbiology, risk factors, clinical course, diagnosis, and treatment of these infections.
2. Materials and Methods
2.1. Search Protocol/Databases
A methodological protocol was developed based on the guidelines of the Preferred Reported Items for Systematic Reviews and Metanalysis (PRISMA, presented as a Supplementary Material) in order to identify and assess studies relevant to the topic of interest of this review [9,10]. The review of the literature was conducted between October 2022 and November 2022. The systematic review was not registered in Prospero.
An electronic search of the existing literature in PubMed and Scopus databases until 3 November 2022 was conducted using combinations of the following keywords: “fungal”, “candida”, “liver abscess”, “hepatic abscess”, “newborn*”, “neonate*”, and “preterm neonate*”, with Boolean logic operators. Duplicate records were removed by a single researcher (R.S.) using the default settings of EndNote X8 software. Initially, two independent researchers (R.S. and P.K.T.) individually screened the titles and abstracts, and clearly irrelevant articles were excluded. A thorough full-text evaluation was followed by the researchers, resulting in the inclusion of only those studies that met the established inclusion criteria. Disagreements between the two researchers regarding the inclusion of studies were resolved with the contribution of a third author (A.G.T.). Additionally, in order to minimize the possibility of missing any relevant studies, a manual search of the electronic records was performed, while the citations of each retrieved study and of previous systematic reviews pertaining to our topic of interest were also screened.
2.2. Study Selection and Data Extraction
Randomized clinical trials, observational studies, case series, and case reports involving neonatal patients with a documentation of fungal liver abscess were considered eligible for this review. Narrative reviews, systematic reviews, and/or meta-analysis were excluded, as well as studies published in languages other than English. No geographic restrictions were applied. After compiling the relevant studies, the data of interest for each study were extracted into an excel file. The extracted data for each study included: the first author, year of publication or presentation, number of participants, participant characteristics and underlying condition, information on the treatment, complications, and the outcome.
