Microvascular and Endothelial Dysfunction in Prediabetes
Abstract
1. Introduction
2. Prediabetes and Cardiovascular Disease
3. Endothelial Dysfunction in Prediabetes
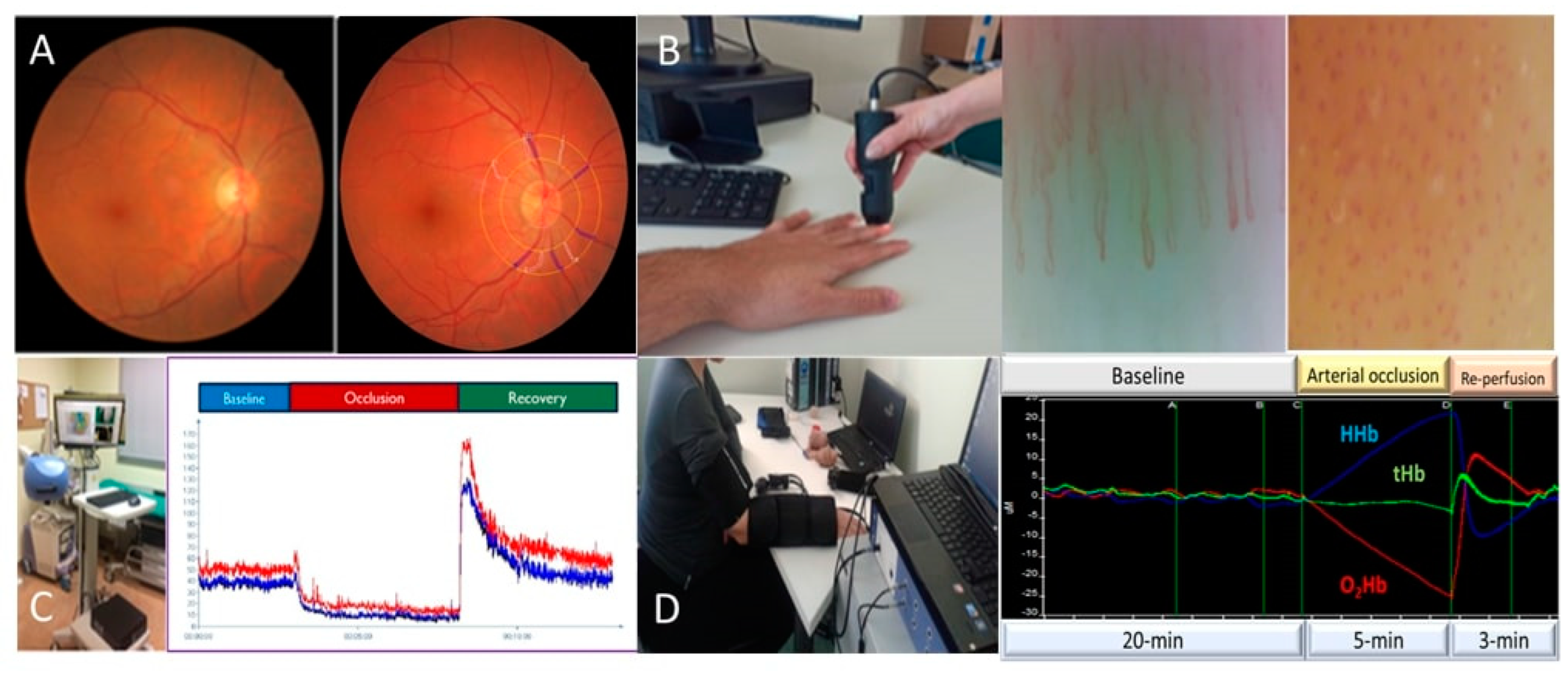
4. Prediabetes and Microcirculation
4.1. Prediabetes and the Retina
4.2. Prediabetes and Albuminuria
4.3. Prediabetes and Skin—Muscle Microcirculation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019 51 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Diabetes Statistics Report; CDC: Atlanta, GA, USA, 2020.
- Care, D. Classification and diagnosis of diabetes: Standards of medical care in diabetesd2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Diabetes Association. 2018. Available online: www.ede.gr (accessed on 8 October 2022).
