Geometric Morphometric Analysis of Mandibular Symphysis Growth between 12 and 15 Years of Age in Class II Malocclusion Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
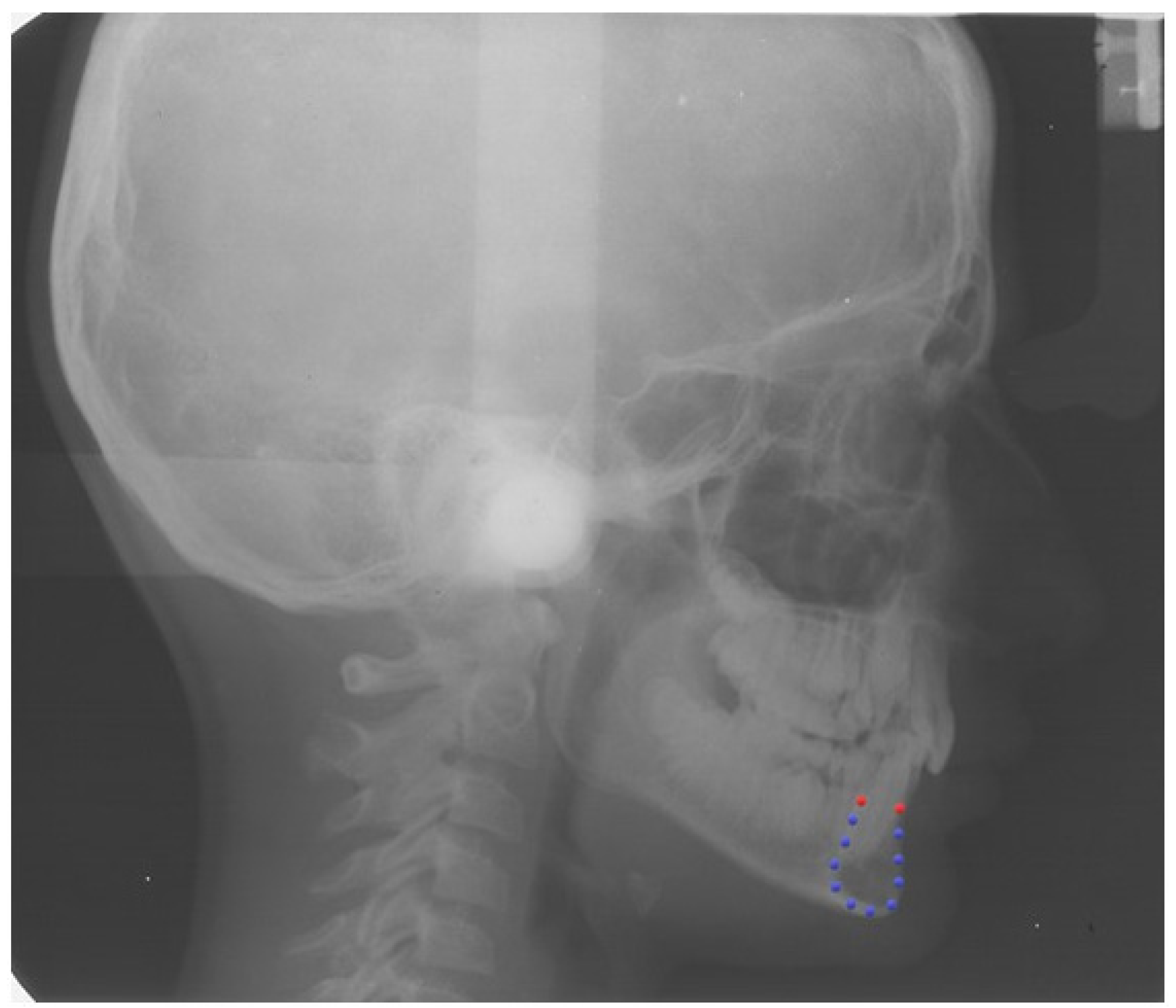
2.2. Definition of the Curve of the Mandibular Symphysis
2.3. Procrustes Superimposition
2.4. Statistical Analysis
3. Results
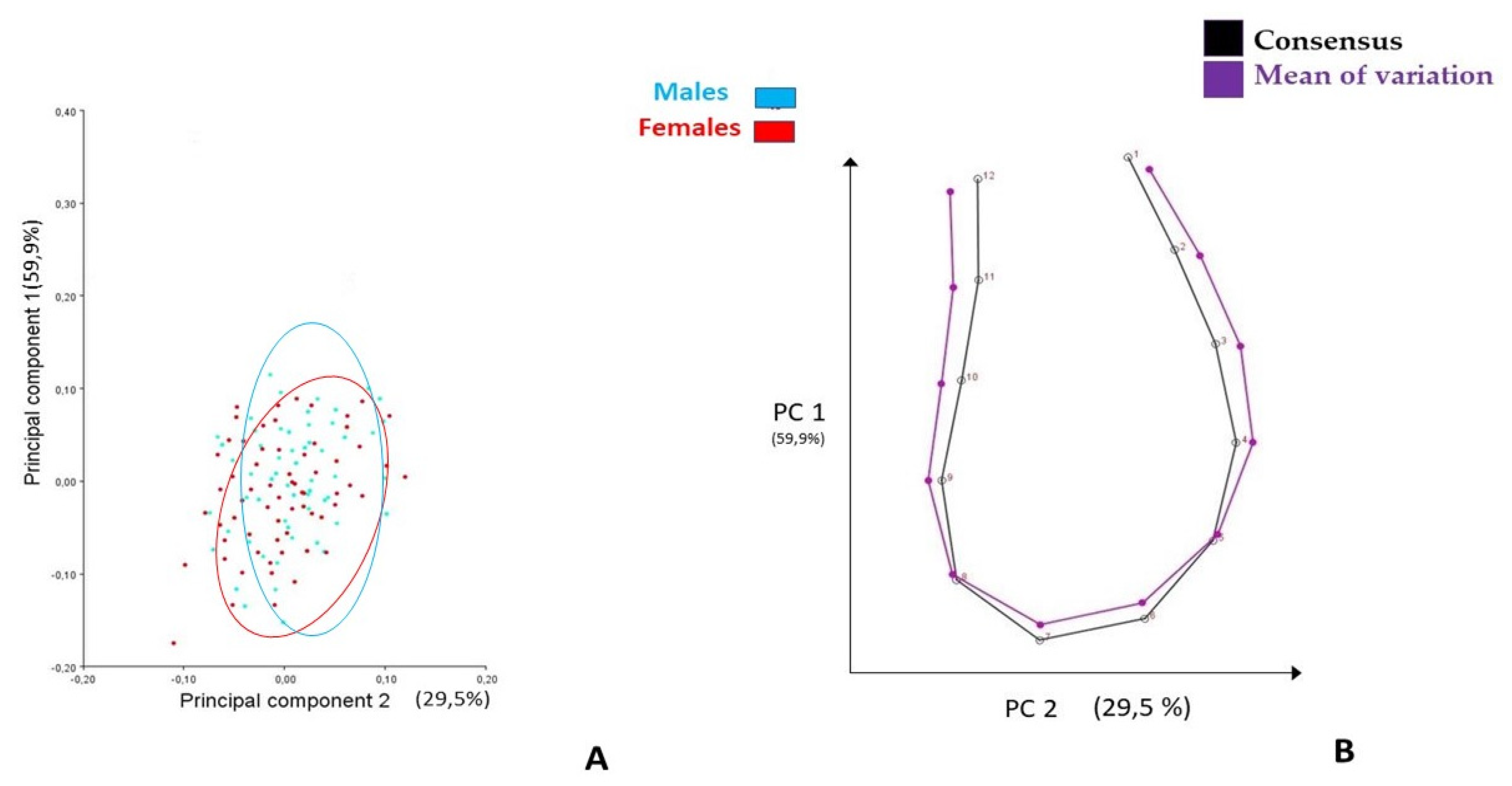
3.1. Generalized Procrustes Superimposition and PCA
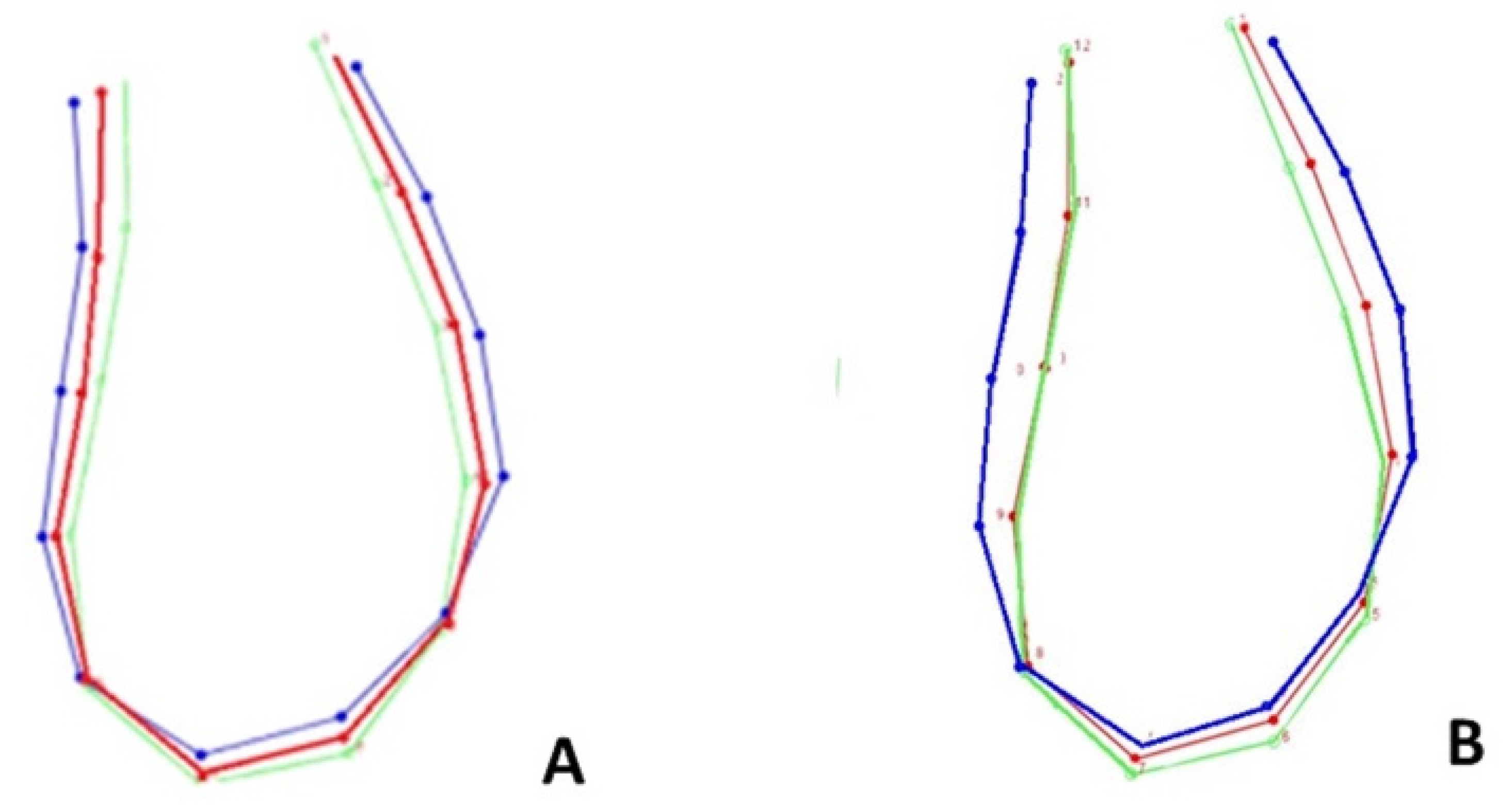
3.2. Age, Sex, and Form Correlation
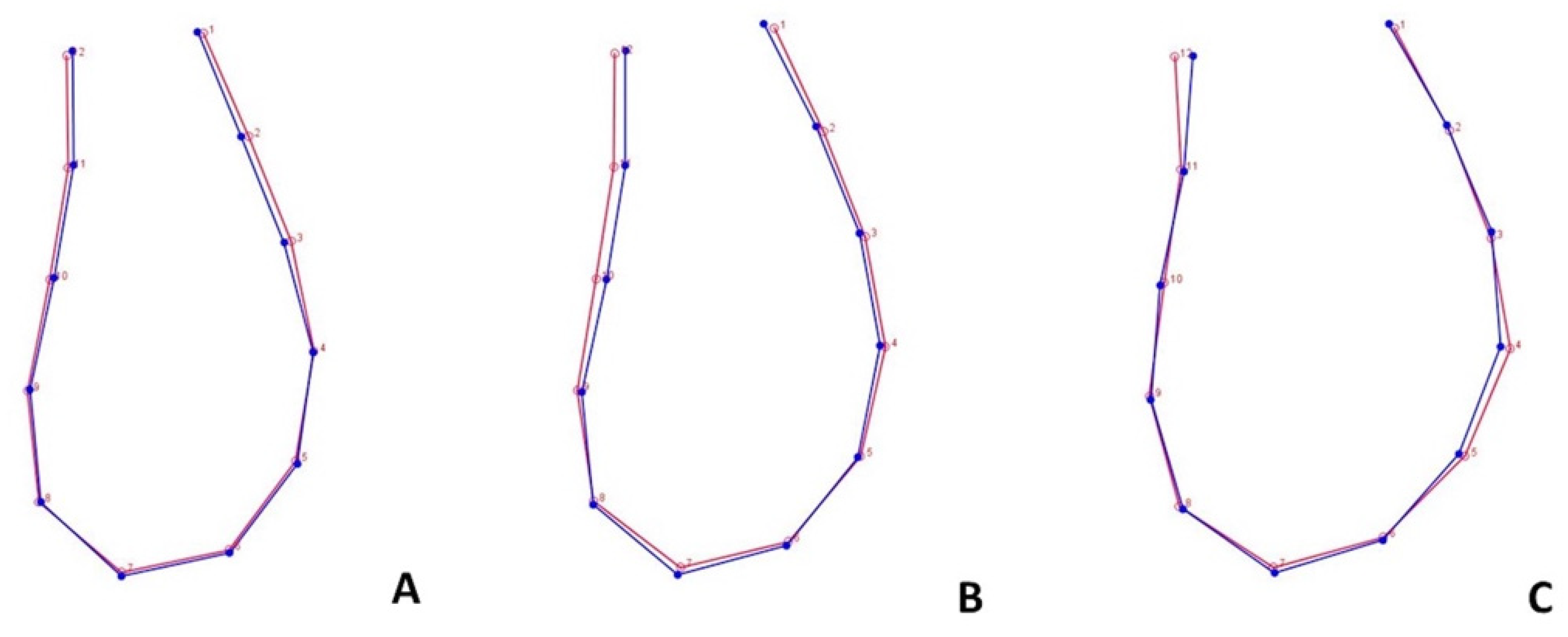
3.3. Form Covariation and Inter-Timing Assessment of Morphological Change
4. Discussion
4.1. Generalized Procrustes Superimposition and PCA
4.2. Age, Sex, and Ms’Form Correlation
4.3. Ms’Form Covariation and Inter-Timing Assessment of Morphological Change
