Abstract
This paper is devoted to the study of the flora of silica-scaled chrysophytes in water bodies of the steppe zone of the Southern Urals (Russia). Twenty-four taxa were identified via scanning and transmission electron microscopy, twenty of which are representatives of the genus Mallomonas Perty, while four are species of the genus Synura Ehrenberg. In the course of the study, a species new to science from the genus Mallomonas, M. baturinae sp. nov., was described. This species belongs to the section Papillosae. The stomatocyst of M. doignonii was described. For the first time in Russia and for the third time since description, M. phasma and M. solea-ferrea var. irregularis were reported in the studied area. Here, their extended description is provided with illustrations of their scales in detail. Some rare taxa for the flora of Russia have been identified: M. doignonii, M. pillula f. exannulata, and M. pillula f. valdiviana. One taxon of the genus Mallomonas has not been identified to a species level and is probably a taxon new to science.
1. Introduction
Chrysophytes are a large group with more than 1200 described species in about 112 genera. They are widespread, predominantly freshwater planktonic protists, diverse in morphology [1]. Chrysophytes are unicellular or colonial algae characterized by heterokont flagella and chloroplasts with chlorophyll a and c, and by their endogenous silicified stomatocysts [1]. A special group of these consists of species whose cells are covered with siliceous structures (scales, bristles, spines). These organisms are represented in different phylogenetic lineages of chrysophytes from the orders Paraphysomonadales, Chromulinales, and Synurales [2]. The order Synurales is the most diverse, including more than 220 species of the genus Mallomonas [3,4] and more than 50 species of the genus Synura [5]. Identification of silica-scaled chrysophytes is based on the study of the ultrastructure of scales and bristles using scanning and transmission electron microscopy (SEM and TEM, respectively). For this group, a species concept based on the ultrastructural features of the silica structures is well-developed, which is in good agreement with molecular data [2,6,7]. This allows the use of chrysophyte algae with silica covers in paleoecological, biogeographical, and monitoring studies [8,9,10].
Silica-scaled chrysophytes of Russia have been studied very unevenly. The first studies of silica-scaled chrysophytes in Russia using electron microscopy began in the second half of the XXth century [11,12,13]. The flora of silica-scaled chrysophytes in Russia today remains insufficiently studied. Using the example of the genus Mallomonas, well studied throughout the world, in the territory of such a vast region as Russia, only 91 species and infraspecific taxa have been identified [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], although the climatic, geographical, and natural diversity of Russia suggests that biodiversity indicators may be significantly higher. For comparison, we can point out that in Europe, which is much smaller in area, 114 species and infraspecific taxa of the genus Mallomonas are known [47]. Almost every study of a new region in Russia leads to the discovery of rare species and taxa new to science or the flora of the country [36,39,40,41,42,43,44,48].
Initially, studies of silica-scaled chrysophytes primarily covered the territory of the central regions of the European part of Russia (reservoirs of the Volga basin) [11,12,13,22]. Until now, most of the work has been carried out in the central and northern regions of European Russia [20,21,22,23,24,25,26,27,28,29,49]. A number of interesting works have been published on the study of rivers, reservoirs, lakes, and swamps in Eastern Siberia, most of which have been carried out in the last decade [30,31,32,33,34,40]. The Ural region and Western Siberia have been studied to a much lesser extent [19,27,35,36,37,39]. However, studies of silica-scaled chrysophytes of the Polar Urals revealed a high diversity and abundant flora, including taxa new to science and to the flora of Russia [27,35,36]. The first data of silica-scaled chrysophytes of the Southern Urals, obtained using electron microscopy, are given in the studies of L.V. Snitko et al. [38,50,51,52,53,54]. However, these results cover only a part of this region excluding the steppe area.
It should be noted that the most important distinguishing feature of climate of the steppe is the limited precipitation. With a precipitation deficit, the importance of water bodies of different origin for the ecosystem increases. In the conditions of the steppe zone, they play a special role, forming the cores of biodiversity concentration [55,56,57].
The strong degradation of steppes as a result of anthropogenic human activity (ploughing, grazing, use of steppe reservoirs for irrigation), which is currently occurring, is the cause of the loss of steppe biodiversity in general, and the biodiversity of water ecosystems in particular [55,56,57]. In this situation, it is necessary to create protected natural areas for the conservation and study of biodiversity.
To date, the flora comprising the silica-scaled chrysophytes in water bodies of the protected natural areas of the Southern Urals, which are not subjected to serious anthropogenic pressure, remains completely unstudied. Numerous studies of chrysophytes from different regions, such as North America [58,59,60], Europe [61,62], Asia [63,64,65,66,67], and Russia [23,24,25,29], have shown that water bodies of the protected areas have a rich and distinctive flora and often include species new to science. The first studies of the protected natural areas of the Southern Urals identified 32 morphotypes of chrysophycean stomatocysts in the flora of Lake Zhurmankol, located on the territory of the Ashchisai Steppe site of the “Orenburgskii” State Nature Reserve, which indicates a wide variety of golden algae in this water body [68,69,70]. In addition, Mallomonas rasilis Dürrschmidt, a new species of chrysophytes for the flora of Russia, was also recorded in Lake Zhurmankol [14]. The data obtained served as a prerequisite for further research on the study of the diversity of the flora of chrysophytes in the natural protected areas of the Southern Urals.
The purpose of this work was to study the diversity of silica-scaled chrysophytes in water bodies of natural protected areas of the steppe zone of the Southern Urals using scanning and transmission electron microscopy.
2. Materials and Methods
The material for the study comprised integrated samples (plankton, epipelon, and epilithon) taken during the open water period from four reservoirs located in the territory of the Aschisai Steppe site (“Orenburgskii” State Nature Reserve, Southern Urals, Russia) and the territory of the “Svetlinskii” Biological Reserve (Southern Urals, Russia) (Figure 1 and Table 1). These reservoirs are small and shallow. Their filling occurs mainly in the spring due to snowmelt; they are fed by rain, and there is no recharge by ground and spring waters [71]. Water replenishment varies greatly over the years and seasons, with some reservoirs completely drying up (lakes Nezametnoe, Zhurmankol, Liman).

Table 1.
List of the sampling sites with environmental variables (T–temperature, S–salinity).
Sampling was carried out in May 2020, April–May 2022, and May 2023. Sampling was carried out in the spring (April, May), since it is known that in temperate latitudes the greatest diversity of chrysophytes is observed in the spring [1,72,73]. In addition, the specified timing of sampling is determined by the temporary boundaries of the existence of the studied reservoirs–reservoirs are ephemeral, drying up quickly. Samples were fixed with a 40% formaldehyde solution and concentrated via the sedimentation method. Water temperature and pH were measured using a portable pH/°C analyzer HI98127 (Hanna Instruments, Inc., Woonsocket, RI, USA), and salinity was measured using an ANION 4100 laboratory analyzer (Infraspak-Analyte, Novosibirsk, Russia).
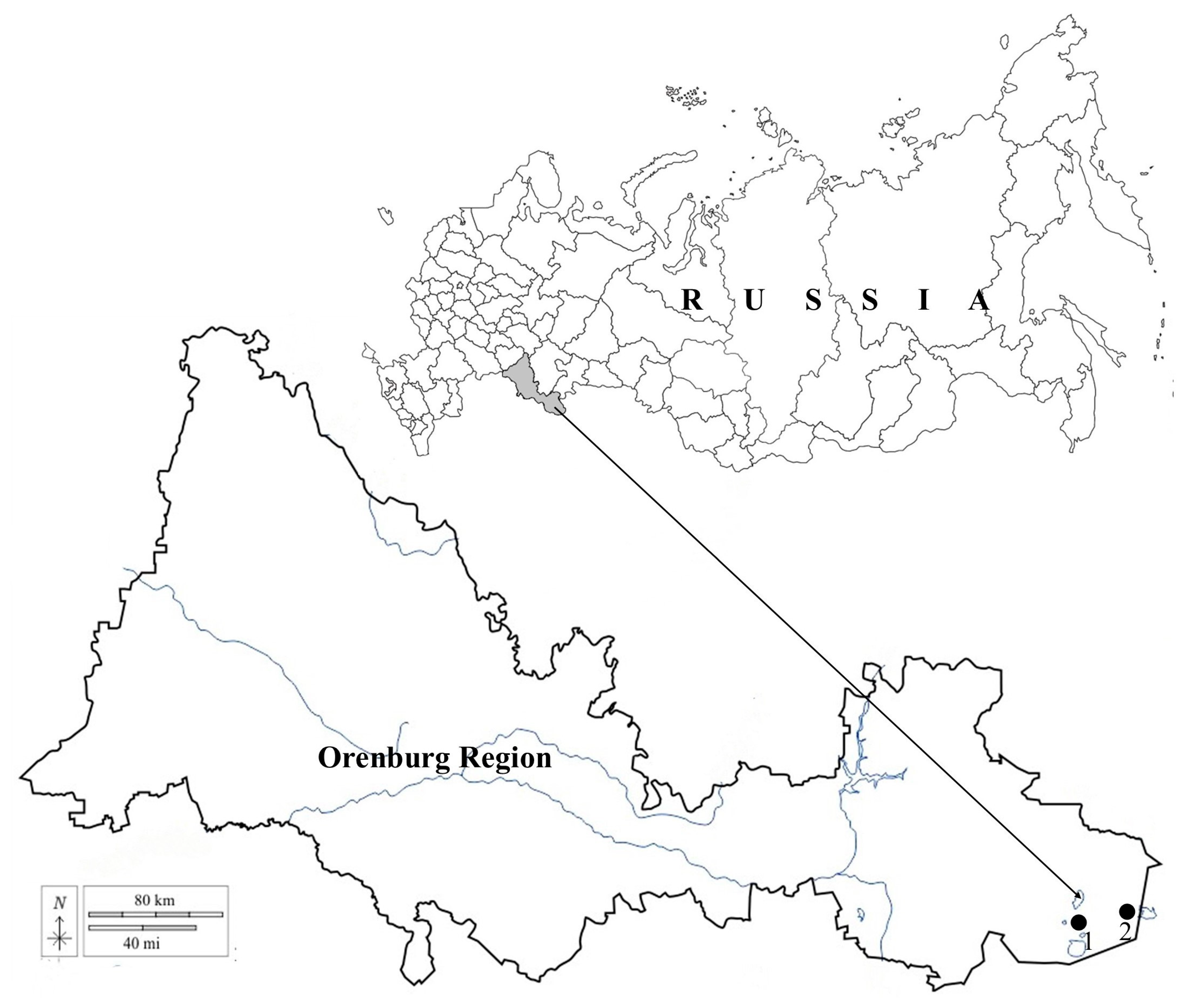
Figure 1.
Schematic map of the study area: (1) “Svetlinskii” Biological Reserve, (2) “Orenburgskii” State Nature Reserve.
Figure 1.
Schematic map of the study area: (1) “Svetlinskii” Biological Reserve, (2) “Orenburgskii” State Nature Reserve.
The study of the species composition of silica-scaled chrysophytes was carried out using scanning electron microscopy (SEM) on a Tescan Mira3 microscope (Tescan Brno, s.r.o, Brno, Czech Republic) at the Gagarin Center for the Identification and Support of Talented Children, (Orenburgskaya oblast) and also using transmission electron microscopy (TEM) on a JEM-1011 transmission electron microscope in the Center of Electron Microscopy at Papanin’s Institute for Biology of Inland Waters, RAS. An aliquot of a sample was washed from the fixative with distilled water via repeated centrifugation (5 min at 3000 rpm) using a Microspin 12 centrifuge (BioSan, Riga, Latvija), then applied to SEM stubs, dried at room temperature, and sputtered with gold using an ion-plasma sputtering system (Quorum Q150R S plus; Quorum Technologies Ltd., London, UK). For studies with the transmission electron microscope (TEM), formvar-coated grids (EMS FF200-Cu-50, Electron Microscopy Sciences, Hatfield, PA, USA) were used.