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Buysschaert, M.; Medina, J.L.; Bergman, M.; Shah, A.; Lonier, J. Prediabetes and associated disorders. Endocrine 2015, 48, 371–393. [Google Scholar] [CrossRef]
- Roglic, G. WHO Global Report on Diabetes. Isbn 2016, 978, 92–94. [Google Scholar]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Pogue, J.; Mann, J.F.E.; Lonn, E.; Dagenais, G.R.; McQueen, M.; Yusuf, S. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: A prospective epidemiological analysis. Diabetologia 2005, 48, 1749–1755. [Google Scholar] [CrossRef]
- DECODE Study Group; European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003, 26, 688–696. [Google Scholar] [CrossRef]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose: The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B.; Wilson, P.W.F. Fasting and postchallenge glycemia and cardiovascular disease risk: The framingham offspring study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef]
- De Vegt, F.; Dekker, J.M.; Ruhé, H.G.; Stehouwer, C.D.A.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: The Hoorn study. Diabetologia 1999, 42, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Pyörälä, K.; Pyörälä, M.; Nissinen, A.; Lindström, J.; Tilvis, R.; Tuomilehto, J. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur. Heart J. 2002, 23, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Tenerz, Å.; Nilsson, G.; Hamsten, A.; Efendíc, S.; Rydén, L.; Malmberg, K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet 2002, 359, 2140–2144. [Google Scholar] [CrossRef]
- Bartnik, M.; Rydén, L.; Ferrari, R.; Malmberg, K.; Pyörälä, K.; Simoons, M.; Standl, E.; Soler-Soler, J.; Öhrvik, J.; Manini, M.; et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: The Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2004, 25, 1880–1890. [Google Scholar] [CrossRef]
- Osei, E.; Fonville, S.; Zandbergen, A.A.M.; Koudstaal, P.J.; Dippel, D.W.J.; den Hertog, H.M. Glucose in prediabetic and diabetic range and outcome after stroke. Acta Neurol. Scand. 2017, 135, 170–175. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Unwin, N. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. Br. J. Surg. 2000, 87, 328–337. [Google Scholar]
- Faghihimani, E.; Darakhshandeh, A.; Feizi, A.; Amini, M. Evaluation of Peripheral Arterial Disease in Prediabetes. Int. J. Prev. Med. 2014, 5, 1099. [Google Scholar] [PubMed]
- Silbernagel, G.; Rein, P.; Saely, C.H.; Engelberger, R.P.; Willenberg, T.; Do, D.D.; Kucher, N.; Baumgartner, I.; Drexel, H. Prevalence of type 2 diabetes is higher in peripheral artery disease than in coronary artery disease patients. Diabetes Vasc. Dis. Res. 2015, 12, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Quigley, F.; Velu, R.; Walker, P.J.; Moxon, J.V. Association of impaired fasting glucose, diabetes and their management with the presentation and outcome of peripheral artery disease: A cohort study. Cardiovasc. Diabetol. 2014, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Tabak, A.G.; Khunti, K.; Valabhji, J.; Majeed, A.; Millett, C.; Vamos, E.P. Association between pre-diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001061. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Gavriilaki, E.; Triantafyllou, A.; Douma, S. Clinical Significance of Endothelial Dysfunction in Essential Hypertension. Curr. Hypertens. Rep. 2015, 17, 85. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gavriilaki, E.; Douma, S.; Gkaliagkousi, E. Endothelial Dysfunction in Patients with Rheumatoid Arthritis: The Role of Hypertension. Curr. Hypertens. Rep. 2020, 22, 56. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr. Hypertens. Rep. 2020, 22, 63. [Google Scholar] [CrossRef]