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skieller, V.; Björk, A.; Linde-Hansen, T. Prediction of mandibular growth rotation evaluated from a longitudinal implant sample. Am. J. Orthod. 1984, 86, 359–370. [Google Scholar] [CrossRef]
- Wainwright, W. Faciolingual tooth movement: Its influence on the root and cortical plate. Am. J. Orthod. 1973, 64, 278–302. [Google Scholar] [CrossRef]
- Djordjevic, J.; Zhurov, A.I.; Richmond, S.; Visigen Consortium. Genetic and Environmental Contributions to Facial Morphological Variation: A 3D Population-Based Twin Study. PLoS ONE 2016, 11, e0162250. [Google Scholar] [CrossRef] [Green Version]
- Hylander, W.L.; Johnson, K.R.; Crompton, A.W. Muscle force recruitment and biomechanical modeling: An analysis of masseter muscle function during mastication inMacaca fascicularis. Am. J. Phys. Anthr. 1992, 88, 365–387. [Google Scholar] [CrossRef]
- Björk, A. Prediction of mandibular growth rotation. Am. J. Orthod. 1969, 55, 585–599. [Google Scholar] [CrossRef]
- Haskell, B.S. The human chin and its relationship to mandibular morphology. Angle Orthod. 1979, 49, 153–166. [Google Scholar] [CrossRef]
- Swasty, D.; Lee, J.; Huang, J.C.; Maki, K.; Gansky, S.A.; Hatcher, D.; Miller, A.J. Cross-sectional human mandibular morphology as assessed in vivo by cone-beam computed tomography in patients with different vertical facial dimensions. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e377–e389. [Google Scholar] [CrossRef]
- Al-Khateeb, S.N.; Al Maaitah, E.F.; Abu Alhaija, E.S.; Badran, S.A. Mandibular symphysis morphology and dimensions in different anteroposterior jaw relationships. Angle Orthod. 