The description of the stomatocyst Mallomonas doignonii Bourrelly was carried out in accordance with the rules of the international working group ISWG (International Statospore Working Group) [74].
Conducting research on the territory of the “Orenburgskii” State Nature Reserve and the territory of the “Svetlinskii” Biological Reserve was agreed upon with the Government of the Orenburg Region and the “Directorate of Specially Protected Natural Territories of the Orenburg Region”.
3. Results
As a result of the studies, 24 taxa of silica-scaled chrysophytes were identified (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 and Table 2). The representatives of the genus Mallomonas Perty were the most numerous (20 taxa), while four taxa belonged to the genus Synura Ehrenberg. Twelve taxa were recorded for the first time in the territory of the Southern Urals, including two new species for Russia. One taxon, of the genus Mallomonas, is a species new to science. Its description is given below. We could not identify one more taxon of the genus Mallomonas to the species level. Presumably, this will also be a new species for science, but additional studies are needed to describe it.
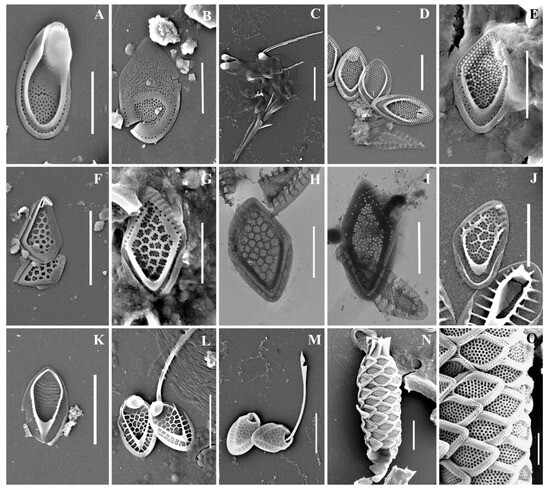
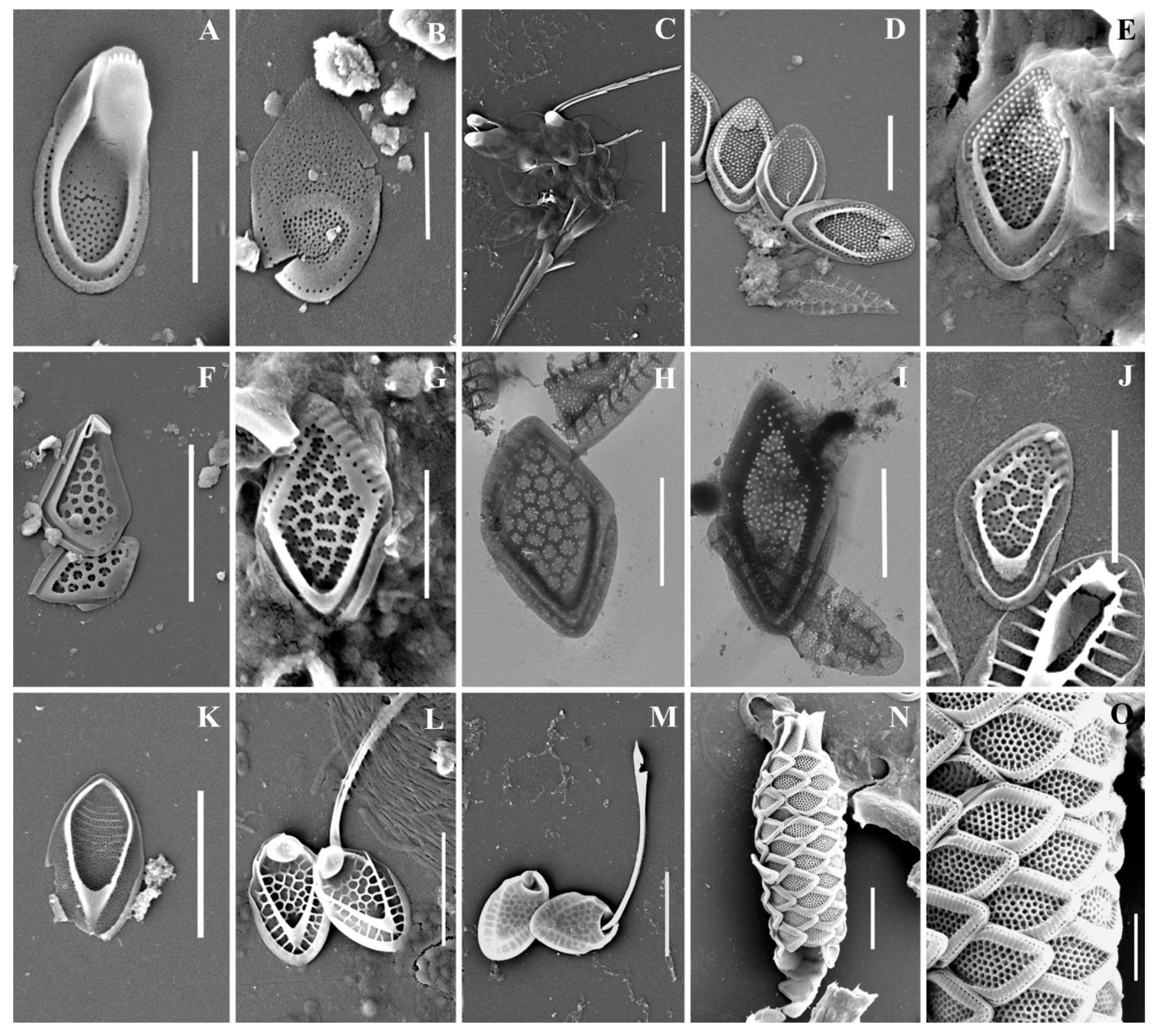
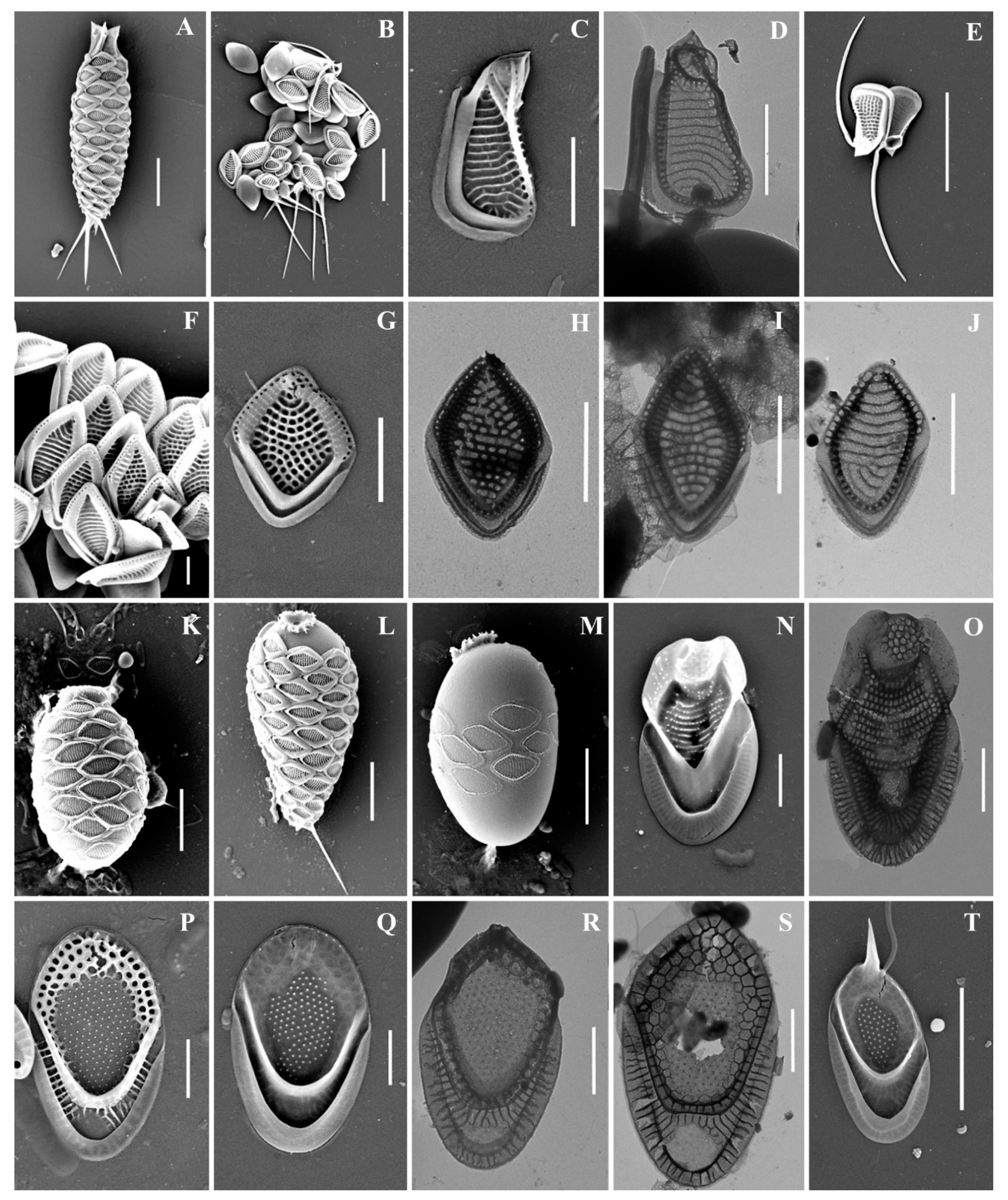
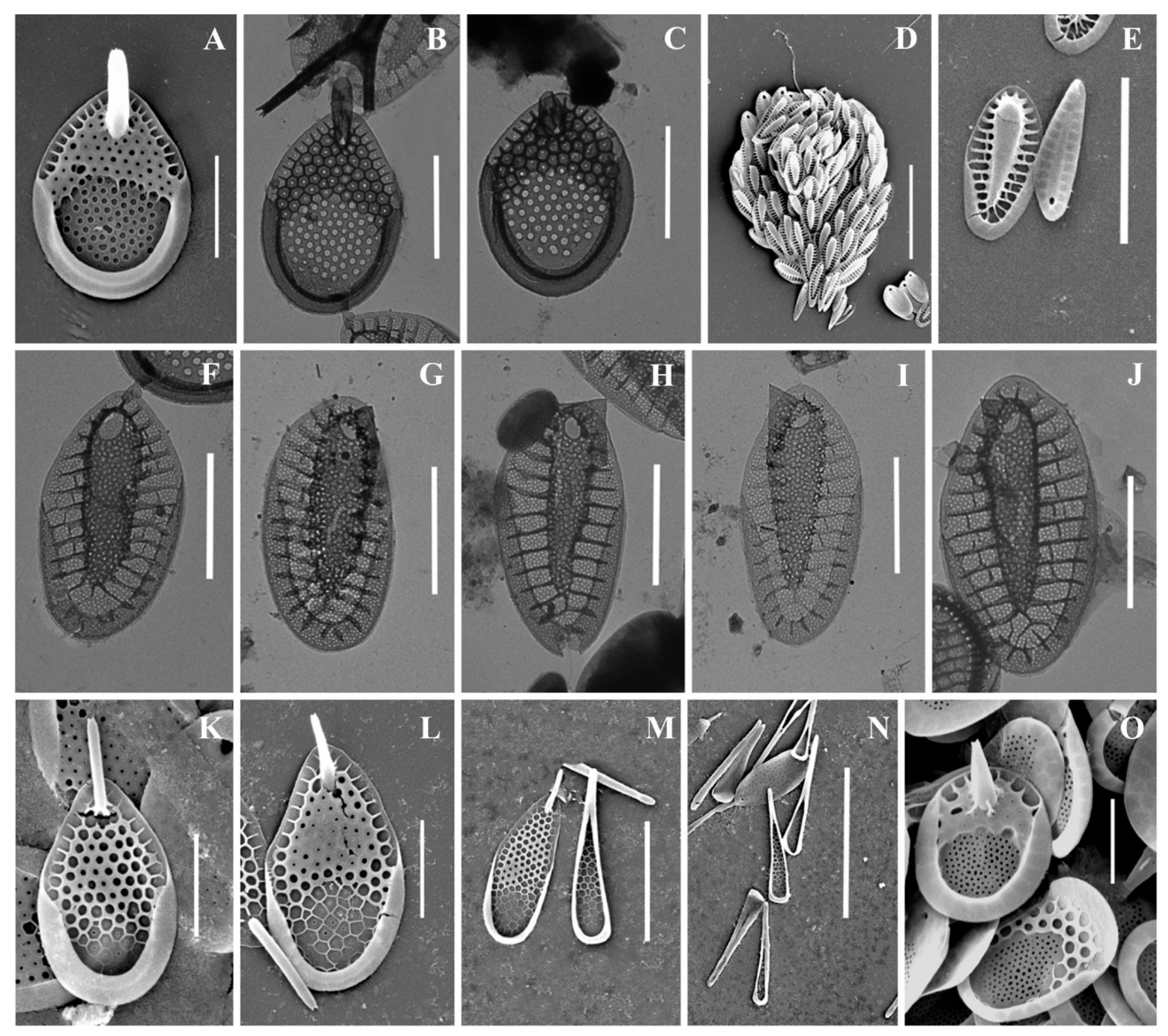
Figure 2.