- Milman, S.; Crandall, J.P. Mechanisms of Vascular Complications in Prediabetes. Med. Clin. North Am. 2011, 95, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Khalil, C.A. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.J.; Webb, D.J. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thr. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Kulkarni, H.; Mamtani, M.; Peralta, J.; Almeida, M.; Dyer, T.D.; Goring, H.H.; Johnson, M.P.; Duggirala, R.; Mahaney, M.C.; Olvera, R.L.; et al. Soluble forms of intercellular and vascular cell adhesion molecules independently predict progression to type 2 diabetes in Mexican American families. PLoS ONE 2016, 11, e0151177. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wang, X.M.; Zhang, X.L. Circulating Vascular Cell Adhesion Molecule-1 (VCAM-1) and Intercellular Adhesion Molecule-1 (ICAM-1): Relationship with carotid artery elasticity in patients with impaired glucose regulation (IGR). Ann. Endocrinol. 2019, 80, 72–76. [Google Scholar] [CrossRef]
- Eliana, F.; Suwondo, P.; Makmun, L.H.; Harbuwono, D.S. AADMA as a marker of endothelial dysfunction in prediabetic women. Acta Med. Indones. 2011, 43, 92–98. [Google Scholar]
- Su, Y.; Liu, X.M.; Sun, Y.M.; Jin, H.B.; Fu, R.; Wang, Y.Y.; Wu, Y.; Luan, Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int. J. Clin. Pract. 2008, 62, 877–882. [Google Scholar] [CrossRef]
- Maschirow, L.; Khalaf, K.; Al-Aubaidy, H.A.; Jelinek, H.F. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes—Biomarkers as a possible tool for early disease detection for rural screening. Clin. Biochem. 2015, 48, 581–585. [Google Scholar] [CrossRef]
- Gupta, A. Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. Br. J. Med. Med. Res. 2012, 2, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.I.; Ambrosio, G.; Pries, A.R.; Struijker-Boudier, H.A.J. Microcirculation in hypertension: A new target for treatment? Circulation 2001, 104, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E.K. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The Twenty-Five-Year Progression of Retinopathy in Persons with Type 1 Diabetes. Ophthalmology 2008, 115, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Cheug, N.; Wang, J.J.; Klein, R.; Couper, D.; Sharrett, A.R.; Wong, T.Y. Diabetic Retinopathy and the Risk of Coronary Heart Disease. Diabetes Care 2007, 30, 1742–1746. [Google Scholar] [CrossRef]
- Cheung, N.; Rogers, S.; Couper, D.J.; Klein, R.; Sharrett, A.R.; Wong, T.Y. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007, 38, 398–401. [Google Scholar] [CrossRef]
- Lamparter, J.; Raum, P.; Pfeiffer, N.; Peto, T.; Höhn, R.; Elflein, H.; Wild, P.; Schulz, A.; Schneider, A.; Mirshahi, A. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: The Gutenberg Health Study. J. Diabetes Complicat. 2014, 28, 482–487. [Google Scholar] [CrossRef]
- Frank, R.N. Diabetic Retinopathy. N. Eng. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef]
- Sairenchi, T.; Iso, H.; Yamagishi, K.; Irie, F.; Okubo, Y.; Gunji, J.; Muto, T.; Ota, H. Mild retinopathy is a risk factor for cardiovascular mortality in Japanese with and without hypertension the Ibaraki Prefectural Health Study. Circulation 2011, 124, 2502–2511. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Doumas, M.; Anyfanti, P.; Gkaliagkousi, E.; Zabulis, X.; Petidis, K.; Gavriilaki, E.; Karamaounas, P.; Gkolias, V.; Pyrpasopoulou, A.; et al. Divergent retinal vascular abnormalities in normotensive persons and patients with never-treated, masked, white coat hypertension. Am. J. Hypertens. 2013, 26, 318–325. [Google Scholar] [CrossRef]
- Anyfanti, P.; Triantafyllou, A.; Gkaliagkousi, E.; Koletsos, N.; Athanasopoulos, G.; Zabulis, X.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Retinal vessel morphology in rheumatoid arthritis: Association with systemic inflammation, subclinical atherosclerosis and cardiovascular risk. Microcirculation 2017, 24, e12417. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Chew, E.; Christophi, C.A.; Davis, M.D.; Fowler, S.; Goldstein, B.J.; Hamman, R.F.; Hubbard, L.D.; Knowler, W.C.; Molitch, M.E. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the diabetes prevention program. Diabet. Med. 2007, 24, 137–144. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wang, J.J.; Wong, T.Y. Retinal Vascular Changes in Pre-Diabetes and Prehypertension. Diabetes Care 2007, 30, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Lye, W.K.