Immune Cell Functionality during Decidualization and Potential Clinical Application
Abstract
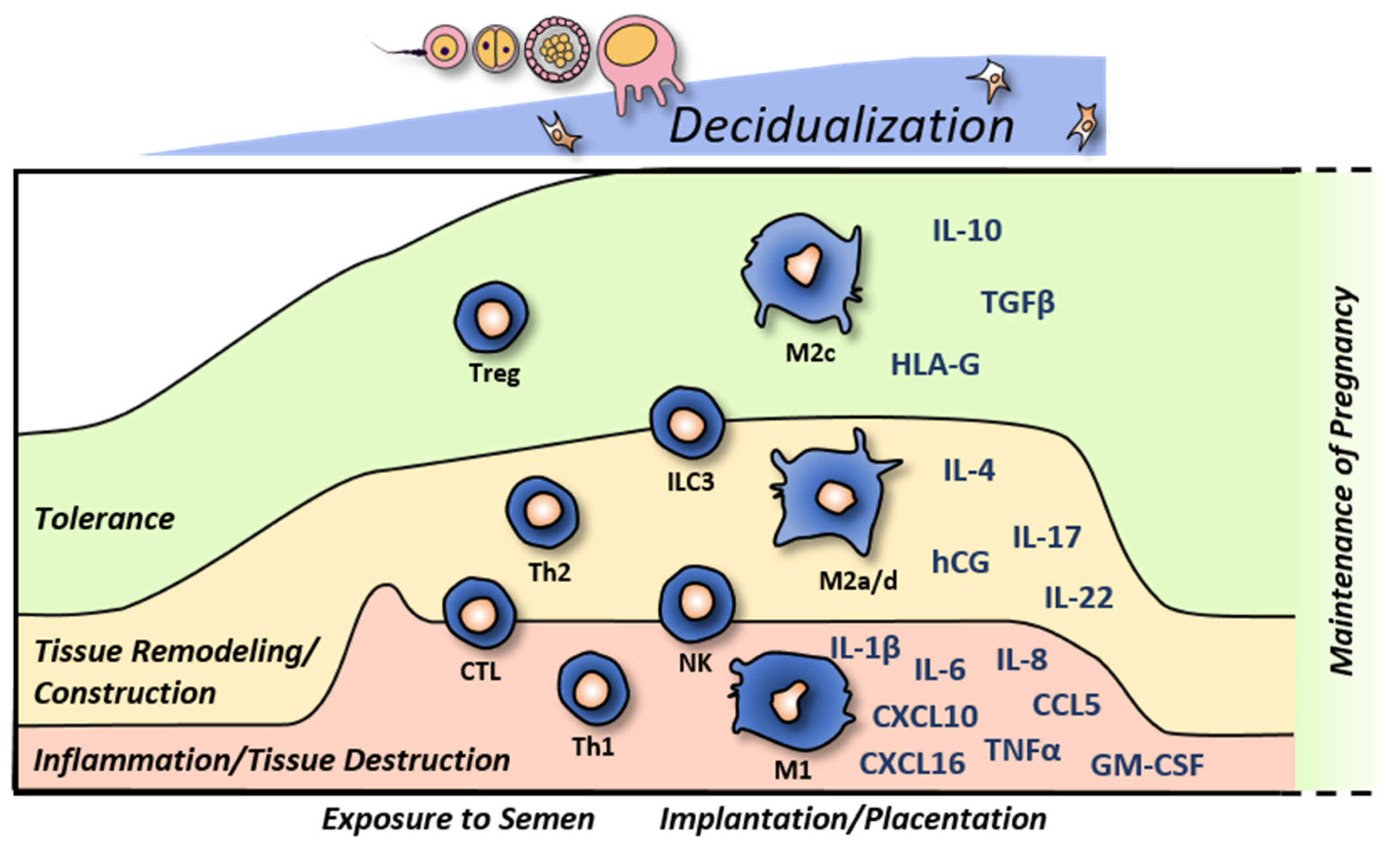
:1. Introduction
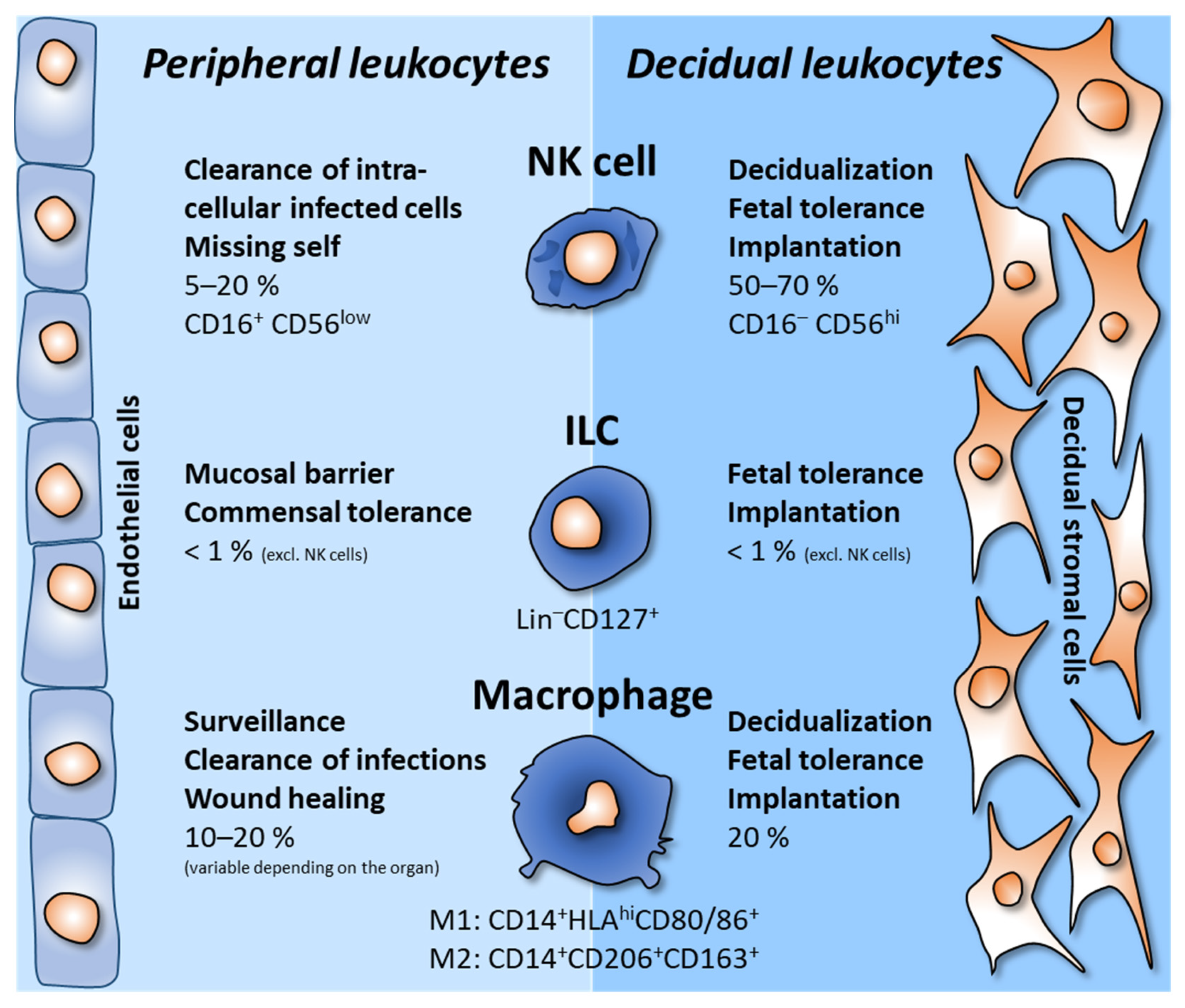
2. Decidual Innate Lymphoid Cells
3. Decidual Macrophages
4. Other Leukocytes in the Decidua
5. Immune Implications in Adverse Pregnancy Outcomes
6. Clinical Significance in Reproductive Medicine
6.1. Targeting Regeneration
6.1.1. Endometrial Scratching
6.1.2. Platelet-Rich Plasma
6.2. Targeting Immune Balance
6.2.1. Diagnostic Tools
6.2.2. Interventions
Glucocorticoids
Intralipid
Tumor Necrosis Factor α Inhibition
Intravenous Immunoglobulin
Tacrolimus
Heparin
Granulocyte Colony Stimulating Factor
Intrauterine Injection of hCG
Seminal Plasma and Paternal Antigens
| Diagnostics | Interventions | Objectives |
|---|---|---|
| NK cell count Plasma cell count Treg cell count Th1:Th2 ratios [194,195,202,203,204,205] | Glucocorticoids [219], intrauterine application of phospholipid-stabilized soybean oil [225,232], anti-TNFα [235,236,237], hCG infusion [276,277,278], immunoglobulins [247,248,249], tacrolimus [252], heparin [257,258,259], G-CSF [267,269,270] Immunization with partner antigens | Balanced tolerogenic Micromilieu Balanced inflammatory micromilieu |
| Microbiome [197] | Antibiotics, Pre- and Probiotics | Modify colonizers |
| Recurrent implantation failure Thin endometrial lining | Scratching [171,172,173,174,175,176,177,178], PRP infusion [183,184,185,186,187,188], G-CSF [267,269,270] | Wound-healing-like Decidualization, Regeneration |
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chavan, A.R.; Griffith, O.W.; Wagner, G.P. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 2017, 47, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.C.; Haslam, S.M.; Dell, A.; Clark, G.F. The human fetoembryonic defense system hypothesis: Twenty years on. Mol. Asp. Med. 2016, 51, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Haque, N. Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol. 2020, 39, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.; Muzzio, D.; Soldati, R.; Fest, S.; Zenclussen, A.C. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol. Reprod. 2013, 89, 90. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef]
- Ozturk, S.; Demir, R. Particular functions of estrogen and progesterone in establishment of uterine receptivity and embryo implantation. Histol. Histopathol. 2010, 25, 1215–1228. [Google Scholar] [CrossRef]
- Liao, H.Q.; Han, M.T.; Cheng, W.; Zhang, C.; Li, H.; Li, M.Q.; Zhu, R. Decidual-derived RANKL facilitates macrophages accumulation and residence at the maternal-fetal interface in human early pregnancy. Am. J. Reprod. Immunol. 2021, 86, e13406. [Google Scholar] [CrossRef]
- Kajimura, S.; Aida, K.; Duan, C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc. Natl. Acad. Sci. USA 2005, 102, 1240–1245. [Google Scholar] [CrossRef]
- Gordon, S.M. Interleukin-15 in Outcomes of Pregnancy. Int. J. Mol. Sci. 2021, 22, 11094. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liang, X.; Liang, X.H.; Wang, T.S.; Qi, Q.R.; Deng, W.B.; Sha, A.G.; Yang, Z.M. The mesenchymal-epithelial transition during in vitro decidualization. Reprod. Sci. 2013, 20, 354–360. [Google Scholar] [CrossRef]
- Afshar, Y.; Jeong, J.W.; Roqueiro, D.; DeMayo, F.; Lydon, J.; Radtke, F.; Radnor, R.; Miele, L.; Fazleabas, A. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012, 26, 282–294. [Google Scholar] [CrossRef]
- Camden, A.J.; Szwarc, M.M.; Chadchan, S.B.; DeMayo, F.J.; O’Malley, B.W.; Lydon, J.P.; Kommagani, R. Growth regulation by estrogen in breast cancer 1 (GREB1) is a novel progesterone-responsive gene required for human endometrial stromal decidualization. Mol. Hum. Reprod. 2017, 23, 646–653. [Google Scholar] [CrossRef]
- Freis, A.; Renke, T.; Kammerer, U.; Jauckus, J.; Strowitzki, T.; Germeyer, A. Effects of a hyperandrogenaemic state on the proliferation and decidualization potential in human endometrial stromal cells. Arch. Gynecol. Obstet. 2017, 295, 1005–1013. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Edelshain, B.; Schatz, F.; Lockwood, C.J.; Taylor, H.S. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction 2012, 143, 531–538. [Google Scholar] [CrossRef]
- Macdonald, L.J.; Sales, K.J.; Grant, V.; Brown, P.; Jabbour, H.N.; Catalano, R.D. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol. Hum. Reprod. 2011, 17, 626–636. [Google Scholar] [CrossRef]
- Adams, N.R.; Vasquez, Y.M.; Mo, Q.; Gibbons, W.; Kovanci, E.; DeMayo, F.J. WNK lysine deficient protein kinase 1 regulates human endometrial stromal cell decidualization, proliferation, and migration in part through mitogen-activated protein kinase 7. Biol. Reprod. 2017, 97, 400–412. [Google Scholar] [CrossRef]
- Voorzanger-Rousselot, N.; Goehrig, D.; Journe, F.; Doriath, V.; Body, J.J.; Clezardin, P.; Garnero, P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br. J. Cancer 2007, 97, 964–970. [Google Scholar] [CrossRef]
- Abir, R.; Ao, A.; Zhang, X.Y.; Garor, R.; Nitke, S.; Fisch, B. Vascular endothelial growth factor A and its two receptors in human preantral follicles from fetuses, girls, and women. Fertil. Steril. 2010, 93, 2337–2347. [Google Scholar] [CrossRef]
- Boutros, T.; Chevet, E.; Metrakos, P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol. Rev. 2008, 60, 261–310. [Google Scholar] [CrossRef]
- Wang, J.; Fu, L.; Gu, F.; Ma, Y. Notch1 is involved in migration and invasion of human breast cancer cells. Oncol. Rep. 2011, 26, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.C.; Selam, F.B.; Taylor, H.S. HOXA10 regulates p53 expression and matrigel invasion in human breast cancer cells. Cancer Biol. Ther. 2004, 3, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Chen, L.; Phillips, T.A.; Azzola, D.; Sedlmayr, P.; Hunt, J.S. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod. 2003, 68, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Y.; Ding, C.; Zhang, R.; Duan, T.; Zhou, Q. Decreased B7-H3 promotes unexplained recurrent miscarriage via RhoA/ROCK2 signaling pathway and regulates the secretion of decidual NK cellsdagger. Biol. Reprod. 2023, 108, 504–518. [Google Scholar] [CrossRef]