Mallomonas taxa from Southern Urals, Russia: (A–C) M. akrokomos, (A) apical scale, (B) body scale, (C) whole cell; (D,E) M. annulata; (F–J) M. alata, (F) apical scales, (G–I) body scales, (J) caudal scale; (K) M. costata; (L,M) M. crassisquama; (N,O) M. eoa. Scale bars: (C,F,K–N): 5 µm, (A,B,D,E,G–J,O): 2 µm. SEM: (A–G,J–O). TEM: (H,I).
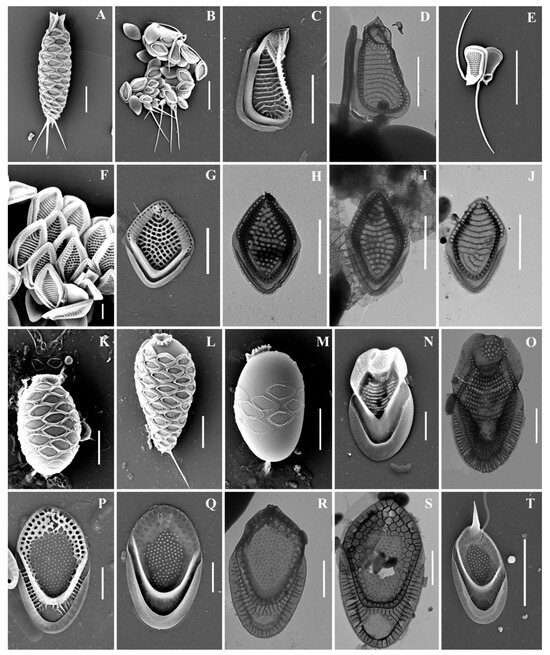
Figure 3.
Mallomonas taxa from Southern Urals, Russia: (A–M) M. doignonii, (A) whole cell, (B) different types of scales, (C–E) collar scales, (F–J) body scales, (K–M) stomatocyst 7 Ignatenko et Yatsenko-Stepanova; (N,O) M. lelymene; (P–T) M. insignis, (P–S) body scales, (T) apical scale. Scale bars: (A,B,E,K,L): 5 µm, (C,D,G–J,M–T): 2 µm, (F): 1 µm. SEM: (A–C,E–G,K–N,P,Q,T); TEM: (D,H–J,O,R,S).
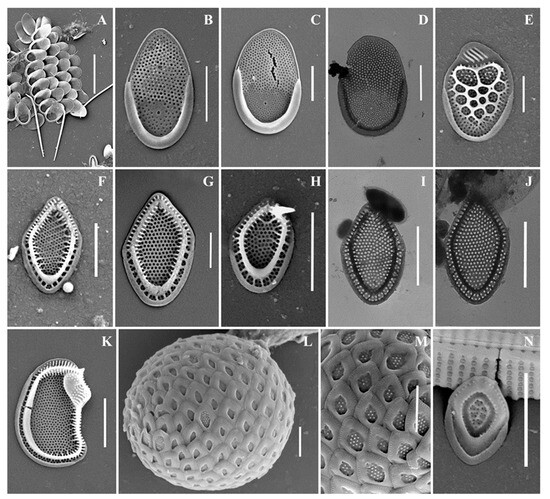
Figure 4.
Mallomonas taxa from Southern Urals, Russia: (A–D) M. matvienkoae, (A) scales with bristles, (B–D) body scales; (E) M. multiunca; (F–K) M. phasma, (F,G,I,J) body scales, (K) collar scale, (H) rear scale, one of the anterior submarginal ribs terminates in a short spine; (L,M) M. pillula f. exannulata; (N) M. pillula f. valdiviana. Scale bars: (A): 10 µm, (B–D,F,H–N): 2 µm, (E,G): 1 µm. SEM: (A–C,E–H,K–N); TEM: (D,I,J).
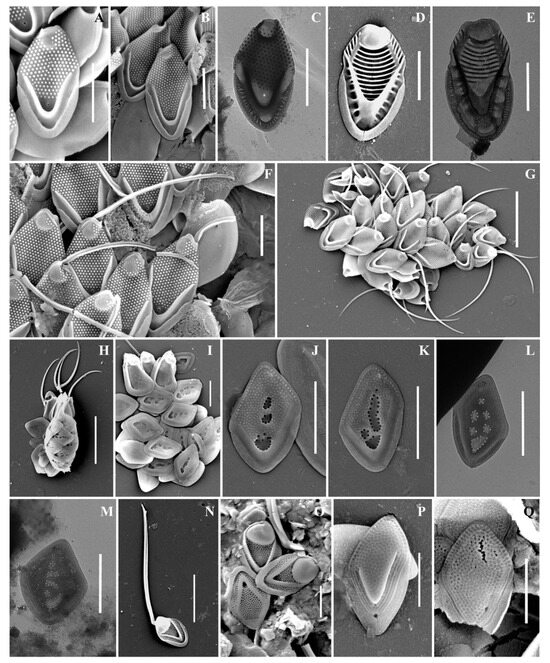
Figure 5.
Mallomonas taxa from Southern Urals, Russia: (A–C,F,G) M. rasilis, (A–C) body scales, (F,G) scales with bristles; (D,E) M. striata; (H–M) M. solea-ferrea var. irregularis, (H) whole cell, (I) collar and body scales, (J–M) body scales; (N,O) M. tonsurata; (P,Q) Mallomonas sp. Scale bars: (G,H,N): 5 µm, (A–F,J–M,O–Q): 2 µm. SEM: (A,B,D,F–K,N–Q); TEM: (C,E,L,M).
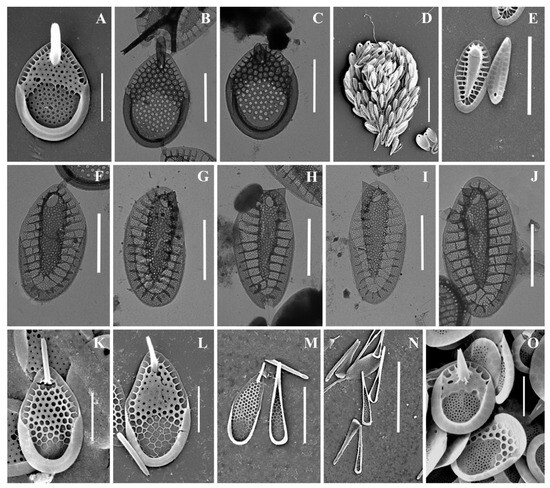
Figure 6.
Synura taxa from Southern Urals, Russia: (A–C) S. curtispina; (D–J) S. petersenii sensu lato, (D) whole cell, (E) body and caudal scales, (F–J) body scales; (K–N) S. mollispina, (K,L) body scales, (M,N) caudal scales. (O) S. uvella. Scale bars: (D,N): 10 µm, (E,M): 5 µm, (A–C,F–L,O): 2 µm. SEM: (A,D,E,K–O); TEM: (B,C,F–J).

Table 2.
List of silica-scaled chrysophytes (Chrysophyceae, Synurales) found in water bodies of natural protected areas of the steppe zone of the Southern Urals, Russia (bold type indicates new taxa for the flora of the Southern Urals; “+”/“–” presence/absence of the species in the studied water body).
Description of the new species.
Description:
Stramenopiles Patterson
Chrysophyceae Pascher
Synurales Andersen
Mallomonadaceae Diesing
Mallomonas Perty
Mallomonas baturinae sp. nov. Ignatenko, Gusev & Yatsenko-Stepanova (Figure 7A–L).