2014, 84, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.S.; Shin, S.M.; Yamaguchi, T.; Maki, K.; Wu, T.-J.; Ko, C.-C.; Kim, Y.-I. Relationship between the maxillofacial skeletal pattern and the morphology of the mandibular symphysis: Structural equation modeling. Korean J. Orthod. 2019, 49, 170–180. [Google Scholar] [CrossRef]
- Laganà, G.; Palmacci, D.; Ruvolo, G.; Cozza, P.; Paoloni, V. 3D evaluation of maxillary morphology in Marfan growing subjects: A controlled clinical study. Prog. Orthod. 2019, 20, 12. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P.; Weber, G.; Bookstein, F. Regional dissociated heterochrony in multivariate analysis. Ann. Anat. Anat. Anz. 2004, 186, 463–470. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.J. Changing Times, Changing Places: Heterochrony and Heterotopy-Beyond Heterochrony: The Evolution of Development. Edited by Miriam Zelditch. Wiley-Liss, New York. 2001. 371 pages. Cloth $99.95.-The odyssey of heterochrony is exceedingly curious (Gould 1977: p. 221). Paleobiology 2002, 28, 551–558. [Google Scholar]
- Bookstein, F.L. Principal warps: Thin-plate splines and the decomposition of deformations. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 567–585. [Google Scholar] [CrossRef] [Green Version]
- Gerber, S.; Neige, P.; Eble, G.J. Combining ontogenetic and evolutionary scales of morphological disparity: A study of early Jurassic ammonites. Evol. Dev. 2007, 9, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Thompson DJTotRSoE. Morphol. Math. 1915, 50, 857–895.