3. Results
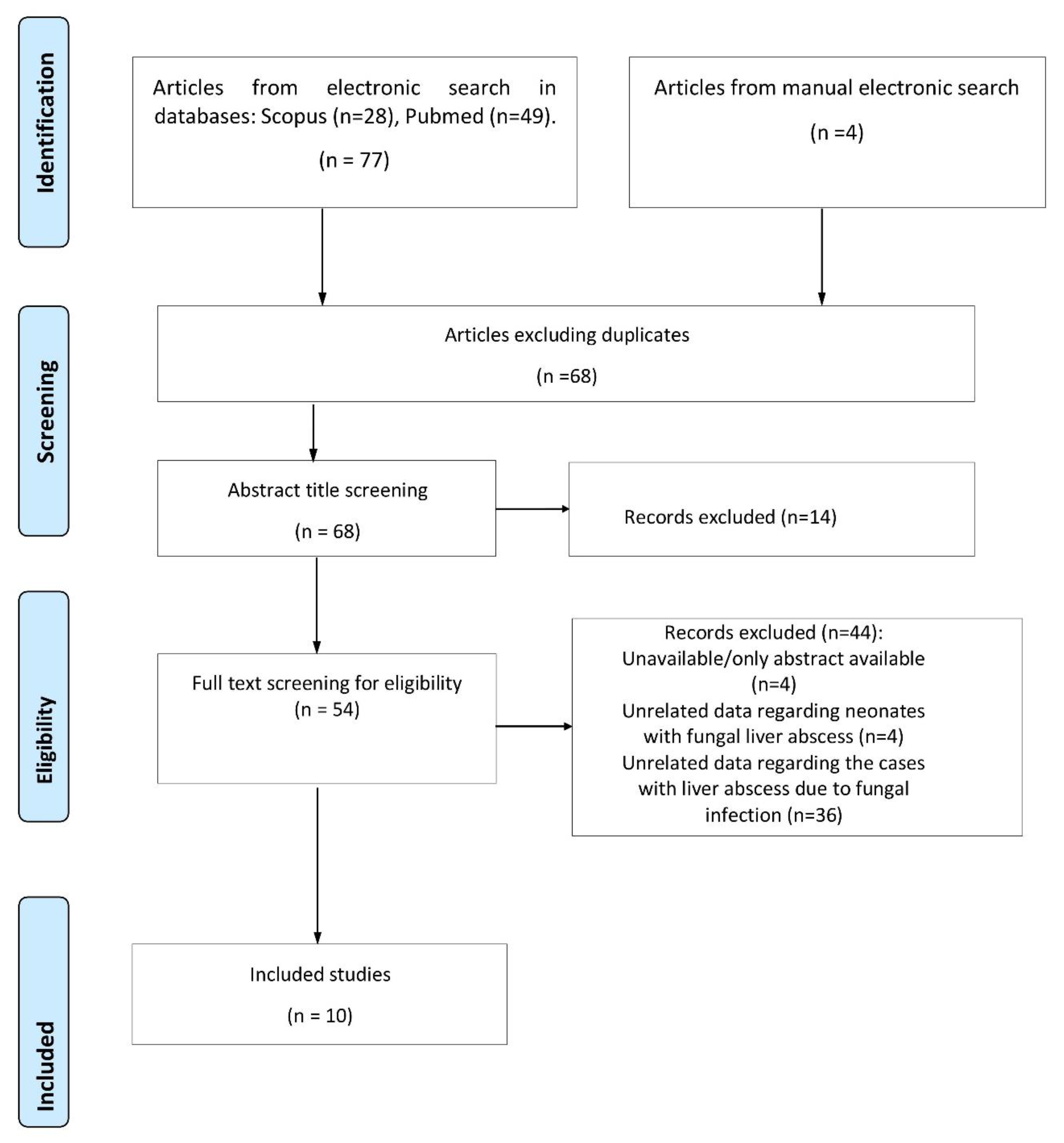
Our systematic review yielded a total of 77 studies. After a thorough evaluation, 10 studies describing 19 patients met the inclusion criteria and were incorporated in our study. Detailed information on the study selection process is presented in Figure 1.
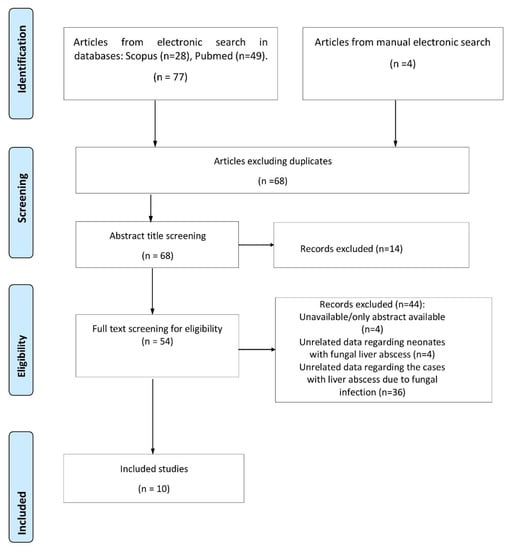
Figure 1.
Flow chart of study selection process.
Detailed data for each patient regarding the demographics, clinical presentation, treatment, and outcome of the infection are presented in Table 1.

Table 1.
Characteristics of individual studies.
The majority of the included neonates were preterm (14/15 neonates with a reported gestational age, 93%), with a median gestational age of 29 weeks (range 24 weeks to term). The median patient age at the presentation was 22.5 (range 7–59) days (Table 2).

Table 2.
Demographics of the study population.
A male predominance 2:1 was shown in studies reporting data on the patient’s sex (eight male vs. four female neonates). Besides prematurity, several other predisposing factors were identified, including total parenteral nutrition (TPN) administration (12/13 neonates, 92.3%), umbilical venous catheter placement (13/13 cases, 100%), mechanical ventilation (7/8 neonates, 87.5%), and previous broad-spectrum antibiotic administration (11/12 cases, 91.7%). Four infants (21%) had a history of a previous surgical abdominal intervention; two due to necrotizing enterocolitis (NEC), one due to duodenal atresia, and one due to ileal atresia. None of the neonates had previously received antifungal prophylaxis.
Candida spp. were the most commonly isolated pathogens (isolated in 94.7% of cases), with Candida albicans accounting for 11/19 cases (57.9%), Candida parapsilosis for 1 (5.2%) case, Candida glabrata for 1 (5.2%) case, and unidentified Candida species for 5/19 cases (26.3%). Only one case of a non-Candida pathogen (Malassezia) was reported (Table 3).

Table 3.
Microbiology of fungal species and co-cultured bacterial pathogens.