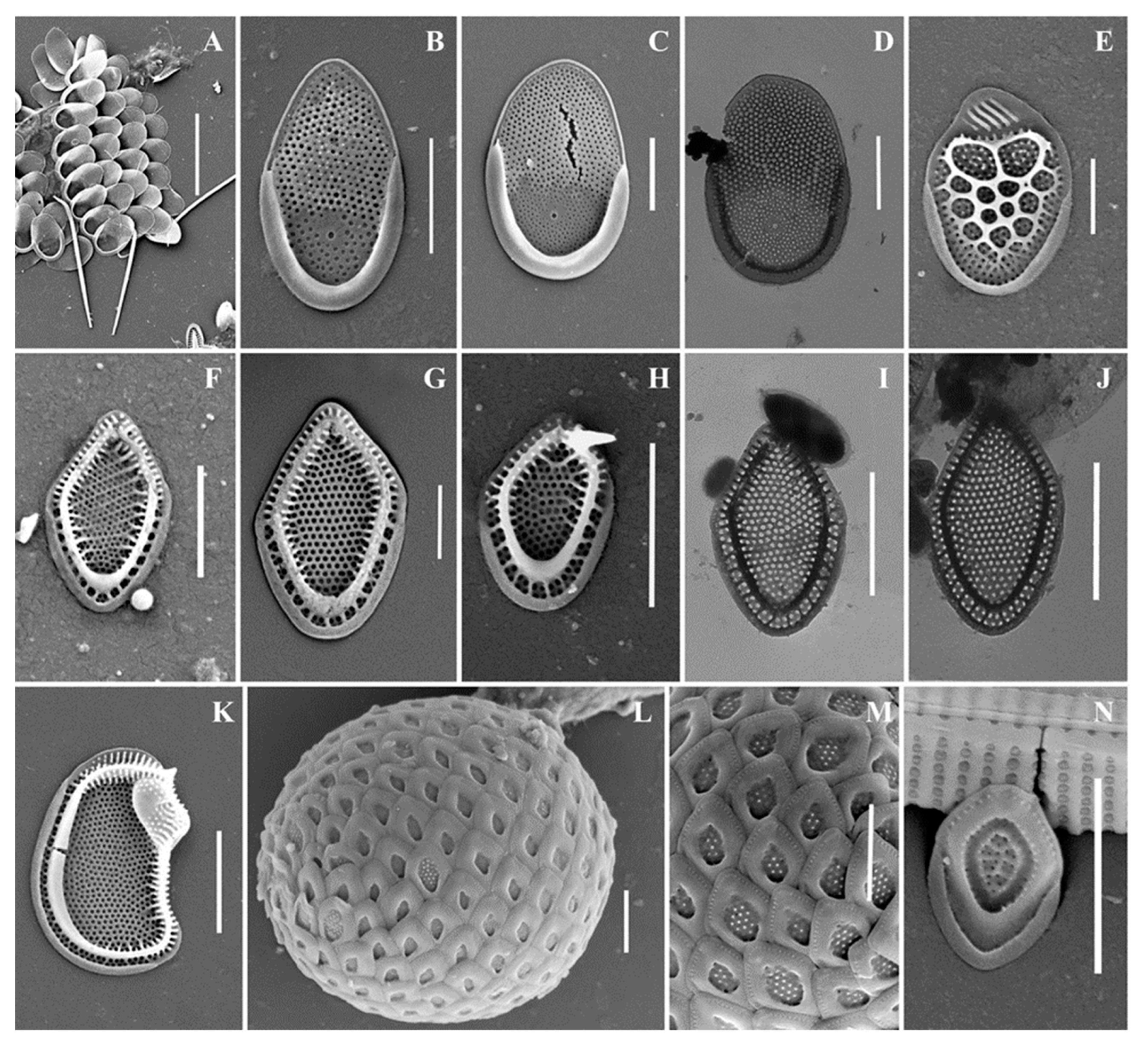
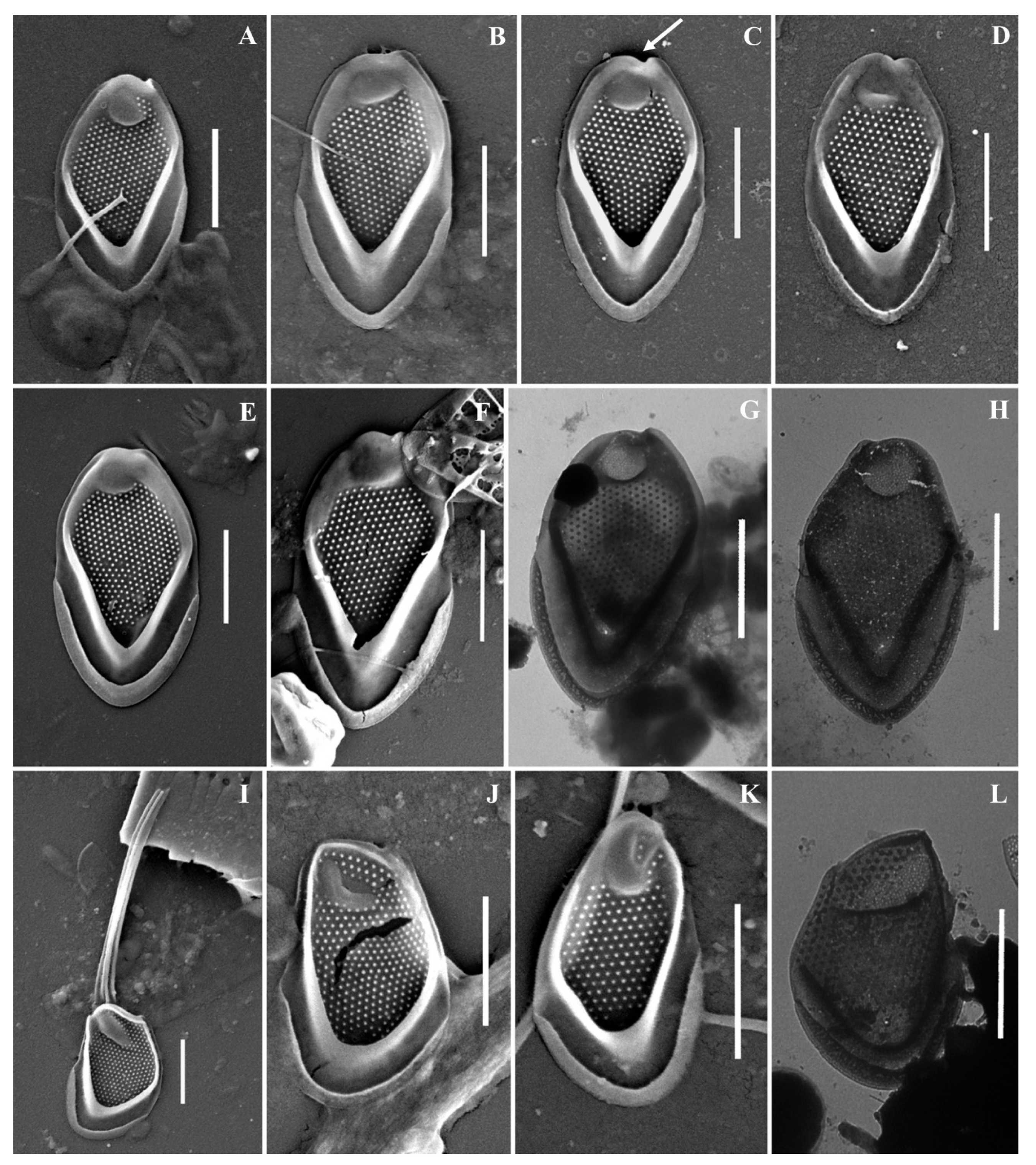
Cell dimensions unknown. The body scales are oval, often slightly asymmetrical, 4.6–6.2 × 2.5–3.3 μm, tripartite, with a dome, a V-rib, anterior submarginal ribs, and posterior rim (Figure 7A–H). The anterior submarginal rib and the anterior flange on the right side of the scale form a protrusion that extends further than the central part of the distal end. As a result, at the distal margin there is a notch at the right side of the scale (Figure 7C,D). The dome is circular, occasionally oval, smooth, and surrounded by the arms of the anterior submarginal ribs or joined with them. The shield has densely and regularly spaced papillae. A distinct, rimmed base-plate pore is situated in the proximal area of the shield at the base of the V-rib (Figure 7A,E). The V-rib is conspicuous with an acute angle, and slightly hooded. The distal ends of the arms of the V-rib become continuous with the anterior submarginal ribs. The anterior submarginal ribs are well-developed, wide, and raised above the shield. Anterior flanges are narrow and smooth. The posterior flange is smooth. The posterior rim is narrow and smooth. Apical scales are smaller than the body scales, asymmetrical with an elongated dome, 4.1–4.3 × 2.6–2.9 μm (Figure 7I–L). The bristle is 7.6 μm long, slightly curved, with longitudinal ribs and a tooth at the apex (Figure 7I). Cysts were not observed.
Holotype specimen: Portion of a single gathering of cells on SEM stub number 53_I_1 deposited at the Herbarium of the Steppe Institute of the Ural Branch of the Russian Academy of Sciences, Orenburg (ORIS). Material is from the Lake Nezametnoe sampled by M.E. Ignatenko on 29 April 2022. Figure 7D is a representative scale from the type specimen.
Type locality: RUSSIA. Orenburg Region: Lake Nezametnoe, Ashchisai steppe, “Orenburgskii” State Nature Reserve, 51°01′20.7″ N, 61°13′29.9″ E, 29 April 2022.
Etymology: The species is named in honor of Vera Nikolaevna Baturina (1927–2007) for her contribution to the study of the algal flora of the Ural.
Distribution and habitat: This species was found at the type locality and Lake Liman (51°02′19″ N 60°47′09″ E). Mallomonas baturinae was found at pH ranged from 6.5 to 8.6 and temperature 11.8–14.8 °C (Table 1 and Table 2).
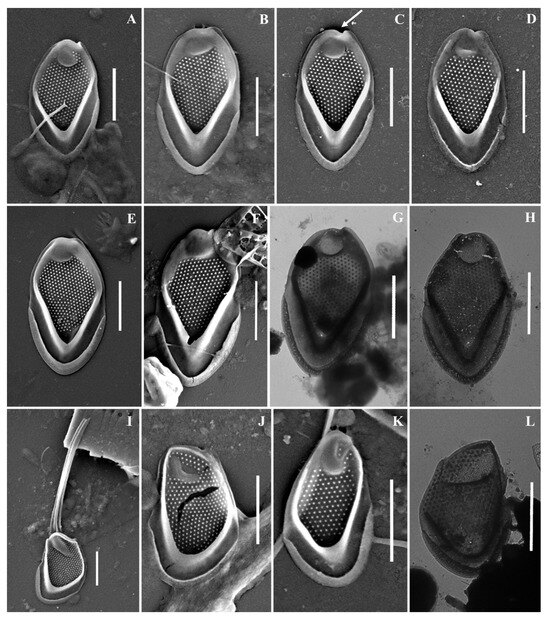
Figure 7.
Mallomonas baturinae sp. nov.: (A–H) body scales, (I–L) apical scales. The arrow marks the notch at the distal end of the scale. Scale bars: 2 µm. SEM: (A–F,I–K); TEM: (G,H,L).
Figure 7.
Mallomonas baturinae sp. nov.: (A–H) body scales, (I–L) apical scales. The arrow marks the notch at the distal end of the scale. Scale bars: 2 µm. SEM: (A–F,I–K); TEM: (G,H,L).
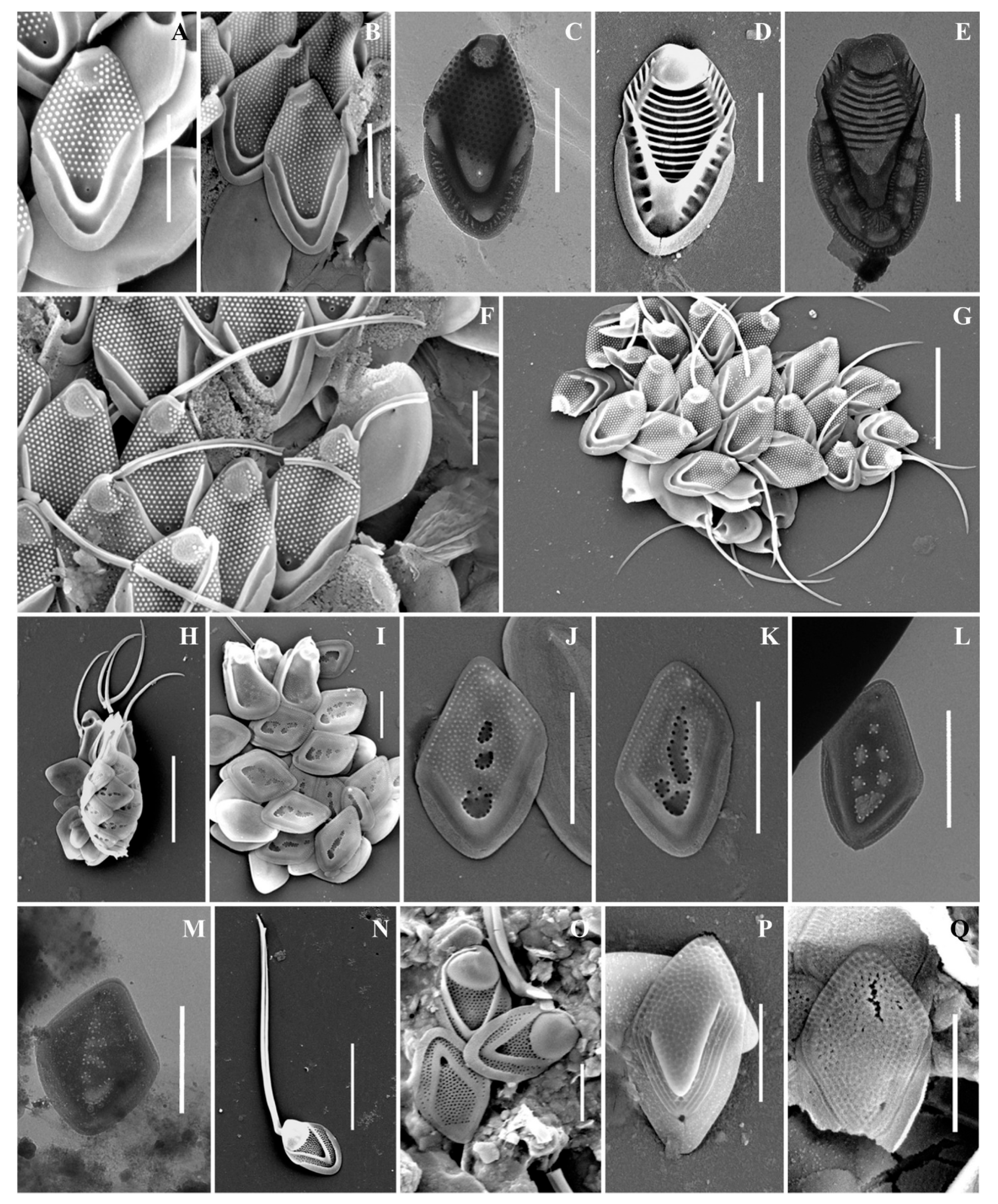
During the research, a cyst of Mallomonas doignonii was also discovered for the first time. We provide a formal description below.