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Wang, J.J.; Mitchell, P.; Shaw, J.E.; Selvin, E.; Sharrett, A.R.; et al. Retinal microvascular calibre and risk of diabetes mellitus: A systematic review and participant-level meta-analysis. Diabetologia 2015, 58, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Huru, J.M.; Leiviskä, I.; Saarela, V.; Johanna Liinamaa, M. Prediabetes influences the structure of the macula: Thinning of the macula in the Northern Finland Birth Cohort. Br. J. Ophthalmol. 2021, 105, 1731–1737. [Google Scholar] [CrossRef]
- Ikram, M.K.; Janssen, J.A.M.J.L.; Roos, A.M.E.; Rietveld, I.; Witteman, J.C.M.; Breteler, M.M.B.; Hofman, A.; Van Duijn, C.M.; De Jong, P.T.V.M. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: The Rotterdam Study. Diabetes 2006, 55, 506–510. [Google Scholar] [CrossRef]
- Kifley, A.; Wang, J.J.; Cugati, S.; Wong, T.; Mitchell, P. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: The blue mountains eye study. Microcirculation 2008, 15, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wang, J.J.; Islam, F.M.A.; Mitchell, P.; Tapp, R.J.; Zimmet, P.Z.; Simpson, R.; Shaw, J.; Wong, T.Y. Retinal arteriolar narrowing predicts incidence of diabetes. Diabetes 2008, 57, 536–539. [Google Scholar] [CrossRef]
- Tien, Y.W.; Shankar, A.; Klein, R.; Klein, B.E.K.; Hubbard, L.D. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch. Intern. Med. 2005, 165, 1060–1065. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Richey Sharrett, A.; Schmidt, M.I.; Pankow, J.S.; Couper, D.J.; Klein, B.E.K.; Hubbard, L.D.; Duncan, B.B. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. J. Am. Med. Assoc. 2002, 287, 2528–2533. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.; Wang, Y.; Chu, Z.; Rk, W.; Lu, L.; Zou, H. Early Retinal Microvasculopathy in Prediabetic Patients and Correlated Factors. Ophthalmic Res. 2022, 66, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Ratra, D.; Angayarkanni, N.; Dalan, D.; Prakash, N.; Kaviarasan, K.; Thanikachalam, S.; Das, U. Quantitative analysis of retinal microvascular changes in prediabetic and diabetic patients. Indian J. Ophthalmol. 2021, 69, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Ratra, D.; Nagarajan, R.; Dalan, D.; Prakash, N.; Kuppan, K.; Thanikachalam, S.; Das, U.; Narayansamy, A. Early structural and functional neurovascular changes in the retina in the prediabetic stage. Eye 2021, 35, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.D.; Arango, F.J.; Parra, M.M.; Sánchez-Ávila, R.M.; Parra-Serrano, G.A.; Hoyos, A.T.; Granados, S.J.; Viteri, E.J.; Gaibor-Santos, I.; Perez, Y. Early microvascular changes in patients with prediabetes evaluated by optical coherence tomography angiography. Ther. Adv. Ophthalmol. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Peng, R.P.; Zhu, Z.Q.; Shen, H.Y.; Lin, H.M.; Zhong, L.; Song, S.Q.; Liu, T.; Ling, S.Q. Retinal Nerve and Vascular Changes in Prediabetes. Front. Med. 2022, 9, 777646. [Google Scholar] [CrossRef]
- Lott, M.E.J.; Slocomb, J.E.; Shivkumar, V.; Smith, B.; Quillen, D.; Gabbay, R.A.; Gardner, T.W.; Bettermann, K. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Act. Ophthalmol. 2013, 91, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Care, D. Microvascular complications and foot care: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S151–S167. [Google Scholar]
- American Diabetes Association. 9. Microvascular Complications and Foot Care. Diabetes Care 2014, 38, S58–S66. [Google Scholar]