Fungi were isolated from blood cultures in 17/19 (89.5%) cases, from fluid aspirated from the abscess in 8/19 (42.1%) cases, and from cerebrospinal fluid in 2/19 (10.5%) cases (Table 4). In one case, even though no pathogen was cultured in blood and abscess fluid, a microscopic examination of the abscess fluid (stained with calcofluor white) revealed hyphae and yeast-like cells indicative of Malassezia [18].

Table 4.
Diagnosis of fungal infections.
A clinical presentation consistent with sepsis (often with a prolonged course) was reported in most cases, whereas other symptoms such as liver enlargement were reported in some neonates. The abscess was identified on abdominal ultrasonography (U/S) in all cases except for one, in which the abscess was discovered during abdominal surgery; the relevant data were not available for four of the cases. Four neonates with positive findings on U/S underwent abdominal computed tomography (CT) for a further evaluation. Additional lesions in organs other than the liver were present in four cases; a brain abscess in two cases, a subphrenic abscess in one case, and a gallbladder lesion (attributed to the rupture of the abscess into the biliary tree) in another case. Concomitant infection with another microorganism was also reported in four cases, involving Acinetobacter baumanii, Klebsiella spp., methicillin-resistant Staphylococcus aureus, and Xanthomonas maltophilia (Table 3). The abscesses were located predominantly in the right lobe (eight in the right lobe vs. three in the left lobe; the location of the abscess in the remaining cases was not specified). The majority involved one solitary lesion (8/12 neonates, 66.7%), whereas multiple abscesses were reported in 4 (33.3%) neonates; no data were available for the remaining cases (Table 4).
The antifungal therapy differed significantly among the cases, with amphotericin B (AMB) being the most commonly used agent. Monotherapy with different formulations of AMB (liposomal, deoxycholate, or unspecified) was reported in 8 out of the 15 cases with available data on the administered antifungal agents (53%). Other treatment regimens included a combination of AMB and flucytosine (1/15 neonates, 6.6%), fluconazole and subsequently AMB (1/15 neonates, 6.6%), monotherapy with micafungin (1/15 neonates, 6.6%), fluconazole followed by caspofungin (1/15 neonates, 6.6%), and a combination of fluconazole, AMB, and caspofungin (1/15 neonates, 6.6%). In one noteworthy case, AMB was injected inside the abscess in addition to the intravenous administration of micafungin and AMB, leading to resolution of the infection. The median duration of an antifungal administration (excluding infants who expired) was 34 days (range 28–42). Additional invasive treatment was required in 4/19 cases (21%), including the placement of a percutaneous pigtail catheter in 3 cases and open surgical drainage in 1 case. The infection resolved in the majority of cases (12/15 neonates, 80%) and only 3 (20%) neonates died; data on the outcome were not available in 4 cases (Table 5).

Table 5.
Treatment strategies and survival rate.
4. Discussion
This systematic review investigated the existing literature regarding fungal liver abscess in the neonatal population. Candida spp. were identified as the most common causative pathogens (94.7%), while prematurity, broad-spectrum antibiotic administration, umbilical vein catheterization, and prolonged parenteral nutrition were identified as common predisposing factors. The presenting symptoms were often vague and non-specific, but the majority of neonates exhibited clinical manifestations consistent with sepsis. Conservative therapy with antifungal agents was the mainstay of treatment, with AMB being the most common agent (47%), while abscess drainage was required in four cases (21%). The infection was resolved in the majority of the studied cases (80%).
Neonatal IFI are life-threatening opportunistic infections, reported as the third most common cause for late-onset sepsis in VLBW infants. The rate of these infections varies greatly among different settings, with a reported median rate of 7.5% in ELBW neonates [20]. Candida species are responsible for the vast majority of IFI, with C. albicans accounting for the majority of cases (60–75%), followed by C. parapsilosis (20–30%), C. krusei, and other non-albicans species [1]. C. auris is an emerging pathogen that has caused significant concern worldwide since it was first described in 2009 due to its resistance to antifungal therapy and reported high mortality rate. Data on its incidence in the neonatal population are sparse with only a few reported cases, but the real impact could be underestimated due to misidentification by conventional microbiological assays [21]. The risk of IFI is higher in low-weight infants, with 86% of all cases occurring in neonates under 1000 g, while the mortality rate ranges from 25 to 55% [22,23]. Moreover, neonates who overcome these infections commonly present with late sequelae such as hearing or/and visual impairment, cerebral palsy, and mental retardation [24,25]. In 2004, Stoll et al. reported that neurodevelopmental impairment was observed during follow-up in 57% of ELBW neonates that survived IFI [25]. Timely diagnosis is extremely challenging as symptoms can be subtle and clinical presentation may be indistinguishable from other infections.