Stomatocyst 7 Ignatenko & Yatsenko-Stepanova, this paper (Figure 3K–M).
SEM description: The stomatocyst is oval (13.5–13.7 × 8.8–9.3 μm) with an unornamented surface. The collar is obliquely conical; the basal diameter of the collar is 2.6–2.8 µm. The collar may be somewhat displaced relative to the central axis of the stomatocyst. The edge of the collar consists of a series of irregularly shaped outgrowths, similar to tentacles. The pore is 0.54 µm in diameter.
Locality: RUSSIA, Orenburg region, “Orenburgskii” State Nature Reserve, Lake Nezametnoe, 30 April 2022; leg. M. Ignatenko, T. Yatsenko-Stepanova.
Picture-file number: 9_2, 29, 100.
Number of observed specimens: 3.
Biological affinity: Mallomonas doignonii.
We also found two species of the genus Mallomonas new to the flora of Russia: M. phasma and M. solea-ferrea var. irregularis. Both taxa are very rare; these discoveries are the third in the world for these species. Therefore, we provide their extended description and illustrate in detail.
Mallomonas phasma(Figure 4F–K). Three types of scales were found: collar scales (Figure 4K), body scales (Figure 4F,G,I,J), and rear scales (Figure 4H). The collar scale is asymmetrical with strongly convex dorsal margin, and measures 4.4 × 2.8 µm. The anterior submarginal rib of the collar scale on the dorsal side is widened, ending in a short spine on the edge from the side of the dome. The dome is wide, covered with ribs and papillae. The base-plate pores are evenly spaced on the shield. The posterior and anterior flanges with the base-plate pores and struts extending from the submarginal ribs both to the edges and to the shield. Body scales are rhombic, measuring 3.1–3.8 × 2.0–2.5 µm, without dome, and with well-developed anterior submarginal ribs. The anterior submarginal ribs have struts extending towards the anterior flanges and shield. The shield is covered with densely and evenly spaced base-plate pores. Sometimes there are thickenings of the secondary siliceous layer, extending from the struts and forming a network structure. The posterior flange has base-plate pores. The rear scale (2.8 × 1.9 µm) is slightly asymmetrical, characterized by the presence of a small spine formed by the continuation of the anterior submarginal rib.
Mallomonas solea-ferrea var. irregularis (Figure 5H–M). Cells are ovoid (6.8–10.5 × 3.5–5.2 µm). Bristles are smooth and slightly curved. Collar scales are trapezoid in shape with a well-developed dome, covered with small papillae, and measure 3.0–3.3 × 1.8–1.9 µm. Posterior scales measure 1.4–2.1 × 1.0–1.3 µm. Body scales are rhombic and without dome, measuring 2.9–3.1 × 1.8–2.2 µm. The anterior submarginal ribs on the body scales are not pronounced. The shield is covered with longitudinal rows of small papillae. On the surface of the shield there are irregularly shaped depressions containing pores, most often located along the edges of the depressions.
Since the chrysophytes of the studied region had been hitherto virtually unstudied, and the water bodies of the steppe zone as a whole had been poorly studied, we present a description of new findings of the class Chrysophyceae for the flora of the region with a discussion of their distribution.
Mallomonas alata (Figure 2F–J) is widespread, eurythermal, and indifferent to water acidity (pH) species [4,47]. It has been registered in North and South America, Great Britain, Europe, Asia, and Australia [4]. In the territory of Russia, it was found in water bodies of St. Petersburg and Leningradskaya Oblast [15], Nizhegorodskaya Oblast [28,49], Neneczkii Autonomous Okrug [20], Yamalo-Neneczkii Autonomous Okrug [19], the Republic of Komi [20,35], Krasnoyarskii Krai [17], and the Republic of Buryatia [32].
Mallomonas annulata (Figure 2D,E) is a widespread species recorded in North and South America, Great Britain, Europe, Asia, and Australia [4]. In the territory of Russia, the species was recorded in the water bodies of St. Petersburg and Leningradskaya Oblast [11,15,27], Yamalo-Neneczkii Autonomous Okrug [19], and the Republic of Sakha (Yakutia) [34].
Mallomonas costata (Figure 2K) is a widespread species known in North America, Great Britain, Europe, Asia, and Australia [4]. In the territory of Russia, it was recorded in the water bodies of St. Petersburg, Leningradskaya Oblast [15], Nizhegorodskaya Oblast [28,49], Neneczkii Autonomous Okrug [20], the Republic of Komi [20,35], Krasnoyarskii Krai [45], and the Republics of Buryatia [31,32] and Sakha (Yakutia) [33].
Mallomonas doignonii (Figure 3A–M) was recorded in the middle latitudes of the Northern Hemisphere: North America, European countries, and Great Britain [4]. To date, the only known discovery of M. doignonii has been in Russia, at the mouth of the River Barguzin, Republic of Buryatia [32]. We present here the second discovery of this species in the country.
Mallomonas eoa (Figure 2N,O) is a widespread species known in North and South America, Great Britain, Europe, Asia, and Australia [4]. In the territory of Russia, M. eoa was previously found in the water bodies of the Republic of Karelia [11], St. Petersburg and Leningradskaya Oblast [15], Yaroslavskaya Oblast, Nizhegorodskaya Oblast [12,13], the Republics of Buryatia [32] and Sakha (Yakutia) [18,34], Bolshezemelskaya tundra [20], Yamalo-Neneczkii Autonomous Okrug [19], and reservoirs of the Volga basin [13].
Mallomonas insignis (Figure 3P–T) is a widespread species, recorded in North America, Great Britain, Europe, Asia, and Australia [4]. On the territory of Russia, M. insignis was found in water bodies of the Yamalo-Neneczkii Autonomous Okrug [19], Yaroslavskaya Oblast [13], Nizhegorodskaya Oblast [28], Permskiy Krai [13], Krasnoyarskii Krai [17], and the Republic of Buryatia [31,32].
Mallomonas lelymene (Figure 3N,O) is a widespread species known in North and South America, Great Britain, Europe, and Asia [4]. Discoveries of M. lelymene in Russia have not been numerous. Previously, the species was identified in small water bodies in the Neneczkii Autonomous Okrug, Arkhangelskaya Oblast [20], Vladimirskaya Oblast [21], and the Republic of Sakha (Yakutia) [34].
Mallomonas pillula f. exannulata (Figure 4L,M) is known in North America and Europe [4,5]. In the territory of Russia, this species was found in two localities: the Republic of Komi [36] and the Nizhegorodskaya Oblast [28].
Mallomonas pillula f. valdiviana (Figure 4N) has been recorded in North and South America, Europe, and Asia [4,47]. In Russia, M. pillula f. valdiviana was found in the Republics of Komi [20,36] and Sakha (Yakutia) [34].
Mallomonas sp. (Figure 5P,Q). The body scales are rhomboidal and slightly asymmetrical, measuring 3.3–3.6 × 2.2–2.4 µm. The shield is patterned with a reticulated meshwork and has scattered papillae. The proximal part of the shield is raised above the posterior submarginal rib. The anterior flange is narrow with struts. The posterior submarginal rib area consists of several (usually three) fine ribs on each side. The posterior flange is smooth, and the posterior border is wide.
Synura curtispina (Figure 6A–C) is a widespread species known in North and South America, Europe, Asia, Africa, and Australia [4]. In the territory of Russia, S. curtispina was recorded in water bodies of St. Petersburg and Leningradskaya Oblast [15,26], Nizhegorodskaya Oblast [49], Neneczkii Autonomous Okrug [20], Republic of Komi [20,35], Yamalo-Neneczkii Autonomous Okrug [17], and the Republic of Sakha (Yakutia) [18,34]. Like other species of the genus, the S. curtispina morphotype forms several clades on the phylogenetic tree and requires further study [75,76].
4. Discussion
Previously, 15 taxa of Mallomonas and 13 taxa of Synura were recorded in diverse water bodies in the montane-forest zone of the eastern foothills of the Southern Urals and the steppe zone of the Southern Urals using electron microscopy [14,38,50,51,52,53,54,68]. The results obtained in this study made it possible to supplement this list with 13 new taxa, one of which is described as new to science and two (M. phasma, M. solea-ferrea var. irregularis) are the first records in Russia.
Newly described Mallomonas baturinae should be placed in the section Papillosae based on the presence of papillae on the shield and the presence of the dome on all types of scales. The main distinctive features of the newly described species are the notch at the right side of the scale on the distal margin, which is formed by the protrusion of anterior flange and submarginal rib, the wide and raised anterior submarginal ribs, and the structure of apical scales with an elongated dome. Within this large section, M. baturinae is most similar to M. paxillata (D.E. Bradley) L.S. Péterfi & Momeu. Scales of both species have a similar size range and a protrusion formed by an anterior flange and submarginal rib adjacent to the dome from one side. However, this protrusion forms a prominent and sharp tooth on the scales of M. paxillata [4,75]. The scales of M. baturinae only have a rounded protrusion on the right side, which forms a characteristic notch at the distal margin behind the dome. The second important difference between the two species is the structure of the anterior flanges and submarginal ribs. The scales of M. paxillata do not have clearly separated anterior submarginal ribs; in their place, there are elevations of the shield covered with papillae. The anterior flanges on M. paxillata scales are also covered with papillae. The scales of M. baturinae have clearly defined anterior submarginal ribs, which are wide, devoid of papillae, raised high above the shield, and surround the dome or are joined with it. Anterior flanges are narrow and smooth on M. baturinae scales. M. baturinae is somewhat similar to M. calceolus D.E. Bradley [76], M. kalinae Rezácova [77], and M. furtiva Gusev, Certnerová, Škaloudová & Škaloud [78] in having anterior submarginal ribs and smooth anterior flanges. However, the body scales of M. baturinae have the notch on the distal margin and are larger (4.6–6.2 × 2.5–3.3 μm) than those of M. calceolus (3–4 × 1–2 μm), M. kalinae (3.7–3.9 × 1.7–2.0 μm), and M. furtiva (3.6–4.3 × 2.2–2.5 μm). The scales of M. calceolus are also distinguished from M. baturinae by a lower density of papillae on the shield and much less-developed anterior submarginal ribs. M. kalinae and M. furtiva are distinguished from M. baturinae by less-developed anterior submarginal ribs on the scales and by bristle ultrastructure. M. kalinae has smooth pointed bristles and M. furtiva has serrated bristles. In both species, domes are shifted towards the distal margin of scales, while on M. baturinae scales, the dome is usually surrounded by the wide anterior submarginal ribs and slightly recessed from the distal margin. The scale, which can be attributed as M. baturinae, was found in Ob River under the epithet M. kalinae ([19], Figure 5I).