- Krstic, J.; Deutsch, A.; Fuchs, J.; Gauster, M.; Gorsek Sparovec, T.; Hiden, U.; Krappinger, J.C.; Moser, G.; Pansy, K.; Szmyra, M.; et al. (Dis)similarities between the Decidual and Tumor Microenvironment. Biomedicines 2022, 10, 1065. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Sethi, B.K.; Chanukya, G.V.; Nagesh, V.S. Prolactin and cancer: Has the orphan finally found a home? Indian J. Endocrinol. Metab. 2012, 16, S195–S198. [Google Scholar] [CrossRef]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simon, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knofler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Helige, C.; Ahammer, H.; Moser, G.; Hammer, A.; Dohr, G.; Huppertz, B.; Sedlmayr, P. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: A quantitative evaluation. Hum. Reprod. 2014, 29, 8–17. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and during Viral Infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Bartmann, C.; Segerer, S.E.; Rieger, L.; Kapp, M.; Sutterlin, M.; Kammerer, U. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am. J. Reprod. Immunol. 2014, 71, 109–119. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Garcia-Flores, V.; Romero, R.; Gomez-Lopez, N. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front. Immunol. 2018, 9, 2396. [Google Scholar] [CrossRef]
- Wang, P.; Liang, T.; Zhan, H.; Zhu, M.; Wu, M.; Qian, L.; Zhou, Y.; Ni, F. Unique metabolism and protein expression signature in human decidual NK cells. Front. Immunol. 2023, 14, 1136652. [Google Scholar] [CrossRef]
- Keskin, D.B.; Allan, D.S.; Rybalov, B.; Andzelm, M.M.; Stern, J.N.; Kopcow, H.D.; Koopman, L.A.; Strominger, J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3378–3383. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, M.; Frincu, F.; Plotogea, M.; Mehedintu, C. Recurrent Abortion and the Involvement of Killer-Cell Immunoglobulin-like Receptor (KIR) Genes, Activated T Cells, NK Abnormalities, and Cytokine Profiles. J. Clin. Med. 2023, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.R.; Meissner, T.B.; Tilburgs, T.; Strominger, J.L. HLA-G: At the Interface of Maternal-Fetal Tolerance. Trends Immunol. 2017, 38, 272–286. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.; Lukassen, H.G.; van Lierop, M.J.; Wijnands, F.; Mosselman, S.; Braat, D.D.; Joosten, I. Membrane-bound HLA-G activates proliferation and interferon-gamma production by uterine natural killer cells. Mol. Hum. Reprod. 2004, 10, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Houser, B.L.; Nicotra, M.L.; Strominger, J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5767–5772. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- De Oliveira, L.G.; Lash, G.E.; Murray-Dunning, C.; Bulmer, J.N.; Innes, B.A.; Searle, R.F.; Sass, N.; Robson, S.C. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta 2010, 31, 595–601. [Google Scholar] [CrossRef]
- Robson, A.; Harris, L.K.; Innes, B.A.; Lash, G.E.; Aljunaidy, M.M.; Aplin, J.D.; Baker, P.N.; Robson, S.C.; Bulmer, J.N. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012, 26, 4876–4885. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamamoto, T.; Yamazaki, M.; Murase, T.; Matsuno, T.; Chishima, F. Natural Cytotoxicity Receptors in Decidua Natural Killer Cells of Term Normal Pregnancy. J. Pregnancy 2018, 2018, 4382084. [Google Scholar] [CrossRef]
- Du, M.R.; Wang, S.C.; Li, D.J. The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Kurian, N.K.; Rao, K.A. Cytokines, NK cells and regulatory T cell functions in normal pregnancy and reproductive failures. Am. J. Reprod. Immunol. 2023, 89, e13667. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Montaldo, E.; Croxatto, D.; Loiacono, F.; Canegallo, F.; Venturini, P.L.; Moretta, L.; Mingari, M.C. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 2015, 8, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Doisne, J.M.; Balmas, E.; Boulenouar, S.; Gaynor, L.M.; Kieckbusch, J.; Gardner, L.; Hawkes, D.A.; Barbara, C.F.; Sharkey, A.M.; Brady, H.J.; et al. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J. Immunol. 2015, 195, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Einenkel, R.; Ehrhardt, J.; Hartmann, K.; Kruger, D.; Muzzio, D.O.; Zygmunt, M. Hormonally controlled ILC antigen presentation potential is reduced during pregnancy. Reproduction 2020, 160, 155–169. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Silva, P.; Panaitescu, B.; Theis, K.R.; Arif, A.; Hassan, S.S.; Gomez-Lopez, N. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am. J. Reprod. Immunol. 2018, 79, e12820. [Google Scholar] [CrossRef]
- Vazquez, J.; Chasman, D.A.; Lopez, G.E.; Tyler, C.T.; Ong, I.M.; Stanic, A.K. Transcriptional and Functional Programming of Decidual Innate Lymphoid Cells. Front. Immunol. 2019, 10, 3065. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Vacca, P.; Vitale, C.; Munari, E.; Cassatella, M.A.; Mingari, M.C.; Moretta, L. Human Innate Lymphoid Cells: Their Functional and Cellular Interactions in Decidua. Front. Immunol. 2018, 9, 1897. [Google Scholar] [CrossRef]
- Pongcharoen, S.; Niumsup, P.; Sanguansermsri, D.; Supalap, K.; Butkhamchot, P. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am. J. Reprod. Immunol. 2006, 55, 291–300. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Li, M.Q.; Li, D.J.; Jin, L.P. IL-22 secreted by decidual stromal cells and NK cells promotes the survival of human trophoblasts. Int. J. Clin. Exp. Pathol. 2013, 6, 1781–1790. [Google Scholar]
- O’Hern Perfetto, C.; Fan, X.; Dahl, S.; Krieg, S.; Westphal, L.M.; Bunker Lathi, R.; Nayak, N.R. Expression of interleukin-22 in decidua of patients with early pregnancy and unexplained recurrent pregnancy loss. J. Assist. Reprod. Genet. 2015, 32, 977–984. [Google Scholar] [CrossRef]
- Perricone, R.; De Carolis, C.; Giacomelli, R.; Guarino, M.D.; De Sanctis, G.; Fontana, L. GM-CSF and pregnancy: Evidence of significantly reduced blood concentrations in unexplained recurrent abortion efficiently reverted by intravenous immunoglobulin treatment. Am. J. Reprod. Immunol. 2003, 50, 232–237. [Google Scholar] [CrossRef]
- Robertson, S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef]