LA in neonates are rare entities and often present as a complication of sepsis, NEC, or surgical abdomen. Diagnosis is elusive as the symptoms are vague and non-specific. Laboratory tests usually include elevated infection markers and transaminases [7]. Four possible mechanisms of pathogen invasion into the liver and subsequent abscess formation are described in the literature: (a) hematogenous spread, (b) ascending infection through the umbilical and portal veins or (c) the biliary tree, and (d) contiguous spread from the adjacent structures [4,7,8].
Very few cases of fungal LA have been reported in the literature. Our results indicate that preterm neonates are most affected by these infections (93% of all cases) since the immaturity of the innate immune system and underdeveloped physical barriers render them at a higher risk for invasive infections. Skin or mucosal colonization with fungal pathogens during hospitalization is a recognized risk factor for the later development of IFI. Neonates with candidemia are reportedly six times more likely to have previously experienced a colonization by the same species [26]. About 88% of fungal colonizations are reported to occur during the first days of life, in sites such as the anus, oral cavity, and umbilicus [27]. A colonized skin and GI tract can become the primary source of the translocation of pathogens through damaged or compromised epithelium and mucosa, respectively, leading to the systemic dissemination of these pathogens [28,29]. The administration of broad-spectrum antibiotics causes alterations in the normal intestinal microbiota and facilitates the invasion by pathogenic species in the setting of a prolonged NICU hospitalization [30]. In line with this, most neonates in our study had previously received broad-spectrum antibiotics.
Abdominal instrumentation and indwelling devices, particularly umbilical venous catheters (UVC), have been identified as important risk factors for the development of LA. Due to the unique anatomy of umbilical vessels and the presence of patent ductus venosus in neonates, the UVC tip can migrate and subsequently lie inside the liver. Moreover, the use of a mal-positioned UVC to infuse hypertonic solutions, such as parenteral nutrition and lipids, can lead to topical necrosis of the hepatic tissue, creating ideal conditions for the formation of an abscess [31]. The association between UVC placement and LA formation is also supported by the results of our study, with 13 of our cases concerning infants who had a history of UVC placement.
An intriguing finding was that four of the patients included in our study exhibited concurrent infection with fungal and bacterial pathogens. This condition has been extensively studied during the last years in adult populations, with various reports raising the percentage of bacteremia in hospitalized patients with candidemia to 27–67% [32,33]. The hypothesis that fungal infection carries an increased risk of bacterial coinfection could have serious implications on the treatment choices and management of neonates with fungal liver abscess.
Abscesses located in other organs, most notably the brain, are often discovered in neonates initially diagnosed with LA, a fact mirrored by the data in our study. The underlying pathophysiological mechanism is thought to be through metastatic septic emboli in the circulation. In a review by Benjamin et al., end-organ damage in neonates with candidemia was reported with a median prevalence of endophthalmitis 3%, meningitis 16%, ventriculitis/brain abscess 4%, endocarditis 5%, renal abscess 5%, and hepatosplenic abscess 1% [34].
The diagnosis of hepatic abscesses in neonates is elusive as the symptoms can be vague and/or non-specific. Therefore, a high index of suspicion is necessary in order to achieve a timely diagnosis and treatment. Common laboratory findings include leukocytosis, thrombocytopenia, elevated acute phase reactants, and altered hepatic enzymes, although jaundice is rare [7]. Radiological findings that should alert the clinician are elevated hemidiaphragm, ipsilateral pleural effusion, and the appearance of enclosed air inside the liver on an X-ray [35]. All neonates with culture-proven fungaemia, as well as those with prolonged sepsis not responding to conventional treatment (especially if umbilical lines remain in situ), should be evaluated for the possible presence of localized lesions such as liver abscess.