Among the taxa found in this study, about half belong to cosmopolitan (M. akrokomos, M. matvienkoae, M. rasilis, M. tonsurata) or widespread species (M. costata, M. crassisquama, M. striata, Synura curtispina, S. petersenii, S. uvella) [79]. At the same time, rare species were found: M. phasma and M. solea-ferrea var. irregularis. To date, only two records of M. phasma are known in Europe [73,80]. M. phasma was first described in 1960 in shallow peat bogs in the southeast of England [80]. Later, Němcová et al. [73] reported this species in four water bodies of various types in southwestern France. Previously, it was considered that M. phasma was endemic to Europe [73], but our discovery in the Asian part of Russia expands the range of the species and suggests the possibility of finding M. phasma in other regions of the world. Based on the example of M. phasma, we consider it necessary to note that the classification of rare species as endemic is often approximate, since, perhaps, their detection only in one region is a consequence of insufficient knowledge. M. solea-ferrea var. irregularis was first described in 2013 from water bodies of the Czech Republic [81], and for a long time there was no information about its records in other localities. The second discovery of M. solea-ferrea var. irregularis was registered in 2022 far outside of Europe. The species was recorded in Indonesia, in the high-mountain swamp reservoir of the island of New Guinea [66]. In the present study, we report the third record of M. solea-ferrea var. irregularis, which indicates a fairly wide range of species distribution. The specimens of M. solea-ferrea var. irregularis from Southern Urals differ from Czech populations only in their slightly smaller cell sizes (6.8–10.5 × 3.5–5.2 µm versus 9.0–11.8 × 4.0–5.9 µm).
The variability in the ultrastructural organization of the scales and differences in the morphology of the bristles of the morphotypes of M. rasilis that were found in the Southern Urals should be noted separately. According to the original description, M. rasilis has scales with a dome without papillae and unilaterally serrated bristles [82]. At the same time, scales with a dome partially covered with papillae and with smooth bristles were reported from South Korea [83]. The specimens found in Vietnam were distinguished by the presence of smooth bristles and a dome completely covered with papillae [78]. Scales of M. rasilis with a dome completely covered with papillae have also been reported from Madagascar [84], the Czech Republic [62], and Hungary [85]. In our study, two morphotypes of M. rasilis were found. One of them had smooth bristles and scales with a dome partially covered with papillae (Figure 5G). The second also had scales with a dome partially covered with papillae, but the bristles were unilaterally serrated (Figure 5F). The size of the scales of the second morphotype was larger than the size indicated in the description of M. rasilis (4.1–5.5 × 2.5–2.9 µm versus 3.8–4.1 × 2.1–2.5 µm). In addition, in the case of the second morphotype, along with the typical ornamentation of the shield, there were many scales with the shield completely covered with papillae, including the area of the pore at the base of the V-rib (Figure 5B). Morphological variability, as a response to changes in environmental factors, has been noted in a number of papers. For example, Siver and Skogstad [86] found a clear relationship between water temperature and bristle morphology in Mallomonas crassisquama. Using the example of M. tonsurata, M. kalinae, Synura petersenii, S. curtispina, a tendency was noted to reduce the size of the scales and the length of the bristles, as well as to change the shape of the scales in response to an increase in temperature of cultivation [87,88,89]. A change in the shape of the scales of S. echinulata Korshikov was revealed under the combined action of illumination and temperature, as well as in the pH gradient of the environment [90].
We were unable to correlate the morphological variability of scales and bristles of M. rasilis that we identified with the ecological adaptation of the species to environmental conditions, since in one sample (Lake Zhurmankol, May 2020) we simultaneously recorded cells covered with scales with smooth bristles, and cells covered with scales with serrated bristles. This observation suggests that the differences in the morphology of the setae of M. rasilis in this case are not an ecological adaptation but represent the presence of different morphotypes of this species in the sample. A large number of morphological variations within the morphotype of M. rasilis indicates the need for molecular studies, which may confirm the significant polymorphism of the species, or allow the species to be divided into separate taxa.
During the study, a few Mallomonas scales were found that we could not identify to the species level. Mallomonas sp. (Figure 5P,Q) can be assigned to the Torquatae section. According to the scale ultrastructure, Mallomonas sp. is similar to M. favosa K.H. Nicholls. M. favosa f. favosa is considered a cosmopolitan species [79]. Initially, M. favosa was described in Canada [91] and was later found in other parts of the world [4,67,92,93]. The main difference between M. favosa and our discovery is the structure of the posterior submarginal rib. M. favosa has the well-delimited posterior submarginal rib, while Mallomonas sp. has three or more fine ribs in this area on each arm. We found only two scales of Mallomonas sp., which was not enough to describe a new species, and this organism requires additional studies.
During studies of the flora of silica-scaled chrysophytes in the water bodies of the steppe zone of the Southern Urals, several interesting stomatocysts were found [68,69,70]. Stomatocysts are the endogenous siliceous resting stage of the chrysophyte life cycle. Stomatocysts are formed in response to endogenous or exogenous signals, ensuring the preservation of the chrysophyte population [94]. Currently, about 2000 morphotypes of stomatocysts have been described from different regions of the world [95], and only a small number (10–15%) of them are correlated with the vegetative stage [96,97,98]. Stomatocysts, along with other siliceous structures such as scales and bristles, are widely used in paleolimnological studies [95,96]. Unlike scales and bristles, which are preserved for a short geological period, stomatocysts are more heavily silicified and are present in older sediments [1,99]. However, the lack of relationship between most currently known stomatocyst morphotypes and their vegetative stages limits the use of stomatocysts in studying the evolutionary history of chrysophytes [96]. In view of the above, determining the biological identity of stomatocysts (correlation with the vegetative stage) is undoubtedly relevant.
During the study, we discovered and described stomatocyst 7 Ignatenko & Yatsenko-Stepanova (Figure 3K–M); we reliably established that it belongs to M. doignonii. This morphotype has a significant similarity to stomatocyst 17, Pang & Wang, 2013, produced by another representative of the Torquatae section–M. eoa [100,101]. Despite the existing postulate about the species specificity of chrysophycean stomatocysts [96], a high similarity of stomatocysts was also noted among representatives of the genus Synura. In particular, it was shown that, due to the morphological simplicity, Synura cysts can be almost identical to each other, as well as to stomatocysts of other golden algae [102]. The main difference between the stomatocysts of M. doignonii and M. eoa is the asymmetry of the collar of the cyst of M. doignonii; however, the small number of observed specimens (n = 3) does not allow us to reliably judge the diagnostic significance of this feature; it therefore requires further observations. The high degree of morphological similarity of the stomatocysts of M. eoa and M. doignonii can cause identification errors and, as a result, distort the information of the range and biogeography of these species.
The genus Synura in our study was represented by a smaller number of taxa than Mallomonas. We found four species from sections Synura and Petersenianae. S. curtispina is noted as a new species for the flora of the Southern Urals.
Representatives of the S. petersenii sensu lato species complex were also found in our study. The S. petersenii s.l. complex includes 17 genetically characterized species, as well as a number of taxa belonging to the section Petersenianae that are still unexplored in detail to date; their phylogenetic position is still unknown [5,103]. Due to the high morphological similarity, the identification of the species of the S. petersenii s.l. complex is difficult. For the correct identification of these species, it is necessary to involve molecular methods. In our study, we were unable to identify the detected scales down to the species level without molecular data, and we identified them as S. petersenii s.l. At the same time, we consider it necessary to publish their images to demonstrate the diversity of silica-scaled chrysophytes in the waterbodies of the steppe zone of the Southern Urals.
5. Conclusions
The study of only a small number of steppe water bodies made it possible to discover a new species for science: Mallomonas baturinae. Seasonal study of algae made it possible to identify and describe the M. doignonii stomatocyst. During our studies, new and very interesting findings were revealed in terms of the biogeography of algae. In particular, steppe water bodies became the third known habitat for two very rare taxa of the genus Mallomonas: M. phasma and M. solea-ferrea var. irregularis, which were also new to the flora of Russia. Additionally, among the species that were rare among the flora of Russia were M. doignonii, M. pillula f. exannulata, and M. pillula f. valdiviana. The diversity of M. rasilis morphotypes was revealed. The significant quantity of new data obtained in the study of steppe water bodies of protected natural areas indicates the need for further study of the region.
Author Contributions
M.I., sampling, sample preparation, SEM observations, identification, writing and drafting of manuscript; E.G., TEM observations, identification, writing and drafting of manuscript; T.Y.-S., sampling, sample preparation, identification, writing and editing of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by a grant from the Russian Science Foundation (project No. 23-24-10056).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Olga G. Kalmykova, the Senior Researcher of the Institute of Steppe; the Ural Branch of the Russian Academy of Sciences; Orenburg Federal Research Center; and Semenov A. A. Director of the “Directorate of Specially Protected Natural Territories of the Orenburg Region”, for their help with sampling and permissions. We thank the anonymous referees for their thoughtful comments, which helped us to further improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kristiansen, J.; Škaloud, P. Chrysophyta. In Handbook of the Protists, 2nd ed.; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 331–366. [Google Scholar] [CrossRef]
- Škaloud, P.; Kristiansen, J.; Škaloudová, M. Developments in the Taxonomy of Silica-Scaled Chrysophytes–From Morphological and Ultrastructural to Molecular Approaches. Nord. J. Bot. 2013, 31, 385–402. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 5 November 2023).