- Halimi, J.M.; Hadjadj, S.; Aboyans, V.; Allaert, F.A.; Artigou, J.Y.; Beaufils, M.; Berrut, G.; Fauvel, J.P.; Gin, H.; Nitenberg, A.; et al. Microalbuminuria and urinary albumin excretion: French clinical practice guidelines. Diabetes Metab. 2007, 33, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Mann, J.F.E.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Hallé, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. J. Am. Med. Assoc. 2001, 286, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Tapp, R.J.; Shaw, J.E.; Zimmet, P.Z.; Balkau, B.; Chadban, S.J.; Tonkin, A.M.; Welborn, T.A.; Atkins, R.C. Albuminuria is evident in the early stages of diabetes onset: Results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am. J. Kidney Dis. 2004, 44, 792–798. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Narayan, K.M.; Weisman, D.; Golden, S.H.; Jaar, B.G. Association between prediabetes and risk of chronic kidney disease: A systematic review and meta-analysis. Diabet. Med. 2016, 33, 1615–1624. [Google Scholar] [CrossRef]
- Jung, D.H.; Byun, Y.S.; Kwon, Y.J.; Kim, G.S. Microalbuminuria as a simple predictor of incident diabetes over 8 years in the Korean Genome and Epidemiology Study (KoGES). Sci. Rep. 2017, 7, 15445. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xu, C.; Wan, Q. Association between microalbuminuria and outcome of non-diabetic population aged 40 years and over: The reaction study. Prim. Care Diabetes 2020, 14, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Makhlough, A.; Yousefi, A.; Kashi, Z.; Abediankenari, S. Correlation between prediabetes conditions and microalbuminuria. Nephrourol. Mon. 2013, 5, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Marrero, D.; Ma, Y.; Ackermann, R.; Narayan, K.M.V.; Barrett-Connor, E.; Watson, K.; Knowler, W.C.; Horton, E.S. Value of urinary albumin-to-creatinine ratio as a predictor of type 2 diabetes in pre-diabetic individuals. Diabetes Care 2008, 31, 2344–2348. [Google Scholar] [CrossRef]
- Schroijen, M.A.; de Mutsert, R.; Dekker, F.W.; de Vries, A.P.J.; de Koning, E.J.P.; Rabelink, T.J.; Rosendaal, F.R.; Dekkers, O.M. The association of glucose metabolism and kidney function in middle-aged adults. Clin. Kidney J. 2021, 14, 2383–2390. [Google Scholar] [CrossRef]
- Won, J.C.; Lee, Y.J.; Kim, J.M.; Han, S.Y.; Noh, J.H.; Ko, K.S.; Rhee, B.D.; Kim, D.J. Prevalence of and factors associated with albuminuria in the Korean adult population: The 2011 Korea National Health and Nutrition Examination Survey. PLoS ONE 2013, 8, e83273. [Google Scholar] [CrossRef]
- Markus, M.R.P.; Ittermann, T.; Baumeister, S.E.; Huth, C.; Thorand, B.; Herder, C.; Roden, M.; Siewert-Markus, U.; Rathmann, W.; Koenig, W.; et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: The KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 234–242. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, K.J.; Kim, B.Y.; Jung, C.H.; Mok, J.O.; Kang, S.K.; Kim, H.K. Prediabetes is not independently associated with microalbuminuria in Korean general population: The Korea National Health and Nutrition Examination Survey 2011-2012 (KNHANES V-2,3). Diabetes Res. Clin. Pract. 2014, 106, e18–e21. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Ciolkiewicz, M.; Klimiuk, P.A.; Sierakowski, S. Clinical significance of nailfold capillaroscopy in systemic lupus erythematosus: Correlation with endothelial cell activation markers and disease activity. Scand. J. Rheumatol. 2009, 38, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Grassi, W.; Matucci Cerinic, M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003, 48, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Anyfanti, P.; Angeloudi, E.; Dara, A.; Arvanitaki, A.; Bekiari, E.; Kitas, G.D.; Dimitroulas, T. Nailfold Videocapillaroscopy for the Evaluation of Peripheral Microangiopathy in Rheumatoid Arthritis. Life 2022, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Anyfanti, P.; Pyrpasopoulou, A.; Triantafyllou, G.; Aslanidis, S.; Douma, S. Capillary rarefaction as an index for the microvascular assessment of hypertensive patients. Curr. Hypertens. Rep. 2015, 17, 33. [Google Scholar] [CrossRef]