Ultrasonography is the preferred imaging modality in neonates for the evaluation of lesions in solid organs since it lacks ionizing radiation, while it is also a pain-free procedure without any need for patient sedation. Moreover, the advent of portable ultrasound machines allows for bedside imaging in the NICU setting. Unfortunately, there are no pathognomonic signs for hepatic abscesses. These lesions usually appear as areas of increased echogenicity, although they can be hypoechoic depending on the chronicity of the infection [15]. Although fungal abscesses have been frequently reported to present as multiple small abscesses scattered in the hepatic parenchyma [36], in most of the neonates included in our study, these lesions presented as solitary lesions. In case of solitary hepatic abscess, the right lobe is more commonly affected due to its larger volume and the increased amount of blood it receives since the flow from the superior mesenteric vein goes preferentially to the right [37,38]. Monitoring with serial ultrasonography is needed in order to evaluate for possible complications, and to assess the response to treatment [7]. Further imaging with CT or MRI can provide more detailed information regarding the location and the extend of the lesion, and can be valuable, especially in case of preoperative planning [12].
Although several medications and interventions have been used for the treatment of neonatal liver fungal abscesses, the optimal therapeutic approach remains debatable. Three classes of antifungal agents are commonly used for the treatment of neonatal fungal infections [39]; polyenes, azoles, and echinocandins. Unfortunately, studies delineating the pharmacokinetics of these medications in the neonatal population are lacking. AMB has been extensively used in the NICU setting due to its favorable safety profile and good tolerance in neonates. Fluconazole, a first-generation triazole, is an alternative agent that is commonly used due to its high efficacy in Candida isolates, its excellent cerebrospinal fluid penetration, and its urinal excretion as an active drug resulting in high concentrations [40]. However, newer antifungal agents such as micafungin and caspofungin have gained ground over recent years, especially in cases where treatment with the traditional agents is precluded due to toxicity or drug resistance [41]. Although C. albicans isolates maintain a low incidence of fluconazole resistance (0.5–2%), other species such as C. parapsilosis and C. glabrata display increasing resistance patterns (2–6% and 11–13%, respectively) or are innately resistant, as is the case with C. krusei [42]. Additionally, the emergence of C. auris, resistant to azoles (more than 90% of isolates), AMB, and even to echinocandins, poses new challenges given the limited antifungal arsenal at our disposal [43]. Developing antifungal resistance is a growing public health emergency, especially in vulnerable populations, such as neonates. Combined efforts to understand the mechanisms of antifungal resistance and to implement antifungal stewardship programs is of the utmost importance in order to combat this new threat.
Even though conservative treatment with antifungal agents is the first line of treatment of fungal liver abscesses, refractory cases may require invasive interventions such as percutaneous aspirations and/or surgical treatment for the successful resolution of the infection [39]. This approach is feasible only in solitary abscesses and remains challenging in neonates, especially in those born prematurely and with an extremely low birth weight. Among the neonates included in our review, four required abscess drainage, one by open surgical procedure and three by percutaneous placement of a pigtail catheter. Interestingly, in one of these cases, intralesional administration of AMB was additionally performed, resulting in the successful resolution of the infection.
Antifungal prophylaxis in preterm neonates is an area of ongoing controversy, with each institution following a different protocol. Prophylactic fluconazole and oral nystatin have both proven to be successful in the prevention of neonatal IFI. Although there is robust evidence supporting the effective and safe use of fluconazole as antifungal prophylaxis, concerns regarding the potential development of antifungal resistance limits its universal use [44]. A recent nationwide retrospective study in Japan reported that only 43% of medical facilities routinely prescribed antifungal prophylaxis for high-risk neonates [45]. It is noteworthy that none of the neonates included in our review had received antifungal prophylaxis.
Fungal liver abscesses are associated with a high mortality rate in this vulnerable population. Although the data are limited, previous studies have estimated the mortality rate of liver abscess due to Candida spp. to be at about 50% [14]. However, the mortality rate in our study was significantly lower (20%), probably reflecting the recent advances in imaging technologies and antifungal agents which result in prompt diagnosis and more effective treatment strategies.
This study has certain limitations. Since a fungal liver abscess is a very rare clinical condition in neonates and the relevant data from the literature are scarce, only case reports and case series were assessed for our systematic review. When searching the literature, no randomized clinical trials nor observational studies were found, and therefore the risk of bias could not be avoided. The results of our study may not be representative of all of the neonatal population due to heterogeneity of clinical presentation, type of treatment, and evaluation of the outcome, and thus they should be interpreted with caution.