- Einenkel, R.; Ehrhardt, J.; Zygmunt, M.; Muzzio, D.O. Oxygen regulates ILC3 antigen presentation potential and pregnancy-related hormone actions. Reprod. Biol. Endocrinol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef]
- Wei, C.Y.; Li, M.Q.; Zhu, X.Y.; Li, D.J. Immune status of decidual macrophages is dependent on the CCL2/CCR2/JAK2 pathway during early pregnancy. Am. J. Reprod. Immunol. 2021, 86, e13480. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: A possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012, 143, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Kabawat, S.E.; Mostoufi-Zadeh, M.; Driscoll, S.G.; Bhan, A.K. Implantation site in normal pregnancy. A study with monoclonal antibodies. Am. J. Pathol. 1985, 118, 76–84. [Google Scholar] [PubMed]
- Pan, Y.; Yang, L.; Chen, D.; Hou, H.; Zhang, M.; Chen, M.; Ning, F.; Lu, Q.; Zhao, M.; Li, L.; et al. Decidual macrophage derived MMP3 contributes to extracellular matrix breakdown in spiral artery remodeling in early human pregnancy. J. Reprod. Immunol. 2022, 150, 103494. [Google Scholar] [CrossRef]
- Sun, F.; Wang, S.; Du, M. Functional regulation of decidual macrophages during pregnancy. J. Reprod. Immunol. 2021, 143, 103264. [Google Scholar] [CrossRef]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef]
- Ding, J.; Yang, C.; Zhang, Y.; Wang, J.; Zhang, S.; Guo, D.; Yin, T.; Yang, J. M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J. Cell. Mol. Med. 2021, 25, 2136–2147. [Google Scholar] [CrossRef]
- Sunderkotter, C.; Steinbrink, K.; Goebeler, M.; Bhardwaj, R.; Sorg, C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994, 55, 410–422. [Google Scholar] [CrossRef]
- Mor, G.; Abrahams, V.M. Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol. 2003, 1, 119. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Aldo, P.; You, Y.; Ding, J.; Kaislasuo, J.; Petersen, J.F.; Lokkegaard, E.; Peng, G.; Paidas, M.J.; Simpson, S.; et al. Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. J. Leukoc. Biol. 2020, 108, 983–998. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015, 194, 1534–1544. [Google Scholar] [CrossRef]
- Aldo, P.B.; Racicot, K.; Craviero, V.; Guller, S.; Romero, R.; Mor, G. Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am. J. Reprod. Immunol. 2014, 72, 270–284. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhou, W.J.; Hou, X.X.; Fu, Q.; Li, D.J. Correction: Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell. Mol. Immunol. 2019, 16, 313. [Google Scholar] [CrossRef]
- Parasar, P.; Guru, N.; Nayak, N.R. Contribution of macrophages to fetomaternal immunological tolerance. Hum. Immunol. 2021, 82, 325–331. [Google Scholar] [CrossRef]
- Houser, B.L. Decidual macrophages and their roles at the maternal-fetal interface. Yale J. Biol. Med. 2012, 85, 105–118. [Google Scholar]
- Heikkinen, J.; Mottonen, M.; Komi, J.; Alanen, A.; Lassila, O. Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 2003, 131, 498–505. [Google Scholar] [CrossRef]
- Unal, E.R.; Cierny, J.T.; Roedner, C.; Newman, R.; Goetzl, L. Maternal inflammation in spontaneous term labor. Am. J. Obstet. Gynecol. 2011, 204, 223.E1–223.E5. [Google Scholar] [CrossRef]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biol. Reprod. 2012, 86, 39. [Google Scholar] [CrossRef]
- Gardner, L.; Moffett, A. Dendritic cells in the human decidua. Biol. Reprod. 2003, 69, 1438–1446. [Google Scholar] [CrossRef]
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal-fetal interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; van der Mast, B.J.; Nagtzaam, N.M.; Roelen, D.L.; Scherjon, S.A.; Claas, F.H. Expression of NK cell receptors on decidual T cells in human pregnancy. J. Reprod. Immunol. 2009, 80, 22–32. [Google Scholar] [CrossRef]
- Wang, S.; Sun, F.; Li, M.; Qian, J.; Chen, C.; Wang, M.; Zang, X.; Li, D.; Yu, M.; Du, M. The appropriate frequency and function of decidual Tim-3+CTLA-4+CD8+ T cells are important in maintaining normal pregnancy. Cell Death Dis. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.; Zenclussen, A.C.; Jensen, F. The role of B cells in pregnancy: The good and the bad. Am. J. Reprod. Immunol. 2013, 69, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, U.; Eggert, A.O.; Kapp, M.; McLellan, A.D.; Geijtenbeek, T.B.; Dietl, J.; van Kooyk, Y.; Kampgen, E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 2003, 162, 887–896. [Google Scholar] [CrossRef]
- Gori, S.; Soczewski, E.; Fernandez, L.; Grasso, E.; Gallino, L.; Merech, F.; Colado, A.; Borge, M.; Perez Leiros, C.; Salamone, G.; et al. Decidualization Process Induces Maternal Monocytes to Tolerogenic IL-10-Producing Dendritic Cells (DC-10). Front. Immunol. 2020, 11, 1571. [Google Scholar] [CrossRef]
- Qin, D.; Xu, H.; Chen, Z.; Deng, X.; Jiang, S.; Zhang, X.; Bao, S. The peripheral and decidual immune cell profiles in women with recurrent pregnancy loss. Front. Immunol. 2022, 13, 994240. [Google Scholar] [CrossRef]
- Hou, R.; Huang, R.; Zhou, Y.; Lin, D.; Xu, J.; Yang, L.; Wei, X.; Xie, Z.; Zhou, Q. Single-cell profiling of the microenvironment in decidual tissue from women with missed abortions. Fertil. Steril. 2023, 119, 492–503. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2022, 24, 132. [Google Scholar] [CrossRef]
- Lai, N.; Fu, X.; Hei, G.; Song, W.; Wei, R.; Zhu, X.; Guo, Q.; Zhang, Z.; Chu, C.; Xu, K.; et al. The Role of Dendritic Cell Subsets in Recurrent Spontaneous Abortion and the Regulatory Effect of Baicalin on It. J. Immunol. Res. 2022, 2022, 9693064. [Google Scholar] [CrossRef]
- Esparvarinha, M.; Madadi, S.; Aslanian-Kalkhoran, L.; Nickho, H.; Dolati, S.; Pia, H.; Danaii, S.; Taghavi, S.; Yousefi, M. Dominant immune cells in pregnancy and pregnancy complications: T helper cells (TH1/TH2, TH17/Treg cells), NK cells, MDSCs, and the immune checkpoints. Cell Biol. Int. 2023, 47, 507–519. [Google Scholar] [CrossRef]
- Genest, G.; Banjar, S.; Almasri, W.; Beauchamp, C.; Benoit, J.; Buckett, W.; Dzineku, F.; Gold, P.; Dahan, M.H.; Jamal, W.; et al. Immunomodulation for unexplained recurrent implantation failure: Where are we now? Reproduction 2023, 165, R39–R60. [Google Scholar] [CrossRef]
- Mukherjee, N.; Sharma, R.; Modi, D. Immune alterations in recurrent implantation failure. Am. J. Reprod. Immunol. 2023, 89, e13563. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef]