- Antonios, T.F.T.; Singer, D.R.J.; Markandu, N.D.; Mortimer, P.S.; MacGregor, G.A. Structural skin capillary rarefaction in essential hypertension. Hypertension 1999, 33, 998–1001. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gkaliagkousi, E.; Triantafyllou, A.; Zabulis, X.; Dolgyras, P.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Dermal capillary rarefaction as a marker of microvascular damage in patients with rheumatoid arthritis: Association with inflammation and disorders of the macrocirculation. Microcirculation 2018, 25, e12451. [Google Scholar] [CrossRef]
- Uyar, S.; Balkarli, A.; Erol, M.K.; Yeşil, B.; Tokuç, A.; Durmaz, D.; Görar, S.; Çekin, A.H. Assessment of the relationship between diabetic retinopathy and nailfold capillaries in type 2 diabetics with a noninvasive method: Nailfold videocapillaroscopy. J. Diabetes Res. 2016, 2016, 7592402. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Cicco, G.; Cignarelli, A.; Garruti, G.; Laviola, L.; Giorgino, F. Computerized video-capillaroscopy alteration related to diabetes mellitus and its complications. In Oxygen Transport to Tissue XL; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1072, pp. 363–368. [Google Scholar]
- Hsu, P.C.; Liao, P.Y.; Chang, H.H.; Chiang, J.Y.; Huang, Y.C.; Lo, L.C. Nailfold capillary abnormalities are associated with type 2 diabetes progression and correlated with peripheral neuropathy. Med. (United States) 2016, 95, e5714. [Google Scholar] [CrossRef]
- Barchetta, I.; Riccieri, V.; Vasile, M.; Stefanantoni, K.; Comberiati, P.; Taverniti, L.; Cavallo, M.G. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet. Med. 2011, 28, 1039–1044. [Google Scholar] [CrossRef]
- Rajaei, A.; Dehghan, P.; Farahani, Z. Nailfold Capillaroscopy Findings in Diabetic Patients (A Pilot Cross-Sectional Study). Open J. Pathol. 2015, 05, 65–72. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Zarzycki, W.; Dubicki, A.; Moskal, D.; Kosztyła-Hojna, B.; Hryniewicz, A. Clinical usefulness of videocapillaroscopy and selected endothelial cell activation markers in people with Type 1 diabetes mellitus complicated by microangiopathy. Adv. Med. Sci. 2017, 62, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Irving, R.J.; Walker, B.R.; Noon, J.P.; Watt, G.C.M.; Webb, D.J.; Shore, A.C. Microvascular correlates of blood pressure, plasma glucose, and insulin resistance in health. Cardiovasc. Res. 2002, 53, 271–276. [Google Scholar] [CrossRef]
- Serné, E.H.; Stehouwer, C.D.A.; Ter Maaten, J.C.; Ter Wee, P.M.; Rauwerda, J.A.; Donker, A.J.M.; Gans, R.O.B. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation 1999, 99, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef]
- Koletsos, N.; Gkaliagkousi, E.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Dolgyras, P.; DIpla, K.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology 2021, 60, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Margouta, A.; Anyfanti, P.; Lazaridis, A.; Nikolaidou, B.; Mastrogiannis, K.; Malliora, A.; Patsatsi, A.; Triantafyllou, A.; Douma, S.; Doumas, M.; et al. Blunted Microvascular Reactivity in Psoriasis Patients in the Absence of Cardiovascular Disease, as Assessed by Laser Speckle Contrast Imaging. Life 2022, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Anyfanti, P.; Gavriilaki, E.; Dolgyras, P.; Nikolaidou, B.; Dimitriadou, A.; Lazaridis, A.; Mastrogiannis, K.; Koletsos, N.; Triantafyllou, A.; Dimitroulas, T.; et al. Skin microcirculation dynamics are impaired in patients with rheumatoid arthritis and no cardiovascular comorbidities. Clin. Exp. Rheumatol. 2023. [Google Scholar] [CrossRef]
- Dolgyras, P.; Lazaridis, A.; Anyfanti, P.; Gavriilaki, E.; Koletsos, N.; Triantafyllou, A.; Nikolaidou, B.; Galanapoulou, V.; Douma, S.; Gkaliagkousi, E. Microcirculation dynamics in systemic vasculitis: Evidence of impaired microvascular response regardless of cardiovascular risk factors. Rheumatology 2022, keac652. [Google Scholar] [CrossRef]