5. Conclusions
Fungal liver abscess in the neonatal population is a rare clinical entity, with only 19 reported cases in the literature. Due to the non-specific clinical and laboratory findings, diagnosis is challenging as it requires a high index of suspicion, especially in neonates with predisposing risk factors such as prematurity, umbilical vein catheter placement, prolonged administration of broad-spectrum antibiotics, and total parenteral nutrition. Although these infections are severe and are associated with a high mortality rate, recent advances in imaging have facilitated a prompt diagnosis, resulting in improved eradication rates. A multidisciplinary approach with the collaboration of neonatology, interventional radiology, and surgery experts is necessary to provide the best available care for this vulnerable population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13010167/s1. Reference [46] has cited in Supplementary Materials.
Author Contributions
R.S., P.K.T. and A.E.T. conceptualized the review. R.S., P.K.T., D.P., S.B., D.H., A.K., K.A.T., G.K.N., N.I., A.G.V., A.G.T. and A.E.T. conducted the review with P.K.T., A.G.T. and R.S. who wrote the first draft of the article. A.G.T., A.E.T., S.B., D.P., A.K., G.K.N. and N.I. reviewed all versions of the article and contributed to the interpretation and the structure of the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pammi, M. Epidemiology and Risk Factors for Candida Infection in Neonates. Available online: https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-candida-infection-in-neonates?sectionName=RISK%20FACTORS%20FOR%20INVASIVE%20CANDIDIASIS&search=neonate%20fungal&topicRef=5024&anchor=H8&source=see_link# (accessed on 9 November 2022).
- Calley, J.L.; Warris, A. Recognition and diagnosis of invasive fungal infections in neonates. J. Infect. 2017, 74 (Suppl. S1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Benjamin, D.K., Jr.; Smith, P.B. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clin. Perinatol. 2015, 42, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.W.; Sriram, B.; Tan-Kendrick, A.P.; Rajadurai, V.S. Neonatal hepatic abscess in preterm infants: A rare entity? Ann. Acad. Med. Singap. 2005, 34, 558–564. [Google Scholar] [PubMed]
- Bosnalı, O.; Moralıoğlu, S.; Cerrah Celayir, A.; Pektaş, O. Liver abscess: Increasing occurrence in premature newborns. J. Neonatal Surg. 2013, 2, 23. [Google Scholar] [CrossRef]
- Geetha, O.; Cherie, C.; Natalie, T.W.H.; Merchant, K.; Chien, C.M.; Chandran, S. Streptococcus gallolyticus subspecies pasteurianus causing early onset neonatal sepsis complicated by solitary liver abscess in a preterm infant. Access Microbiol. 2021, 3, 000200. [Google Scholar] [CrossRef]
- Semerci, S.Y.; Babayigit, A.; Cebeci, B.; Buyukkale, G.; Cetinkaya, M. Hepatic Abscesses in Preterm Infants: Report of Three Cases and Review of the Literature. J. Trop. Pediatr. 2016, 62, 255–260. [Google Scholar] [CrossRef]
- Sajjad Ur, R.; Abid, G.; Muhammad Hasan, A.; Abdullah, J. Liver Abscess in a Term Baby: A Case Report and Review of Literature. Dr. Sulaiman Al Habib Med. J. 2019, 1, 27–29. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- Noyola, D.E.; Fernandez, M.; Moylett, E.H.; Baker, C.J. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin. Infect. Dis. 2001, 32, 1018–1023. [Google Scholar] [CrossRef]