- Loeb, L. Über die experimentelle Erzeugung von Knoten von Deciduagewebe in dem Uterus des Meerschweinchens nach stattgefundener Copulation. Zent. Allg. Pathol. Pathol. Anat. 1907, 18, 563–565. [Google Scholar]
- Barash, A.; Dekel, N.; Fieldust, S.; Segal, I.; Schechtman, E.; Granot, I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003, 79, 1317–1322. [Google Scholar] [CrossRef]
- Karow, W.G.; Gentry, W.C.; Skeels, R.F.; Payne, S.A. Endometrial biopsy in the luteal phase of the cycle of conception. Fertil. Steril. 1971, 22, 482–495. [Google Scholar] [CrossRef]
- Han, X.; Hu, L. The effect of endometrial scratch on pregnancy outcomes of frozen-thawed embryo transfer: A propensity score-matched study. Gynecol. Endocrinol. 2022, 38, 39–44. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, Z.; Hu, L. Comparing the effects of endometrial injury in the luteal phase and follicular phase on in vitro fertilization treatment outcomes. Front. Endocrinol. 2022, 13, 1004265. [Google Scholar] [CrossRef]
- Ueno, J.; Salgado, R.M.; Ejzenberg, D.; Carvalho, F.M.H.; Veiga, E.C.A.; Soares Junior, J.M.; Baracat, E.C. Is the length of time between endometrial scratching and embryo transfer important for pregnancy success? An observational study. Rev. Assoc. Med. Bras. 2023, 69, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Tamar, A.M.; Martha, L.R.; Carlos, H.N.; Deborah, C.B.; Benjamin, S. Hysteroscopic endometrial peeling as a different approach to endometrial scratching. Case series report. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102195. [Google Scholar] [CrossRef] [PubMed]
- Mahran, A.; Ibrahim, M.; Bahaa, H. The effect of endometrial injury on first cycle IVF/ICSI outcome: A randomized controlled trial. Int. J. Reprod. Biomed. 2016, 14, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Rashwan, H.; AbdelAziz, S.; Ramadan, W.; Mostafa, W.A.I.; Metwally, A.A.; Katta, M. Randomized controlled trial of the effect of endometrial injury on implantation and clinical pregnancy rates during the first ICSI cycle. Int. J. Gynaecol. Obstet. 2018, 140, 211–216. [Google Scholar] [CrossRef]
- Madhuri, M.S.; Thyagaraju, C.; Naidu, A.; Dasari, P. The effect of endometrial scratching on pregnancy rate after failed intrauterine insemination: A Randomised Controlled Trail. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 268, 37–42. [Google Scholar] [CrossRef]
- Liang, Y.; Han, J.; Jia, C.; Ma, Y.; Lan, Y.; Li, Y.; Wang, S. Effect of Endometrial Injury on Secretion of Endometrial Cytokines and IVF Outcomes in Women with Unexplained Subfertility. Mediat. Inflamm. 2015, 2015, 757184. [Google Scholar] [CrossRef]
- Guven, S.; Kart, C.; Unsal, M.A.; Yildirim, O.; Odaci, E.; Yulug, E. Endometrial injury may increase the clinical pregnancy rate in normoresponders undergoing long agonist protocol ICSI cycles with single embryo transfer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 173, 58–62. [Google Scholar] [CrossRef]
- van Hoogenhuijze, N.E.; van Eekelen, R.; Mol, F.; Schipper, I.; Groenewoud, E.R.; Traas, M.A.F.; Janssen, C.A.H.; Teklenburg, G.; de Bruin, J.P.; van Oppenraaij, R.H.F.; et al. Economic evaluation of endometrial scratching before the second IVF/ICSI treatment: A cost-effectiveness analysis of a randomized controlled trial (SCRaTCH trial). Hum. Reprod. 2022, 37, 254–263. [Google Scholar] [CrossRef]
- Huang, S.Y.; Wang, C.J.; Soong, Y.K.; Wang, H.S.; Wang, M.L.; Lin, C.Y.; Chang, C.L. Site-specific endometrial injury improves implantation and pregnancy in patients with repeated implantation failures. Reprod. Biol. Endocrinol. 2011, 9, 140. [Google Scholar] [CrossRef]
- Gibreel, A.; Badawy, A.; El-Refai, W.; El-Adawi, N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: A randomized controlled trial. J. Obstet. Gynaecol. Res. 2013, 39, 680–684. [Google Scholar] [CrossRef]
- Kara, M.; Aydin, T.; Turktekin, N.; Karacavus, S. Efficacy of the local endometrial injury in patients who had previous failed IVF-ICSI outcome. Iran. J. Reprod. Med. 2012, 10, 567–570. [Google Scholar]
- Nastri, C.O.; Ferriani, R.A.; Raine-Fenning, N.; Martins, W.P. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: A randomized controlled trial. Ultrasound Obstet. Gynecol. 2013, 42, 375–382. [Google Scholar] [CrossRef]
- Parsanezhad, M.E.; Dadras, N.; Maharlouei, N.; Neghahban, L.; Keramati, P.; Amini, M. Pregnancy rate after endometrial injury in couples with unexplained infertility: A randomized clinical trial. Iran. J. Reprod. Med. 2013, 11, 869–874. [Google Scholar]
- Singh, N.; Toshyan, V.; Kumar, S.; Vanamail, P.; Madhu, M. Does endometrial injury enhances implantation in recurrent in-vitro fertilization failures? A prospective randomized control study from tertiary care center. J. Hum. Reprod. Sci. 2015, 8, 218–223. [Google Scholar] [CrossRef]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Ishikawa, T. Clinical background affecting pregnancy outcome following local endometrial injury in infertile patients with repeated implantation failure. Gynecol. Endocrinol. 2016, 32, 587–590. [Google Scholar] [CrossRef]
- Kanazawa, E.; Nakashima, A.; Yonemoto, K.; Otsuka, M.; Yoshioka, N.; Kuramoto, T.; Mitao, H.; Imaishi, H.; Komai, K.; Ushijima, K. Injury to the endometrium prior to the frozen-thawed embryo transfer cycle improves pregnancy rates in patients with repeated implantation failure. J. Obstet. Gynaecol. Res. 2017, 43, 128–134. [Google Scholar] [CrossRef]
- Siristatidis, C.; Kreatsa, M.; Koutlaki, N.; Galazios, G.; Pergialiotis, V.; Papantoniou, N. Endometrial injury for RIF patients undergoing IVF/ICSI: A prospective nonrandomized controlled trial. Gynecol. Endocrinol. 2017, 33, 297–300. [Google Scholar] [CrossRef]
- Reljic, M.; Knez, J.; Kovac, V.; Kovacic, B. Endometrial injury, the quality of embryos, and blastocyst transfer are the most important prognostic factors for in vitro fertilization success after previous repeated unsuccessful attempts. J. Assist. Reprod. Genet. 2017, 34, 775–779. [Google Scholar] [CrossRef]
- Helmy, M.E.E.; Maher, M.A.; Elkhouly, N.I.; Ramzy, M. A randomized trial of local endometrial injury during ovulation induction cycles. Int. J. Gynaecol. Obstet. 2017, 138, 47–52. [Google Scholar] [CrossRef]