- Lazaridis, A.; Triantafyllou, A.; Dipla, K.; Dolgyras, P.; Koletsos, N.; Anyfanti, P.; Aslanidis, S.; Douma, S.; Gkaliagkousi, E. Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens. Res. 2022, 45, 445–454. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Lazaridis, A.; Anyfanti, P.; Stavropoulos, K.; Imprialos, K.; Triantafyllou, A.; Mastrogiannis, K.; Douma, S.; Doumas, M. Assessment of skin microcirculation in primary aldosteronism: Impaired microvascular responses compared to essential hypertensives and normotensives. J. Hum. Hypertens. 2022, 36, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- de Matheus, A.S.; Clemente, E.L.S.; de Lourdes Guimarães Rodrigues, M.; Torres Valença, D.C.; Gomes, M.B.; Alessandra, A.S.; Clemente, E.L.S.; de Lourdes Guimarães Rodrigues, M.; Torres Valença, D.C.; Gomes, M.B. Assessment of microvascular endothelial function in type 1 diabetes using laser speckle contrast imaging. J. Diabetes Complicat. 2017, 31, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Mennes, O.A.; Van Netten, J.J.; Van Baal, J.G.; Steenbergen, W. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol. Meas. 2019, 40, 065002. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Triantafyllou, A.; Grigoriadou, I.; Kintiraki, E.; Triantafyllou, G.A.; Poulios, P.; Vrabas, I.S.; Zafeiridis, A.; Douma, S.; Goulis, D.G. Impairments in microvascular function and skeletal muscle oxygenation in women with gestational diabetes mellitus: Links to cardiovascular disease risk factors. Diabetologia 2017, 60, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.K.; Deysher, D.M.; Wu, E.E.; Barstow, T.J. Reduced insulin sensitivity in young, normoglycaemic subjects alters microvascular tissue oxygenation during postocclusive reactive hyperaemia. Exp. Physiol. 2019, 104, 967–974. [Google Scholar] [CrossRef]
- Soares, R.N.; Reimer, R.A.; Murias, J.M. Changes in vascular responsiveness during a hyperglycemia challenge measured by near-infrared spectroscopy vascular occlusion test. Microvasc. Res. 2017, 111, 67–71. [Google Scholar] [CrossRef] [PubMed]
| Peripheral Organ/Tissue | Non-Invasive Methods for Microvascular Assessment |
|---|---|
| Retinal microvasculature | Retinal photography, for the qualitative and quantitative evaluation of the retinal microvasculature (e.g., evaluation of retinal microvascular diameters) |
| Optical coherence tomography angiography, for assessing with high accuracy the retinal vessels and the macula area | |
| Flickering light stimulus, to assess microvascular responses (specifically, in retinal blood flow and diameters) | |
| Renal microvascular injury | Urinary albumin excretion (UAE), as an index of renal glomerular dysfunction |
| Skin microvascular network | Nailfold capillaroscopy and video capillaroscopy, for the qualitative and quantitative assessment of the dermal microcirculation (e.g., dermal capillary rarefaction) |
| Laser doppler flowmetry (LDF), for the evaluation of dermal microvascular reactivity | |
| Laser Speckle Contrast Imaging (LSCI), as an evolution of older LDF techniques | |
| Peripheral arterial tonometry (PAT), for the evaluation of peripheral arterial vascular tone on the finger | |
| Skeletal muscle microvasculature | Near infrared spectroscopy (NIRS), for monitoring of regional tissue oxygenation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamprou, S.; Koletsos, N.; Mintziori, G.; Anyfanti, P.; Trakatelli, C.; Kotsis, V.; Gkaliagkousi, E.; Triantafyllou, A. Microvascular and Endothelial Dysfunction in Prediabetes. Life 2023, 13, 644. https://doi.org/10.3390/life13030644
Lamprou S, Koletsos N, Mintziori G, Anyfanti P, Trakatelli C, Kotsis V, Gkaliagkousi E, Triantafyllou A. Microvascular and Endothelial Dysfunction in Prediabetes. Life. 2023; 13(3):644. https://doi.org/10.3390/life13030644
Chicago/Turabian StyleLamprou, Stamatina, Nikolaos Koletsos, Gesthimani Mintziori, Panagiota Anyfanti, Christina Trakatelli, Vasileios Kotsis, Eugenia Gkaliagkousi, and Areti Triantafyllou. 2023. "Microvascular and Endothelial Dysfunction in Prediabetes" Life 13, no. 3: 644. https://doi.org/10.3390/life13030644
APA StyleLamprou, S., Koletsos, N., Mintziori, G., Anyfanti, P., Trakatelli, C., Kotsis, V., Gkaliagkousi, E., & Triantafyllou, A. (2023). Microvascular and Endothelial Dysfunction in Prediabetes. Life, 13(3), 644. https://doi.org/10.3390/life13030644