- Cascio, A.; Pantaleo, D.; Corona, G.; Barberi, G.; Delfino, D.; Romeo, O.; Iaria, C.; Barberi, I. Neonatal liver abscesses associated with candidemia: Three cases and review of literature. J. Matern. Fetal Neonatal. Med. 2014, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdeljelil, J.; Saghrouni, F.; Nouri, S.; Geith, S.; Khammari, I.; Fathallah, A.; Sboui, H.; Ben Saïd, M. Neonatal invasive candidiasis in Tunisian hospital: Incidence, risk factors, distribution of species and antifungal susceptibility. Mycoses 2012, 55, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Picone, S.; Manzoni, P.; Bedetta, M.; Mostert, M.; Benjamin, D.K., Jr.; Paolillo, P. Pharmacological resolution of a multiloculated Candida spp. liver abscess in a preterm neonate. Early Hum. Dev. 2013, 89 (Suppl. S1), S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Choudhary, M.; Shastri, S.; Sharma, P.K. Neonatal liver abscesses due to Candida infection in a preterm infant, secondary to malpositioned umbilical lines--a rare entity. Pathog. Glob. Health 2015, 109, 84–87. [Google Scholar] [CrossRef]
- Auriti, C.; Ronchetti, M.P.; Bersani, I.; Gennari, F.; Piersigilli, F. Intrahepatic Administration of Liposomal Amphotericin B (Ambisome) for the Management of a Liver Abscess from Candida albicans in a Preterm Infant. Antimicrob. Agents Chemother. 2018, 62, e01239-18. [Google Scholar] [CrossRef]
- Filippi, L.; Poggi, C.; Gozzini, E.; Meleleo, R.; Mirabile, L.; Fiorini, P. Neonatal liver abscesses due to Candida infection effectively treated with caspofungin. Acta Paediatr. 2009, 98, 906–909. [Google Scholar] [CrossRef]
- Cantey, J.B.; Dallas, S.D.; Cigarroa, F.G.; Quinn, A.F. Malassezia Hepatic Abscess in a Neonate. Pediatr. Infect. Dis. J. 2020, 39, 1043–1044. [Google Scholar] [CrossRef]
- Doerr, C.A.; Demmler, G.J.; Garcia-Prats, J.A.; Brandt, M.L. Solitary pyogenic liver abscess in neonates: Report of three cases and review of the literature. Pediatr. Infect. Dis. J. 1994, 13, 64–69. [Google Scholar] [CrossRef]
- Fridkin, S.K.; Kaufman, D.; Edwards, J.R.; Shetty, S.; Horan, T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 19952004. Pediatrics 2006, 117, 1680–1687. [Google Scholar] [CrossRef]
- Alvarado-Socarras, J.L.; Vargas-Soler, J.A.; Franco-Paredes, C.; Villegas-Lamus, K.C.; Rojas-Torres, J.P.; Rodriguez-Morales, A.J. A Cluster of Neonatal Infections Caused by Candida auris at a Large Referral Center in Colombia. J. Pediatr. Infect. Dis. Soc. 2021, 10, 549–555. [Google Scholar] [CrossRef]
- Leonart, L.P.; Tonin, F.S.; Ferreira, V.L.; Tavares da Silva Penteado, S.; de Araújo Motta, F.; Pontarolo, R. Fluconazole Doses Used for Prophylaxis of Invasive Fungal Infection in Neonatal Intensive Care Units: A Network Meta-Analysis. J. Pediatr. 2017, 185, 129–135.e6. [Google Scholar] [CrossRef]
- Clerihew, L.; Lamagni, T.L.; Brocklehurst, P.; McGuire, W. Invasive fungal infection in very low birthweight infants: National prospective surveillance study. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F188–F192. [Google Scholar] [CrossRef]
- Benjamin, D.K., Jr.; Stoll, B.J.; Fanaroff, A.A.; McDonald, S.A.; Oh, W.; Higgins, R.D.; Duara, S.; Poole, K.; Laptook, A.; Goldberg, R. Neonatal candidiasis among extremely low birth weight infants: Risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006, 117, 84–92. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef]
- Manzoni, P.; Farina, D.; Leonessa, M.; d’Oulx, E.A.; Galletto, P.; Mostert, M.; Miniero, R.; Gomirato, G. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics 2006, 118, 2359–2364. [Google Scholar] [CrossRef]
- Rundjan, L.; Wahyuningsih, R.; Oeswadi, C.A.; Marsogi, M.; Purnamasari, A. Oral nystatin prophylaxis to prevent systemic fungal infection in very low birth weight preterm infants: A randomized controlled trial. BMC Pediatr. 2020, 20, 170. [Google Scholar] [CrossRef]
- Sherman, M.P. New concepts of microbial translocation in the neonatal intestine: Mechanisms and prevention. Clin. Perinatol. 2010, 37, 565–579. [Google Scholar] [CrossRef]