- Olesen, M.S.; Hauge, B.; Ohrt, L.; Olesen, T.N.; Roskaer, J.; Baek, V.; Elbaek, H.O.; Nohr, B.; Nyegaard, M.; Overgaard, M.T.; et al. Therapeutic endometrial scratching and implantation after in vitro fertilization: A multicenter randomized controlled trial. Fertil. Steril. 2019, 112, 1015–1021. [Google Scholar] [CrossRef]
- Tang, Z.; Hong, M.; He, F.; Huang, D.; Dai, Z.; Xuan, H.; Zhang, H.; Zhu, W. Effect of endometrial injury during menstruation on clinical outcomes in frozen-thawed embryo transfer cycles: A randomized control trial. J. Obstet. Gynaecol. Res. 2020, 46, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shi, H.; Fang, L.L.; Su, Y.C. The effect of endometrial injury on reproductive outcomes of frozen-thawed embryo transfer cycles in women with one implantation failure. J. Int. Med. Res. 2020, 48, 300060520913130. [Google Scholar] [CrossRef] [PubMed]
- Acet, F.; Sahin, G.; Goker, E.N.T.; Tavmergen, E. The effect of hysteroscopy and conventional curretage versus no hysteroscopy on live birth rates in recurrent in vitro fertilisation failure: A retrospective cohort study from a single referral centre experience. J. Obstet. Gynaecol. 2022, 42, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Turktekin, N.; Karakus, C.; Ozyurt, R. Comparing the effects of endometrial injury with hysteroscopy or Pipelle cannula on fertility outcome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Yuan, X.; Demirol, A.; Ledger, W.; Li, T.C. Factors affecting the outcome of “endometrial scratch” in women with recurrent implantation failure. J. Reprod. Med. 2014, 59, 39–43. [Google Scholar]
- Maged, A.M.; Al-Inany, H.; Salama, K.M.; Souidan, I.I.; Abo Ragab, H.M.; Elnassery, N. Endometrial Scratch Injury Induces Higher Pregnancy Rate for Women with Unexplained Infertility Undergoing IUI with Ovarian Stimulation: A Randomized Controlled Trial. Reprod. Sci. 2016, 23, 239–243. [Google Scholar] [CrossRef]
- Bahaa Eldin, A.M.; Abdelmaabud, K.H.; Laban, M.; Hassanin, A.S.; Tharwat, A.A.; Aly, T.R.; Elbohoty, A.E.; Elsayed, H.M.; Ibrahim, A.M.; Ibrahim, M.E.; et al. Endometrial Injury May Increase the Pregnancy Rate in Patients Undergoing Intrauterine Insemination: An Interventional Randomized Clinical Trial. Reprod. Sci. 2016, 23, 1326–1331. [Google Scholar] [CrossRef]
- Taneja, J.; Ogutu, D.; Ah-Moye, M. Rare successful pregnancy in a patient with Swyer Syndrome. Case Rep. Women’s Health 2016, 12, 1–2. [Google Scholar] [CrossRef]
- van Hoogenhuijze, N.E.; Mol, F.; Laven, J.S.E.; Groenewoud, E.R.; Traas, M.A.F.; Janssen, C.A.H.; Teklenburg, G.; de Bruin, J.P.; van Oppenraaij, R.H.F.; Maas, J.W.M.; et al. Endometrial scratching in women with one failed IVF/ICSI cycle-outcomes of a randomised controlled trial (SCRaTCH). Hum. Reprod. 2021, 36, 87–98. [Google Scholar] [CrossRef]
- Yu, X.; Gao, C.; Dai, C.; Yang, F.; Deng, X. Endometrial injury increases expression of hypoxia-inducible factor and angiogenesis in the endometrium of women with recurrent implantation failure. Reprod. Biomed. Online 2019, 38, 761–767. [Google Scholar] [CrossRef]
- Metwally, M.; Chatters, R.; Pye, C.; Dimairo, M.; White, D.; Walters, S.; Cohen, J.; Young, T.; Cheong, Y.; Laird, S.; et al. Endometrial scratch to increase live birth rates in women undergoing first-time in vitro fertilisation: RCT and systematic review. Health Technol. Assess. 2022, 26, 1–212. [Google Scholar] [CrossRef]
- Safdarian, L.; Movahedi, S.; Aleyasine, A.; Aghahosaini, M.; Fallah, P.; Rezaiian, Z. Local injury to the endometrium does not improve the implantation rate in good responder patients undergoing in-vitro fertilization. Iran. J. Reprod. Med. 2011, 9, 285–288. [Google Scholar]
- Melnick, A.P.; Murphy, E.M.; Masbou, A.K.; Sapra, K.J.; Rosenwaks, Z.; Spandorfer, S.D. Autologous endometrial coculture biopsy: Is timing everything? Fertil. Steril. 2015, 104, 104–109.e1. [Google Scholar] [CrossRef]
- Shokeir, T.; Ebrahim, M.; El-Mogy, H. Hysteroscopic-guided local endometrial injury does not improve natural cycle pregnancy rate in women with unexplained infertility: Randomized controlled trial. J. Obstet. Gynaecol. Res. 2016, 42, 1553–1557. [Google Scholar] [CrossRef]
- Shahrokh-Tehraninejad, E.; Dashti, M.; Hossein-Rashidi, B.; Azimi-Nekoo, E.; Haghollahi, F.; Kalantari, V. A Randomized Trial to Evaluate the Effect of Local Endometrial Injury on the Clinical Pregnancy Rate of Frozen Embryo Transfer Cycles in Patients with Repeated Implantation Failure. J. Fam. Reprod. Health 2016, 10, 108–114. [Google Scholar]
- Levin, D.; Hasson, J.; Cohen, A.; Or, Y.; Ata, B.; Barzilay, L.; Almog, B. The effect of endometrial injury on implantation and clinical pregnancy rates. Gynecol. Endocrinol. 2017, 33, 779–782. [Google Scholar] [CrossRef]
- Tk, A.; Singhal, H.; Premkumar, S.P.; Acharya, M.; Kamath, M.S.; George, K. Local endometrial injury in women with failed IVF undergoing a repeat cycle: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 109–114. [Google Scholar] [CrossRef]
- Liu, W.; Tal, R.; Chao, H.; Liu, M.; Liu, Y. Effect of local endometrial injury in proliferative vs. luteal phase on IVF outcomes in unselected subfertile women undergoing in vitro fertilization. Reprod. Biol. Endocrinol. 2017, 15, 75. [Google Scholar] [CrossRef]
- Mackens, S.; Racca, A.; Van de Velde, H.; Drakopoulos, P.; Tournaye, H.; Stoop, D.; Blockeel, C.; Santos-Ribeiro, S. Follicular-phase endometrial scratching: A truncated randomized controlled trial. Hum. Reprod. 2020, 35, 1090–1098. [Google Scholar] [CrossRef]
- Kalyoncu, S.; Yazicioglu, A.; Demir, M. Endometrial scratching for poor responders based on the Bologna criteria in ICSI fresh embryo transfer cycles: A preliminary retrospective cohort study. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 47–52. [Google Scholar] [CrossRef]