- Kristiansen, J.; Preisig, H.R. Chrysophyta and Haptophyta Algae, 2nd part: Synurophyceae. In Süsswasserflora Von Mitteleuropa (Freshwater Flora of Central Europe); Büdel, B., Gärtner, G., Krienitz, L., Preisig, H.R., Schagerl, M., Eds.; Spektrum Akademisher Verlag, Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–252. ISBN 978-3-8274-1701-5. [Google Scholar]
- Škaloud, P.; Škaloudová, M.; Jadrná, I.; Bestová, H.; Pusztai, M.; Kapustin, D.; Siver, P.A. Comparing Morphological and Molecular Estimates of Species Diversity in the Freshwater Genus Synura (Stramenopiles): A Model for Understanding Diversity of Eukaryotic Microorganisms. J. Phycol. 2020, 56, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Siver, P.A.; Jo, B.Y.; Kim, J.I.; Shin, W.; Lott, A.M.; Wolfe, A.P. Assessing the Evolutionary History of the Class Synurophyceae (Heterokonta) Using Molecular, Morphometric, and Paleobiological Approaches. Am. J. Bot. 2015, 102, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Scoble, J.M.; Cavalier-Smith, T. Scale Evolution in Paraphysomonadida (Chrysophyceae): Sequence Phylogeny and Revised Taxonomy of Paraphysomonas, New Genus Clathromonas, and 25 New Species. Eur. J. Protistol. 2014, 50, 551–592. [Google Scholar] [CrossRef]
- Smol, J.P. Applications of Chrysophytes to Problems in Paleoecology. In Chrysophyte Algae: Ecology, Phylogeny and Development; Sandgren, C., Smol, J., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 303–330. [Google Scholar] [CrossRef]
- Siver, P.A. The distribution of chrysophytes along environmental gradients: Their use as biological indicators. In Chrysophyte Algae: Ecology, Phylogeny and Development; Sandgren, C., Smol, J., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 232–268. [Google Scholar] [CrossRef]
- Kristiansen, J. Biogeography of Silica-Scaled Chrysophytes. Nova Hedwig. Beih. 2001, 122, 23–29. [Google Scholar]
- Balonov, I.M. Chrysophyta, fam. Synuraceae Lemm., from the Waterbodies in Karelia. Trudy Instituta Biologii Vnutrennich Vod AN SSSR 1979, 42, 1–26. (In Russian) [Google Scholar]
- Balonov, I.M.; Kuzmin, G.V. Electron Microscopic Study of the Species of the Genus Mallomonas Perty (Chrysophyta) from Reservoirs of the Volga Cascade. II. Series Planae Harris et Bradley. Bot. Zhurn. Russ. Bot. J. 1975, 60, 1289–1296. (In Russian) [Google Scholar]
- Balonov, I.M. Species from the Genus Mallomonas Perty (Chrysophyta) from the Waterbodies in the Volga River Basin. Trudy Instituta Biologii Vnutrennich Vod AN SSSR 1978, 35, 75–102. (In Russian) [Google Scholar]
- Ignatenko, M.E.; Yatsenko-Stepanova, T.N.; Urzhumov, A.A. New Records of Mallomonas Species (Chrysophyceae, Synurales) for the Orenburg Region (South Urals, Russia). Nov. Sist. Nizshikh Rast. 2021, 55, 315–323. (In Russian) [Google Scholar] [CrossRef]
- Safronova, T.V. Golden Algae (Chrysophyceae, Synurophyceae) of Specially Protected Natural Territories of the Leningrad Region and St. Petersburg. Doctoral Dissertation, Komarov Botanical Institute of the Russian Academy of Sciences, Saint Peterburg, Russia, 2018; p. 204. (In Russian). [Google Scholar]
- Kulizin, P.V.; Gusev, E.S.; Vodeneeva, E.L.; Okhapkin, A.G. Silica-Scaled Chrysophytes of Some Left-Bank Tributaries of the Cheboksary Reservoir. Inland Water Biol. 2021, 4, 332–342. (In Russian) [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Bukin, Y.S.; Sorokovikova, L.M.; Firsova, A.D.; Tomberg, I.V. Silica-Scaled Chrysophytes in Small Lakes of the Lower Yenisei Basin, the Arctic. Nova Hedwig. Beih. 2018, 107, 315–336. [Google Scholar] [CrossRef]
- Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed. Sustainability 2021, 132, 13768. [Google Scholar] [CrossRef]
- Bessudova, A.; Likhoshway, Y.; Firsova, A.; Mitrofanova, E.; Koveshnikov, M.; Soromotin, A.; Khoroshavin, V.; Kirillov, V. Small Organisms in a Large River: What Provides the High Diversity of Scaled Chrysophytes in the Ob River. Water 2023, 15, 3054. [Google Scholar] [CrossRef]
- Siver, P.A.; Voloshko, L.N.; Gavrilova, O.V.; Getsen, M.V. The Scaled Chrysophyte Flora of the Bolshezemelskaya Tundra (Russia). Nova Hedwig. Beih. 2005, 128, 125–150. [Google Scholar]
- Gusev, E.S. Silica-Scaled Chrysophytes from Karst Lakes in Central Russia. Nova Hedwig. Beih. 2013, 142, 017–025. [Google Scholar]
- Voloshko, L.N.; Gavrilova, O.V. A Checklist of Silica-Scaled Chrysophytes in Russia with an Emphasis on the Flora of Lake Ladoga. Nova Hedwig. Beih. 2001, 122, 147–167. [Google Scholar]
- Safronova, T.V. Species Composition of Chrysophyta in the Waterbodies of the Wetland of International Importance “Mshinskaya Bog System” (Leningrad Region). Bot. Zhurn. 2011, 96, 1037–1052. (In Russian) [Google Scholar]
- Safronova, T.V. New for Flora of Leningrad Region and Russia Species of Chrysophyta. Nov. Sist. Nizshikh Rast. 2012, 46, 60–67. (In Russian) [Google Scholar]
- Safronova, T.V.; Voloshko, L.N. Silica-Scaled Chrysophytes in the Waterbodies of Protected Areas of the North-West of Russia. Nova Hedwig. Beih. 2013, 142, 97–115. [Google Scholar]
- Safronova, T.V. Seasonal Changes of Taxonomic Composition of Chrysophycean Algae (Chrysophyceae, Synurophyceae) in the Ponds of the Botanical Garden of the Komarov Botanical Institute (St. Petersbur). Bot. Zhurn. 2014, 99, 443–458. (In Russian) [Google Scholar]
- Voloshko, L.N. Chrysophycean Algae in Water Bodies of the Northern Russia; Renome: Saint Peterburg, Russia, 2017; p. 380. (In Russian) [Google Scholar]
- Gusev, E.S.; Perminova, O.S.; Guseva, E.E.; Startseva, N.A. The Genus Mallomonas in Small Urban Rivers in Nizhniy Novgorod (Russia). Nova Hedwig. Beih. 2019, 148, 77–88. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shadrina, S.N. New and Noteworthy Species of Chrysophyta for the Russian Flora. Nov. Sist. Nizshikh Rast. 2020, 54, 371–380. [Google Scholar] [CrossRef]
- Bessudova, A.; Likhoshway, Y. Scaled Chrysophyte (Chrysophyceae) of the Boguchany Reservoir. Mod. Sci. Actual Probl. Theory Pract. Ser. Nat. Tech. Sci. 2017, 11, 4–11. (In Russian) [Google Scholar]
- Bessudova, A.Y.; Firsova, A.D.; Tomberg, I.V.; Sorokovikova, L.M.; Likhoshway, Y.V. Biodiversity of Silica-Scaled Chrysophytes in Tributaries of Northern Limit of Lake Baikal. Acta Biol. Sib. 2018, 4, 75–84. [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Sorokovikova, L.M.; Tomberg, I.V.; Likhoshway, Y.V. Silica-Scaled Chrysophytes in Large Tributaries of Lake Baikal. Cryptogam. Algol. 2018, 39, 145–165. [Google Scholar] [CrossRef]
- Gusev, E.S.; Guseva, E.E.; Gabyshev, V.A. Taxonomic Composition of Silica-Scaled Chrysophytes in Rivers and Lakes of Yakutia and Magadanskaya oblast (Russia). Nova Hedwig. Beih. 2018, 147, 105–117. [Google Scholar]
- Bessudova, A.Y.; Firsova, A.D. Silica-Scaled Protista and Stomatocysts in East Siberia. Limnol. Freshw. Biol. 2022, 5, 1663–1670. [Google Scholar] [CrossRef]
- Voloshko, L.N. The Chrysophycean Algae from Glacial Lakes of Polar Ural (Russia). Nova Hedwig. Beih. 2010, 136, 191–212. [Google Scholar] [CrossRef]
- Voloshko, L.N. New Species of the Genus Mallomonas (Chrysophyta, Synurophyceae) from Ponds of Vokkuta Tundra. Bot. Zhurn. 2012, 97, 1090–1098. (In Russian) [Google Scholar] [CrossRef]
- Mitrofanova, E.Y. Phytoplankton of Lake Teletskoye (Altai, Russia): Features of Development and Long-Term Dynamics. Russ. J. Ecol. 2018, 49, 180–185. [Google Scholar] [CrossRef]
- Snitko, L.V.; Snitko, V.P.; Safronova, T.V. Chrysophycean Algae in Waterbodies of the South Urals. II. Genus Mallomonas (Synurophyceae, Mallomonadaceae). Bot. Zhurn. 2020, 105, 368–383. (In Russian) [Google Scholar] [CrossRef]
- Bessudova, A.; Firsova, A.; Hilkhanova, D.; Makarov, M.; Sakirko, M.; Bashenkhaeva, M.; Khanaev, I.; Zakharova, Y.; Likhoshway, Y. Two New Species, Mallomonas baicalensis sp. nov. and M. grachevii sp. nov. (Synurales Chrysophyceae), Found under the Ice of Lake Baikal. Water 2023, 15, 2250. [Google Scholar] [CrossRef]
- Bessudova, A.; Firsova, A.D.; Tomberg, I.V.; Bayramova, E.; Hilkhanova, D.; Bedoshvili, Y.D.; Bashenkhaeva, M.; Kopyrina, L.I.; Zakharova, Y.R.; Likhoshway, Y.V. Two new species of silica-scaled chrysophytes (Chrysophyceae, Synurales) Mallomonas kicherica and M. sibirica water bodies of Eastrn Siberia, Russia. Phytotaxa 2023, 620, 59–69. [Google Scholar] [CrossRef]
- Gusev, E.S.; Kulikovskiy, M.S. A new species of the genus Mallomonas (Chrysophyceae: Synurales), Mallomonas kuzminii sp. nov., from lake Frolikha (Russia, Baikal region). Phytotaxa 2013, 155, 66–70. [Google Scholar] [CrossRef][Green Version]
- Gusev, E.; Martynenko, N. Silica-Scaled Chrysophytes of Teletskoye Lake and Adjacent Area with a Description of a New Species from the Genus Mallomonas. Diversity 2022, 14, 1040. [Google Scholar] [CrossRef]
- Gusev, E.; Nĕmcová, Y.; Kulikovskiy, M. Mallomonas voloshkoae sp. nov. (Synurales, Chrysophyceae) and distribution of M. pechlaneri in mountain lakes of Siberia. Phytotaxa 2022, 530, 221–229. [Google Scholar] [CrossRef]
- Sokolova, I.V.; Shadrina, S.N.; Gogorev, R.M.; Stepanova, V.A. Validation of the name Mallomonas silvicola (Chrysophyceae), a new species for the flora of Russia. Nov. Sist. Nizshikh Rast. 2023, 57, N1–N4. (In Russian) [Google Scholar] [CrossRef]
- Firsova, A.D.; Bessudova, A.Y.; Sorokovikova, L.M.; Tomberg, I.V.; Likhoshway, Y.V. The Diversity of Chrysophycean Algae in an Arctic Zone of River and Sea Water Mixing, Russia. Am. J. Plant Sci. 2015, 6, 2439–2452. [Google Scholar] [CrossRef]
- Bessudova, A.; Galachyants, Y.; Firsova, A.; Hilkhanova, D.; Nalimova, M.; Marchenkov, A.; Mikhailov, I.; Sakirko, M.; Likhoshway, Y. Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir). Life 2023, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Škaloud, P.; Škaloudová, M.; Pichrtová, M.; Němcová, Y.; Kreidlová, J.; Pusztai, M. www.chrysophytes.eu–A Database on Distribution and Ecology of Silica-Scaled Chrysophytes in Europe. Nova Hedwig. Beih. 2013, 142, 141–146. [Google Scholar]
- Kristiansen, J.; Düwel, L.; Wegeberg, S. Silica-scaled chrysophytes from the Taymyr Peninsula, Northern Siberia. Nova Hedwig. Beih. 1997, 65, 337–351. [Google Scholar] [CrossRef]
- Gusev, E.S.; Perminova, O.S.; Startseva, N.A.; Okhapkin, A.G. The Genus Synura (Synurales, Synurophyceae) in Small Urban Rivers of Nizhniy Novgorod. Nov. Sist. Nizshikh Rast. 2017, 51, 57–70. (In Russian) [Google Scholar] [CrossRef]
- Snitko, L.V.; Snitko, V.P.; Blinov, I.A.; Voloshko, L.N. Chrysophycean Algae (Chrysophyceae, Synurophyceae) in Waterbodies of the Eastern Foothills of the South and Middle Urals. Bot. Zhurn. 2016, 101, 1361–1378. (In Russian) [Google Scholar]