- Lachenbruch, P.A. An Almost Unbiased Method of Obtaining Confidence Intervals for the Probability of Misclassification in Discriminant Analysis. Biometrics 1967, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.A.; Atchley, W.R. The Geometry of Canonical Variate Analysis. Syst. Zool. 1981, 30, 268–280. [Google Scholar] [CrossRef]
- Timm, N.H. Applied Multivariate Analysis; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Rohlf, F.J.; Corti, M. Use of Two-Block Partial Least-Squares to Study Covariation in Shape. Syst. Biol. 2000, 49, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solow, B. The Dentoalveolar Compensatory Mechanism: Background and Clinical Implications. Br. J. Orthod. 1980, 7, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, J.; Jiang, J.-H. Morphological analysis of the alveolar bone of the anterior teeth in severe high-angle skeletal Class II and Class III malocclusions assessed with cone-beam computed tomography. PLoS ONE 2019, 14, e0210461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jossvassalli, I.M.; Grebenstein, C.; Topouzelis, N.; Sculean, A.; Katsaros, C. Orthodontic therapy and gingival recession: A systematic review. Orthod. Craniofacial Res. 2010, 13, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, K.; Silva, M.A.G.S.; Normando, D.; Valladares-Neto, J. Factors associated with the morphology of the mandibular symphysis and soft tissue chin. Dent. Press J. Orthod. 2021, 26, e2119347. [Google Scholar] [CrossRef] [PubMed]
- Wehrbein, H.; Bauer, W.; Diedrich, P. Mandibular incisors, alveolar bone, and symphysis after orthodontic treatment. A retrospective study. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.; Sabet, N.E.; Hassan, I. Alveolar bone mapping in subjects with different vertical facial dimensions. Eur. J. Orthod. 2015, 37, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kawamura, A.; Yamaguchi, M.; Kasai, K. Relationship between masticatory function and internal structure of the mandible based on computed tomography findings. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 766–773. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Di Pede, C.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public Health 2022, 19, 988. [Google Scholar] [CrossRef]
- Teo, K.F.; Nor, M.M.; Mohamed, A.M.; Pritam, H.M.H. Geometric morphometric analysis of mandibular symphysis in Class III skeletal base. Anat. Cell Biol. 2022, 55, 433–440. [Google Scholar] [CrossRef]
- Gomes, A.S.; Lima, E.M. Mandibular growth during adolescence. Angle Orthod. 2006, 76, 786–790. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Novelty and “Homology-free” Morphometrics: What’s in a Name? Evol. Biol. 2008, 35, 186–190. [Google Scholar] [CrossRef]
- Bergmann, I.; Hublin, J.-J.; Ben-Ncer, A.; Sbihi-Alaoui, F.Z.; Gunz, P.; Freidline, S.E. The relevance of late MSA mandibles on the emergence of modern morphology in Northern Africa. Sci. Rep. 2022, 12, 8841. [Google Scholar] [CrossRef]
- Giuca, M.R.; Pasini, M.; Drago, S.; Del Corso, L.; Vanni, A.; Carli, E.; Manni, A. Influence of Vertical Facial Growth Pattern on Herbst Appliance Effects in Prepubertal Patients: A Retrospective Controlled Study. Int. J. Dent. 2020, 2020, 1018793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carli, E.; Pasini, M.; Lardani, L.; Giuca, G.; Miceli, M. Impact of self-ligating orthodontic brackets on dental biofilm and periodontal pathogens in adolescents. J. Biol. Regul. Homeost. Agents 2021, 35, 107–115. [Google Scholar] [PubMed]