- Rigos, I.; Athanasiou, V.; Vlahos, N.; Papantoniou, N.; Profer, D.; Siristatidis, C. The Addition of Endometrial Injury to Freeze-All Strategy in Women with Repeated Implantation Failures. J. Clin. Med. 2021, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Ghaemdoust, F.; Ghasemi, M.; Liavaly, M.; Keikha, N.; Dehghan Haghighi, J. The effect of endometrial scratching on reproductive outcomes in infertile women undergoing IVF treatment cycles. J. Obstet. Gynaecol. 2022, 42, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Dain, L.; Ojha, K.; Bider, D.; Levron, J.; Zinchenko, V.; Walster, S.; Dirnfeld, M. Effect of local endometrial injury on pregnancy outcomes in ovum donation cycles. Fertil. Steril. 2014, 102, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.S.M.; Chung, C.H.S.; Chung, J.P.W.; Kong, G.W.S.; Saravelos, S.H.; Cheung, L.P.; Li, T.C. The effect of endometrial scratch on natural-cycle cryopreserved embryo transfer outcomes: A randomized controlled study. Reprod. Biomed. Online 2017, 35, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Tehraninejad, E.S.; Haghiri, M.; Masomi, M.; Sadatmahalleh, S.J.; Arabipoor, A. The effect of endometrial scratch injury on pregnancy outcome in women with previous intrauterine insemination failure: A randomized clinical trial. J. Obstet. Gynaecol. Res. 2017, 43, 1421–1427. [Google Scholar] [CrossRef]
- Eskew, A.M.; Reschke, L.D.; Woolfolk, C.; Schulte, M.B.; Boots, C.E.; Broughton, D.E.; Jimenez, P.T.; Omurtag, K.R.; Keller, S.L.; Ratts, V.S.; et al. Effect of endometrial mechanical stimulation in an unselected population undergoing in vitro fertilization: Futility analysis of a double-blind randomized controlled trial. J. Assist. Reprod. Genet. 2019, 36, 299–305. [Google Scholar] [CrossRef]
- Frantz, S.; Parinaud, J.; Kret, M.; Rocher-Escriva, G.; Papaxanthos-Roche, A.; Creux, H.; Chansel-Debordeaux, L.; Benard, A.; Hocke, C. Decrease in pregnancy rate after endometrial scratch in women undergoing a first or second in vitro fertilization. A multicenter randomized controlled trial. Hum. Reprod. 2019, 34, 92–99. [Google Scholar] [CrossRef]
- Lensen, S.; Osavlyuk, D.; Armstrong, S.; Stadelmann, C.; Hennes, A.; Napier, E.; Wilkinson, J.; Sadler, L.; Gupta, D.; Strandell, A.; et al. A Randomized Trial of Endometrial Scratching before In Vitro Fertilization. N. Engl. J. Med. 2019, 380, 325–334. [Google Scholar] [CrossRef]
- Crosby, D.A.; Glover, L.E.; Downey, P.; Mooney, E.E.; McAuliffe, F.M.; O’Farrelly, C.; Brennan, D.J.; Wingfield, M. The impact of accurately timed mid-luteal endometrial injury in nulligravid women undergoing their first or second embryo transfer. Ir. J. Med. Sci. 2021, 190, 1071–1077. [Google Scholar] [CrossRef]
- Metwally, M.; Chatters, R.; Dimairo, M.; Walters, S.; Pye, C.; White, D.; Bhide, P.; Chater, T.; Cheong, Y.; Choudhary, M.; et al. A randomised controlled trial to assess the clinical effectiveness and safety of the endometrial scratch procedure prior to first-time IVF, with or without ICSI. Hum. Reprod. 2021, 36, 1841–1853. [Google Scholar] [CrossRef]
- Yavangi, M.; Varmaghani, N.; Pirdehghan, A.; Varmaghani, M.; Faryadras, M. Comparison of pregnancy outcome in intrauterine insemination-candidate women with and without endometrial scratch injury: An RCT. Int. J. Reprod. Biomed. 2021, 19, 457–464. [Google Scholar] [CrossRef]
- Farzaneh, F.; Khastehfekr, F. The effect of topical endometrial scratching on pregnancy outcome in women with previous failure of intrauterine insemination: A non-randomized clinical trial. Int. J. Reprod. Biomed. 2021, 19, 465–470. [Google Scholar] [CrossRef]
- Glanville, E.J.; Wilkinson, J.; Sadler, L.; Wong, T.Y.; Acharya, S.; Aziz, N.; Clarke, F.; Das, S.; Dawson, J.; Hammond, B.; et al. A randomized trial of endometrial scratching in women with PCOS undergoing ovulation induction cycles. Reprod. Biomed. Online 2022, 44, 316–323. [Google Scholar] [CrossRef]
- Wong, T.Y.; Lensen, S.; Wilkinson, J.; Glanville, E.J.; Acharya, S.; Clarke, F.; Das, S.; Dawson, J.; Hammond, B.; Jayaprakasan, K.; et al. Effect of endometrial scratching on unassisted conception for unexplained infertility: A randomized controlled trial. Fertil. Steril. 2022, 117, 612–619. [Google Scholar] [CrossRef]
- Bernard, A.; Schumacher, K.; Marsh, C. Endometrial Scratch (Injury): Does Timing Matter? J. Fam. Reprod. Health 2019, 13, 85–88. [Google Scholar] [CrossRef]
- Iriarte, C.; Awosika, O.; Rengifo-Pardo, M.; Ehrlich, A. Review of applications of microneedling in dermatology. Clin. Cosmet. Investig. Dermatol. 2017, 10, 289–298. [Google Scholar] [CrossRef]
- Ersahin, S.S.; Ersahin, A. Endometrial injury concurrent with hysteroscopy increases the expression of Leukaemia inhibitory factor: A preliminary study. Reprod. Biol. Endocrinol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Mrozikiewicz, A.E.; Ozarowski, M.; Jedrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Saravelos, S.H.; Liu, Y.; Huang, J.; Zhang, J.; Li, T.C. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil. Steril. 2017, 107, 136–143.e2. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, S.; Qi, J.; Wang, Y.; Ding, Y.; Zhu, Q.; He, Y.; Lu, Y.; Yao, Y.; Wang, S.; et al. Increased expression of HOXA11-AS attenuates endometrial decidualization in recurrent implantation failure patients. Mol. Ther. 2022, 30, 1706–1720. [Google Scholar] [CrossRef]
- Santamaria, X.; Katzorke, N.; Simon, C. Endometrial ‘scratching’: What the data show. Curr. Opin. Obstet. Gynecol. 2016, 28, 242–249. [Google Scholar] [CrossRef]
- Maged, A.M.; Ogila, A.I.; Mohsen, R.A.; Mahmoud, S.I.; Fouad, M.A.; El Komy, R.O.; Lasheen, Y.; El-Nassery, N.; Dahab, S.; Hussein, E.A. Endometrial scratch injury in infertile women seeking conception through natural or intrauterine insemination cycles: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.; Yang, Y.; Liang, H.; Duan, X.; Gao, Q.; Yin, Z. Impact of endometrial scratching on reproductive outcome in patients: A systematic review and meta-analysis. Medicine 2022, 101, e30150. [Google Scholar] [CrossRef] [PubMed]
- Baradwan, S.; Alshahrani, M.S.; AlSghan, R.; Alkhamis, W.H.; Alsharif, S.A.; Alanazi, G.A.; Abdelwahed, R.M.; Alkholy, E.A.; Fouad, M.; Saleh, M.; et al. The Effect of Endometrial Scratch on Pregnancy Rate in Women with Previous Intrauterine Insemination Failure: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Reprod. Sci. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Vitagliano, A.; Marci, R.; Caserta, D. Endometrial Scratching for Improving Endometrial Receptivity: A Critical Review of Old and New Clinical Evidence. Reprod. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Xu, W.; Yang, J.; Feng, L.; Jia, J. Impact of local endometrial injury on in vitro fertilization/intracytoplasmic sperm injection outcomes: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2019, 45, 57–68. [Google Scholar] [CrossRef]