- Snitko, L.V.; Snitko, V.P.; Blinov, I.A. The Formation and Morphology of Stomatocysts Golden Algae (Chrysophyceae, Synurophyceae) in the Plankton of Water Bodies of the South Urals. Int. J. Appl. Fundam. Res. 2018, 11, 114–118. (In Russian) [Google Scholar] [CrossRef]
- Snitko, L.V.; Snitko, V.P.; Blinov, I.A.; Voloshko, L.N. Chrysophycean Algae in Waterbodies of the South Urals. Genus Chrysosphaerella (Paraphysomonadaceae). Bot. Zhurn. 2019, 104, 587–601. (In Russian) [Google Scholar] [CrossRef]
- Snitko, L.V.; Safronova, T.V.; Blinov, I.A.; Snitko, V.P. New Species of Synura Section Synura (Chrysophyceae, Synurales, Synuraceae) in Waterbodies of the South Urals. Bot. Zhurn. 2021, 106, 1101–1112. (In Russian) [Google Scholar] [CrossRef]
- Snitko, L.V.; Safronova, T.V.; Snitko, V.P. Chrysophycean Algae (Chrysophyceae) in Waterbodies of the South Urals and Transural Plateau. Genus Synura (Synuraceae) Section Petersenianae. Bot. Zhurn. 2022, 107, 333–349. (In Russian) [Google Scholar]
- Chibilev, A.; Levykin, S. Virgin Lands Divided be an Ocean: The Fate of Grasslands in the Northern Hemisphere. Translated by David Moon. Nova Acta Leopold. 2013, 390, 91–103. [Google Scholar]
- Levykin, S.V.; Chibilev, A.A.; Ulyanov, Y.A.; Silantieva, M.M.; Kazachkov, G.V.; Yakovlev, I.G. Environmental and landscape significance of steppe megaprojects. Ukr. J. Ecol. 2019, 9, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Machine, K.V.; Baynard, C.W.; Chibilev, A.A.; Richardson, R.D. Landscape disturbance caused by non-renewable energy production in a semi-arid region: A case study on the Russian steppe. Int. J. Sustain. Dev. World Ecol. 2018, 25, 541–553. [Google Scholar]
- Siver, P.A.; Lott, A.M. Further Observations on the Scaled Chrysophycean and Synurophycean Flora of the Ocala National Forest, Florida, U.S.A. Nord. J. Bot. 2004, 24, 211–233. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. The Scaled Chrysophyte Flora from the Pinelands National Preserve of Southern New Jersey, U.S.A. Nova Hedwig. Beih. 2010, 136, 167–180. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. The Scaled Chrysophyte Flora in Freshwater Ponds and Lakes from Newfoundland, Canada, and Their Relationship to Environmental Variables. Cryptogam. Algol. 2017, 38, 325–347. [Google Scholar] [CrossRef]
- Nĕmcová, Y.; Neustupa, J.; Nováková, S.; Kalina, T. Silica-Scaled Chrysophytes of the Šumava National Park and the Třeboňsko UNESCO Biosphere Reserve (Southern Bohemia, Czech Republic). Nord. J. Bot. 2002, 22, 375–383. [Google Scholar] [CrossRef]
- Nĕmcová, Y. Diversity and Ecology of Silica-Scaled Chrysophytes (Synurophyceae, Chrysophyceae) in the National Nature Monument Swamp and Břehyňský Pond, Czech Republic. Cryptogam. Algol. 2010, 31, 229–243. [Google Scholar]
- Gusev, E.S.; Doan-Nhu, H.; Nguyen-Ngoc, L. Silica-Scaled Chrysophytes from Cat Tien National Park (Dong Nai Province, Vietnam). Nova Hedwig. Beih. 2017, 105, 347–364. [Google Scholar] [CrossRef]
- Gusev, E.S.; Siver, P.A.; Shin, W. Mallomonas bronchartiana Compère Revisited: Two New Species Described from Asia. Cryptogam. Algol. 2017, 38, 3–16. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N.; Tran, H. Studies on Algae from the Order Synurales (Chrysophyceae) in Northern Vietnam. Diversity 2021, 13, 602. [Google Scholar] [CrossRef]
- Gusev, E.; Kapustin, D.; Martynenko, N.; Kulikovskiy, M. Diversity of Silica-Scaled Chrysophytes (Stramenopiles: Chrysophyceae) from Indonesian Papua. Diversity 2022, 14, 726. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N.; Shkurina, N.; Huan, P.T.; Dien, T.D.; Thanh, N.T.H. An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam. Diversity 2023, 15, 183. [Google Scholar] [CrossRef]
- Ignatenko, M.; Yatsenko-Stepanova, T.N.; Kapustin, D. Additions to Chrysophycean Stomatocyst Flora from South Urals Shallow Lake Including Descriptions of Three New Morphotypes. Phytotaxa 2022, 561, 014–026. [Google Scholar] [CrossRef]
- Chibilyov, A.A. The “Orenburgsky” Reservation: History of Organization and Nature Diversity; The Institute of Steppe UB RAS, Orenburg Branch of the Russian Geographical Society: Yekaterinburg, Russia, 2014; p. 139. (In Russian) [Google Scholar]
- Kristiansen, J. Seasonal occurrence of silica-scaled chrysophytes under eutrophic conditions. Hydrobiologia 1988, 161, 171–184. [Google Scholar] [CrossRef]
- Nĕmcová, Y.; Faturova, J.; Škaloud, P. Comparing continental and local distribution patterns of protists: A case study of silica–scaled chrysophytes. Fottea Olomouc 2023, 23, 177–189. [Google Scholar] [CrossRef]
- Cronberg, G.; Sandgren, C.D. A Proposal for the Development of Standardized Nomenclature and Terminology for Chrysophycean Statospores. In Chrysophytes: Aspects and Problems; Cambridge University Press: Cambridge, UK, 1986; pp. 317–328. [Google Scholar]
- Ňemcová, Y.; Kreidlová, J.; Kosová, A.; Neustupa, J. Lakes and Pools of Aquitaine Region (France)–A Biodiversity Hotspot of Synurales in Europe. Nova Hedwig. Beih. 2012, 95, 1–24. [Google Scholar] [CrossRef]
- Jo, B.Y.; Kim, J.I.; Škaloud, P.; Siver, P.A.; Shin, W. Multigene Phylogeny of Synura (Synurophyceae) and Descriptions of Four New Species Based on Morphological and DNA Evidence. Eur. J. Phycol. 2016, 51, 413–430. [Google Scholar] [CrossRef]
- Bradley, D.E. Observations on Some Chrysomonads from Scotland. J. Protozool. 1966, 13, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.E. A Study of the Mallomonas, Synura and Chrysosphaerella of Northern Iceland. J. Gen. Microbiol. 1964, 37, 321–333. [Google Scholar] [CrossRef]
- Rezácova, M. Mallomonas kalinae (Synurophyceae), a New Species of Alga from Northern Bohemia, Czech Republic. Preslia 2006, 78, 353–358. [Google Scholar]
- Gusev, E.S.; Čertnerová, D.; Škaloudová, M.; Škaloud, P. Exploring Cryptic Diversity and Distribution Patterns in the Mallomonas kalinae/rasilis Species Complex with a Description of a New Taxon–Mallomonas furtiva sp. nov. J. Eukaryot. Microbiol. 2018, 65, 38–47. [Google Scholar] [CrossRef]
- Kristiansen, J. Cosmopolitan Chrysophytes. Syst. Geog. Plants 2000, 70, 291–300. [Google Scholar] [CrossRef]
- Harris, K.; Bradley, D.E.; Bradley, D.E. A Taxonomic Study of Mallomonas. J. Gen. Microbiol. 1960, 22, 750–777. [Google Scholar] [CrossRef]
- Němcová, Y.; Kreidlová, J.; Pusztai, M.; Neustupa, J. Mallomonas pumilio Group (Chrysophyceae/Stramenopiles)–A Revision Based on the Scale/Scale-Case Morphology and Analysis of Scale Shape. Nova Hedwig. Beih. 2013, 142, 027–049. [Google Scholar]
- Dürrschmidt, M. Three New Species of Mallomonas (Chrysophyceae, Mallomonadaceae) from Lake Lanalhue, Chile. Nord. J. Bot. 1983, 3, 423–430. [Google Scholar] [CrossRef]
- Kim, J.; Hee Park, Y.; Jung Kim, H. Silica-Scaled Chrysophytes (Synurophyceae) from Jeju Island, Korea. Nova Hedwig. Beih. 2009, 89, 201–218. [Google Scholar] [CrossRef]
- Hansen, P. Silica-Scaled Chrysophyceae and Synurophyceae from Madagascar. Arch. Für Protistenkd. 1996, 147, 145–172. [Google Scholar] [CrossRef]
- Barreto, S. The Silica-Scaled Chrysophyte Flora of Hungary. Nova Hedwig. Beih. 2005, 128, 11–41. [Google Scholar]
- Siver, P.A.; Skogstad, A. Morphological variation and ecology of Mallomonas crassisquama (Chrysophyceae). Nord. J. Bot. 1988, 7, 99–107. [Google Scholar] [CrossRef]
- Pichrtová, M.; Němcová, Y. Effect of temperature on size and shape of silica scales in Synura petersenii and Mallomonas tonsurata (Stramenopiles). Hydrobiologia 2011, 673, 1–11. [Google Scholar] [CrossRef]
- Gutowski, A. Temperature dependent variability of scales and bristles of Mallomonas tonsurata Teiling emend. Krieger (Synurophyceae). Nova Hedwig. Beih. 1996, 114, 125–146. [Google Scholar]
- Řezáčová-Škaloudová, M.; Neustupa, J.; Němcová, Y. Effect of temperature on the variability of silicate structures in Mallomonas kalinae and Synura curtispina (Synurophyceae). Nova Hedwig. Beih. 2010, 136, 55–69. [Google Scholar] [CrossRef]
- Němcová, Y.; Neustupa, J.; Kvíderová, J.; Řezáčová-Škaloudová, M. Morphological plasticity of silica scales of Synura echinulata (Synurophyceae) in crossed gradients of light and temperature–A geometric morphometric approach. Nova Hedwig. Beih. 2010, 136, 21–32. [Google Scholar] [CrossRef]
- Nicholls, K.H. Four new Mallomonas Species of the Torquatae Series (Chrysophyceae). Can. J. Bot. 1984, 62, 1583–1591. [Google Scholar] [CrossRef]
- Dürrschmidt, M.; Croome, R. Mallomonadaceae (Chrysophyceae) from Malaysia and Australia. Nord. J. Bot. 1985, 5, 285–298. [Google Scholar] [CrossRef]
- Wei, Y.X.; Yuan, X.P.; Kristiansen, J. Silica-Scaled Chrysophytes from Hainan, Guangdong Provinces and Hong Kong Special Administrative Region, China. Nord. J. Bot. 2014, 32, 881–896. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Smol, J.P. Chrysophycean stomatocyst flora from south-central Ontario lakes. Can. J. Bot. 1998, 76, 836–862. [Google Scholar]
- Pang, W.; Van de Vijver, B. Freshwater Chrysophycean Stomatocysts from Monte Lauro (Buccheri, Sicily, Italy). Phytotaxa 2021, 494, 177–192. [Google Scholar] [CrossRef]
- Duff, K.E.; Zeeb, B.A.; Smol, J.P. Atlas of Chrysophycean Cysts; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; p. 189. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Zeeb, B.A.; Smol, J.P. Atlas of Chrysophycean Cysts. II; Springer Science & Business Media: Dordrecht, The Netherlands, 2001; p. 169. [Google Scholar] [CrossRef]
- Piątek, J.; Piątek, M. Morphological variability of new chrysophyte stomatocyst forming a single-cyst assemblage in a low-conductivity tropical lake in the Guineo-Congolian rainforest. Phytotaxa 2014, 174, 261–271. [Google Scholar] [CrossRef]
- Siver, P.A.; Wolfe, A.P. Scaled chrysophytes in Middle Eocene lake sediments from Northwestern Canada, including descriptions of six new species. Nova Hedwig. Beih. 2005, 128, 295–308. [Google Scholar]
- Cronberg, G. Development of Cysts in Mallomonas eoa Examined by Scanning Electron Microscopy. Hydrobiologia 1973, 43, 29–38. [Google Scholar] [CrossRef]
- Pang, W.; Wang, Q. Chrysophycean Stomatocysts from the Stone Ponds in the Aershan National Geological Park, China. Nova Hedwig. Beih. 2013, 142, 051–067. [Google Scholar]
- Kapustin, D.A.; Kulikovsky, M.S. Stomatocysts of Species of the Genus Synura (Chrysophyceae, Synurales)–Identical or Species Specificity? In Algae: Problems of Taxonomy and Ecology, Use in Monitoring and Biotechnology; Materials of the VI Russian Scientific Conference with International Participation and the School of Young Scientists: Moscow, Russia, 2022; pp. 23–24. (In Russian) [Google Scholar]
- Škaloud, P.; Škaloudová, M.; Procházková, A.; Nĕmcová, Y. Morphological Delineation and Distribution Patterns of Four Newly Described Species within the Synura petersenii Species Complex (Chrysophyceae, Stramenopiles). Eur. J. Phycol. 2014, 49, 213–229. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).