- Montinaro, F.; Nucci, L.; D’Apuzzo, F.; Perillo, L.; Chiarenza, M.C.; Grassia, V. Oral nonsteroidal anti-inflammatory drugs as treatment of joint and muscle pain in temporomandibular disorders: A systematic review. Cranio 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Nanda, R.S.; Currier, G.F.; Nanda, S.K. Assessment of symphysis morphology as a predictor of the direction of mandibular growth. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sas, B. Oral manifestations in paediatric patients with hepatobiliary diseases: A review. J. Biol. Regul. Homeost. Agents 2021, 35, 117–125. [Google Scholar] [CrossRef]
- Giuca, M.R.; Carli, E.; Lardani, L.; Pasini, M.; Miceli, M.; Fambrini, E. Pediatric obstructive sleep apnea syndrome: Emerging evidence and treatment approach. Sci. World J. 2021, 2021, 5591251. [Google Scholar] [CrossRef]
- Jaju, P.P.; Jaju, S.P. Cone-beam computed tomography: Time to move from ALARA to ALADA. Imaging Sci. Dent. 2015, 45, 263–265. [Google Scholar] [CrossRef]


| Sample Characteristics | N° of Observations |
|---|---|
| Gender | |
| Female Male | 64 56 |
| Age | |
| 12 Years Old (T0) | 120 * |
| 15 Tears Old (T1) | 120 * |
| Vertical Skeletal Pattern | |
| Normodivergent (FMAˆ ≥ 26°) | 46 |
| Hypodivergent (FMAˆ = 25° ± 1°) | 43 |
| Hyperdivergent subjects (FMAˆ ≤ 24°) | 31 |
| T1 PCs | % Variance | Cumulative % |
|---|---|---|
| PC 1. | 60.897 | 60.897 |
| PC 2. | 21.292 | 82.189 |
| PC 3. | 6.619 | 88.808 |
| PC 4. | 6.176 | 94.984 |
| PC 5. | 2.198 | 97.183 |
| PC 6. | 1.001 | 98.184 |
| PC 7. | 0.627 | 98.890 |
| T0 PCs | % Variance | Cumulative % |
| PC 1. | 59.223 | 59.223 |
| PC 2. | 24.123 | 83.346 |
| PC 3. | 6.99 | 90.145 |
| PC 4. | 5.84 | 95.829 |
| PC 5. | 1.551 | 97.381 |
| PC 6. | 0.933 | 98.314 |
| PC 7. | 0.589 | 98.903 |
| Variable | T0 | T1 |
|---|---|---|
| Gender | p-value: 0.56 | p-value: 0.11 |
| Age | p-value: 0.71 |
| Variable | T0 | T1 |
|---|---|---|
| Skeletal pattern | ||
| - Hyper/Hypo - Normo/Hyper - Normo/Hypo | p-value: <0.001 p-value: 0.062 p-value: 0.34 | p-value: <0.001 p-value: 0.073 p-value: 0.38 |
| 2B-PLS | RV: 0,486; p-Value: < 0.001 | Cumulative Covariance |
|---|---|---|
| PLS1 | 87.01% | 87.01% |
| PLS2 | 7.57% | 94.58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, F.; Venezia, P.; Ronsivalle, V.; Lacagnina, C.; Conforte, C.; Isola, G.; Leonardi, R.; Lo Giudice, A. Geometric Morphometric Analysis of Mandibular Symphysis Growth between 12 and 15 Years of Age in Class II Malocclusion Subjects. Life 2023, 13, 543. https://doi.org/10.3390/life13020543
Ruiz F, Venezia P, Ronsivalle V, Lacagnina C, Conforte C, Isola G, Leonardi R, Lo Giudice A. Geometric Morphometric Analysis of Mandibular Symphysis Growth between 12 and 15 Years of Age in Class II Malocclusion Subjects. Life. 2023; 13(2):543. https://doi.org/10.3390/life13020543
Chicago/Turabian StyleRuiz, Ferdinando, Pietro Venezia, Vincenzo Ronsivalle, Calogero Lacagnina, Cristina Conforte, Gaetano Isola, Rosalia Leonardi, and Antonino Lo Giudice. 2023. "Geometric Morphometric Analysis of Mandibular Symphysis Growth between 12 and 15 Years of Age in Class II Malocclusion Subjects" Life 13, no. 2: 543. https://doi.org/10.3390/life13020543