- El-Toukhy, T.; Sunkara, S.; Khalaf, Y. Local endometrial injury and IVF outcome: A systematic review and meta-analysis. Reprod. Biomed. Online 2012, 25, 345–354. [Google Scholar] [CrossRef]
- Lensen, S.F.; Armstrong, S.; Gibreel, A.; Nastri, C.O.; Raine-Fenning, N.; Martins, W.P. Endometrial injury in women undergoing in vitro fertilisation (IVF). Cochrane Database Syst. Rev. 2021, 6, CD009517. [Google Scholar] [CrossRef]
- Aghajanzadeh, F.; Esmaeilzadeh, S.; Basirat, Z.; Mahouti, T.; Heidari, F.N.; Golsorkhtabaramiri, M. Using autologous intrauterine platelet-rich plasma to improve the reproductive outcomes of women with recurrent implantation failure. JBRA Assist. Reprod. 2020, 24, 30–33. [Google Scholar] [CrossRef]
- Hajipour, H.; Farzadi, L.; Latifi, Z.; Keyhanvar, N.; Navali, N.; Fattahi, A.; Nouri, M.; Dittrich, R. An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: Clinical and molecular aspects. Syst. Biol. Reprod. Med. 2021, 67, 177–188. [Google Scholar] [CrossRef]
- Sharara, F.I.; Lelea, L.L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Correa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Maged, A.M.; El-Mazny, A.; Kamal, N.; Mahmoud, S.I.; Fouad, M.; El-Nassery, N.; Kotb, A.; Ragab, W.S.; Ogila, A.I.; Metwally, A.A.; et al. The value of platelet-rich plasma in women with previous implantation failure: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2023. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rouholamin, S.; Rezaeinejad, M.; Maroufizadeh, S.; Sepidarkish, M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103078. [Google Scholar] [CrossRef]
- Anitua, E.; Allende, M.; de la Fuente, M.; Del Fabbro, M.; Alkhraisat, M.H. Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Bioengineering 2023, 10, 303. [Google Scholar] [CrossRef]
- Li, M.; Kang, Y.; Wang, Q.; Yan, L. Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6753. [Google Scholar] [CrossRef]
- Deng, H.; Wang, S.; Li, Z.; Xiao, L.; Ma, L. Effect of intrauterine infusion of platelet-rich plasma for women with recurrent implantation failure: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2023, 43, 2144177. [Google Scholar] [CrossRef]
- Mouanness, M.; Ali-Bynom, S.; Jackman, J.; Seckin, S.; Merhi, Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for Endometrial Receptivity and Thickness: A Literature Review of the Mechanisms of Action. Reprod. Sci. 2021, 28, 1659–1670. [Google Scholar] [CrossRef]
- Kong, X.; Tang, G.; Liu, Y.; Zheng, Z.; Li, Y.; Yan, F. Efficacy of intrauterine infusion therapy before embryo transfer in recurrent implantation failure: A systematic review and network meta-analysis. J. Reprod. Immunol. 2023, 156, 103819. [Google Scholar] [CrossRef]
- Aghajanova, L.; Houshdaran, S.; Balayan, S.; Manvelyan, E.; Irwin, J.C.; Huddleston, H.G.; Giudice, L.C. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J. Assist. Reprod. Genet. 2018, 35, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Luo, S.; Mao, J.; Luo, B.; Wang, J. Effects of intrauterine infusion of platelet-rich plasma on hormone levels and endometrial receptivity in patients with repeated embryo implantation failure. Am. J. Transl. Res. 2022, 14, 5651–5659. [Google Scholar]
- Kieu, V.; Lantsberg, D.; Mizrachi, Y.; Stern, C.; Polyakov, A.; Teh, W.T. A survey study of endometrial receptivity tests and immunological treatments in in vitro fertilisation (IVF). Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Woon, E.V.; Day, A.; Bracewell-Milnes, T.; Male, V.; Johnson, M. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 142, 103189. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Vomstein, K.; Togawa, R.; Bottcher, B.; Hudalla, H.; Strowitzki, T.; Daniel, V.; Kuon, R.J. The impact of previous live births on peripheral and uterine natural killer cells in patients with recurrent miscarriage. Reprod. Biol. Endocrinol. 2019, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Lapides, L.; Varga, I.; Klein, M.; Rybanska, L.; Belusakova, V.; Babal, P. When Less Is More—Pipelle Endometrial Sampling for Quantification of Uterine Natural Killer Cells in Patients with Recurrent Implantation Failure or Habitual Abortion. Physiol. Res. 2022, 71, S65–S73. [Google Scholar] [CrossRef]
- Hartman, S.K.; Symons, W.A.; Yeh, I.-T. Chronic endometritis: How many plasma cells does it take to make the diagnosis? FASEB J. 2011, 25, 1002.13. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Yoon, T.K.; Lee, W.S. Chronic endometritis and infertility. Clin. Exp. Reprod. Med. 2016, 43, 185–192. [Google Scholar] [CrossRef]
- Einenkel, R.; Zygmunt, M.; Muzzio, D.O. Microorganisms in the healthy upper reproductive tract: From denial to beneficial assignments for reproductive biology. Reprod. Biol. 2019, 19, 113–118. [Google Scholar] [CrossRef]
- Vomstein, K.; Feil, K.; Strobel, L.; Aulitzky, A.; Hofer-Tollinger, S.; Kuon, R.-J.; Toth, B. Immunological Risk Factors in Recurrent Pregnancy Loss: Guidelines Versus Current State of the Art. J. Clin. Med. 2021, 10, 869. [Google Scholar] [CrossRef]
- Salih, S.M.; Havemann, L.; Lindheim, S.R. Human Leukocyte Antigen (HLA) Typing in Medically Assisted Reproduction. In Textbook of Assisted Reproduction; Allahbadia, G.N., Ata, B., Lindheim, S.R., Woodward, B.J., Bhagavath, B., Eds.; Springer: Singapore, 2020; pp. 299–306. [Google Scholar]
- Wu, L.; Fang, X.; Lu, F.; Zhang, Y.; Wang, Y.; Kwak-Kim, J. Anticardiolipin and/or anti-beta2-glycoprotein-I antibodies are associated with adverse IVF outcomes. Front. Immunol. 2022, 13, 986893. [Google Scholar] [CrossRef]