- Filioti, J.; Spiroglou, K.; Roilides, E. Invasive candidiasis in pediatric intensive care patients: Epidemiology, risk factors, management, and outcome. Intensive Care Med. 2007, 33, 1272–1283. [Google Scholar] [CrossRef]
- Barton, M.; O’Brien, K.; Robinson, J.L.; Davies, D.H.; Simpson, K.; Asztalos, E.; Langley, J.M.; Le Saux, N.; Sauve, R.; Synnes, A.; et al. Invasive candidiasis in low birth weight preterm infants: Risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infect. Dis. 2014, 14, 327. [Google Scholar] [CrossRef]
- Lam, H.S.; Li, A.M.; Chu, W.C.; Yeung, C.K.; Fok, T.F.; Ng, P.C. Mal-positioned umbilical venous catheter causing liver abscess in a preterm infant. Biol. Neonate 2005, 88, 54–56. [Google Scholar] [CrossRef]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Markou, E.; Zarokostas, K.; Sokou, R.; Trikoupis, I.; Mavrogenis, A.F.; Houhoula, D.; Piovani, D.; Bonovas, S.; et al. Aspergillus spp. osteoarticular infections: An updated systematic review on the diagnosis, treatment and outcomes of 186 confirmed cases. Med. Mycol. 2022, 60, myac052. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K., Jr.; Poole, C.; Steinbach, W.J.; Rowen, J.L.; Walsh, T.J. Neonatal candidemia and end-organ damage: A critical appraisal of the literature using meta-analytic techniques. Pediatrics 2003, 112, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kanner, R.; Weinfeld, A.; Tedesco, F.J. Hepatic abscess-plain film findings as an early aid to diagnosis. Am. J. Gastroenterol. 1979, 71, 432–437. [Google Scholar] [PubMed]
- Di Serafino, M.; Severino, R.; Gioioso, M.; Rossi, E.; Vezzali, N.; Pelliccia, P.; Caprio, M.G.; Acampora, C.; Iorio, R.; Vallone, G. Paediatric liver ultrasound: A pictorial essay. J. Ultrasound 2020, 23, 87–103. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, E.M.; Torres, U.S.; Racy, D.J.; Torres, L.R.; Chojniak, R.; D’Ippolito, G. The “streamline phenomenon” of the portal vein flow and its influence on liver involvement by gastrointestinal diseases: Current concepts and imaging-based review. Abdom. Radiol. 2020, 45, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Chowdhary, S.; Kumar, A.; Ali, M.A. Solitary sterile neonatal liver abscess in an infant. Int. J. Contemp. Pediatr. 2014, 1, 187–189. [Google Scholar]
- Bersani, I.; Piersigilli, F.; Goffredo, B.M.; Santisi, A.; Cairoli, S.; Ronchetti, M.P.; Auriti, C. Antifungal Drugs for Invasive Candida Infections (ICI) in Neonates: Future Perspectives. Front. Pediatr. 2019, 7, 375. [Google Scholar] [CrossRef]
- Turner, K.; Manzoni, P.; Benjamin, D.K.; Cohen-Wolkowiez, M.; Smith, P.B.; Laughon, M.M. Fluconazole pharmacokinetics and safety in premature infants. Curr. Med. Chem. 2012, 19, 4617–4620. [Google Scholar] [CrossRef]
- Natarajan, G.; Lulic-Botica, M.; Aranda, J.V. Refractory neonatal candidemia and high-dose micafungin pharmacotherapy. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2009, 29, 738–743. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Frías-De-León, M.G.; Hernández-Castro, R.; Vite-Garín, T.; Arenas, R.; Bonifaz, A.; Castañón-Olivares, L.; Acosta-Altamirano, G.; Martínez-Herrera, E. Antifungal Resistance in Candida auris: Molecular Determinants. Antibiotics 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Fly, J.H.; Kapoor, S.; Bobo, K.; Stultz, J.S. Updates in the Pharmacologic Prophylaxis and Treatment of Invasive Candidiasis in the Pediatric and Neonatal Intensive Care Units: Updates in the Pharmacologic Prophylaxis. Curr. Treat. Options Infect. Dis. 2022, 14, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Ishiwada, N.; Kitajima, H.; Morioka, I.; Takeuchi, N.; Endo, M.; Watanabe, A.; Kamei, K. Nationwide survey of neonatal invasive fungal infection in Japan. Med. Mycol. 2017, 56, 679–686. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).