- Kwak-Kim, J.Y.; Chung-Bang, H.S.; Ng, S.C.; Ntrivalas, E.I.; Mangubat, C.P.; Beaman, K.D.; Beer, A.E.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Degree of TNF-alpha/IL-10 cytokine elevation correlates with IVF success rates in women undergoing treatment with Adalimumab (Humira) and IVIG. Am. J. Reprod. Immunol. 2011, 65, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Elevated preconception CD56+16+ and/or Th1:Th2 levels predict benefit from IVIG therapy in subfertile women undergoing IVF. Am. J. Reprod. Immunol. 2011, 66, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kwak-Kim, J.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. Immunosuppressive treatment using tacrolimus promotes pregnancy outcome in infertile women with repeated implantation failures. Am. J. Reprod. Immunol. 2017, 78, e12682. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Chouliaras, S. Adjuvants in IVF-evidence for what works and what does not work. Ups. J. Med. Sci. 2020, 125, 144–151. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef]
- Henderson, T.A.; Saunders, P.T.K.; Moffett-King, A.; Groome, N.P.; Critchley, H.O.D. Steroid receptor expression in uterine natural killer cells. J. Clin. Endocr. Metab. 2003, 88, 440–449. [Google Scholar] [CrossRef]
- Thiruchelvam, U.; Maybin, J.A.; Armstrong, G.M.; Greaves, E.; Saunders, P.T.K.; Critchley, H.O.D. Cortisol regulates the paracrine action of macrophages by inducing vasoactive gene expression in endometrial cells. J. Leukoc. Biol. 2016, 99, 1165–1171. [Google Scholar] [CrossRef]
- Horton, J.S.; Yamamoto, S.Y.; Bryant-Greenwood, G.D. Relaxin Modulates Proinflammatory Cytokine Secretion from Human Decidual Macrophages. Biol. Reprod. 2011, 85, 788–797. [Google Scholar] [CrossRef]
- Quenby, S.; Kalumbi, C.; Bates, M.; Farquharson, R.; Vince, G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil. Steril. 2005, 84, 980–984. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, Y.; Zhuang, Y.L.; Zhou, F.L.; Huang, L.L. Mifepristone Increases the Cytotoxicity of Uterine Natural Killer Cells by Acting as a Glucocorticoid Antagonist via ERK Activation. PLoS ONE 2012, 7, e36413. [Google Scholar] [CrossRef]
- Thum, M.Y.; Bhaskaran, S.; Abdalla, H.I.; Ford, B.; Sumar, N.; Bansal, A. Prednisolone suppresses NK cell cytotoxicity in vitro in women with a history of infertility and elevated NK cell cytotoxicity. Am. J. Reprod. Immunol. 2008, 59, 259–265. [Google Scholar] [CrossRef]
- Mahdian, S.; Zarrabi, M.; Moini, A.; Shahhoseini, M.; Movahedi, M. In silico evidence for prednisone and progesterone efficacy in recurrent implantation failure treatment. J. Mol. Model. 2022, 28, 105. [Google Scholar] [CrossRef]
- Kuroda, K.; Venkatakrishnan, R.; Salker, M.S.; Lucas, E.S.; Shaheen, F.; Kuroda, M.; Blanks, A.; Christian, M.; Quenby, S.; Brosens, J.J. Induction of 11beta-HSD 1 and activation of distinct mineralocorticoid receptor- and glucocorticoid receptor-dependent gene networks in decidualizing human endometrial stromal cells. Mol. Endocrinol. 2013, 27, 192–202. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Kamath, M.S.; Keay, S.D.; Macklon, N.S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2022, 6, CD005996. [Google Scholar] [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jin, M.; Yu, D.; Moldenhauer, L.M.; Davies, M.J.; Hull, M.L.; Norman, R.J. Corticosteroid therapy in assisted reproduction—Immune suppression is a faulty premise. Hum. Reprod. 2016, 31, 2164–2173. [Google Scholar] [CrossRef]
- Michael, A.E.; Papageorghiou, A.T. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum. Reprod. Update 2008, 14, 497–517. [Google Scholar] [CrossRef]
- Mayer, K.; Meyer, S.; Reinholz-Muhly, M.; Maus, U.; Merfels, M.; Lohmeyer, J.; Grimminger, F.; Seeger, W. Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J. Immunol. 2003, 171, 4837–4843. [Google Scholar] [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of intralipid’s suppressive effect on NK cell’s functional activity. Am. J. Reprod. Immunol. 2008, 60, 258–263. [Google Scholar] [CrossRef]
- Ledee, N.; Vasseur, C.; Petitbarat, M.; Chevrier, L.; Vezmar, K.; Dray, G.; Cheniere, S.; Lobersztajn, A.; Vitoux, D.; Cassuto, G.N.; et al. Intralipid (R) may represent a new hope for patients with reproductive failures and simultaneously an over-immune endometrial activation. J. Reprod. Immunol. 2018, 130, 18–22. [Google Scholar] [CrossRef]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: Is there any evidence? Reprod. Fertil. 2021, 2, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin. Exp. Reprod. Med. 2021, 48, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, M.P.; Black, N.; Keay, S.; Quenby, S.; Al Wattar, B.H. Intralipid infusion at time of embryo transfer in women with history of recurrent implantation failure: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Placais, L.; Kolanska, K.; Kraiem, Y.B.; Cohen, J.; Suner, L.; Bornes, M.; Sedille, L.; Rosefort, A.; D’Argent, E.M.; Selleret, L.; et al. Intralipid therapy for unexplained recurrent miscarriage and implantation failure: Case-series and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, H.; Lin, X.; Wang, S.; Zhang, S. The effect of intralipid on pregnancy outcomes in women with previous implantation failure in in vitro fertilization/intracytoplasmic sperm injection cycles: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 187–192. [Google Scholar] [CrossRef]
- Coulam, C.B. Intralipid treatment for women with reproductive failures. Am. J. Reprod. Immunol. 2021, 85, e13290. [Google Scholar] [CrossRef]
- Shreeve, N.; Sadek, K. Intralipid therapy for recurrent implantation failure: New hope or false dawn? J. Reprod. Immunol. 2012, 93, 38–40. [Google Scholar] [CrossRef]
- Foyle, K.L.; Sharkey, D.J.; Moldenhauer, L.M.; Green, E.S.; Wilson, J.J.; Roccisano, C.J.; Hull, M.L.; Tremellen, K.P.; Robertson, S.A. Effect of Intralipid infusion on peripheral blood T cells and plasma cytokines in women undergoing assisted reproduction treatment. Clin. Transl. Immunol. 2021, 10, e1328. [Google Scholar] [CrossRef]
- Martini, A.E.; Jasulaitis, S.; Fogg, L.F.; Uhler, M.L.; Hirshfeld-Cytron, J.E. Evaluating the Utility of Intralipid Infusion to Improve Live Birth Rates in Patients with Recurrent Pregnancy Loss or Recurrent Implantation Failure. J. Hum. Reprod. Sci. 2018, 11, 261–268. [Google Scholar] [CrossRef]
- Canella, P.; Barini, R.; Carvalho, P.O.; Razolli, D.S. Lipid emulsion therapy in women with recurrent pregnancy loss and repeated implantation failure: The role of abnormal natural killer cell activity. J. Cell. Mol. Med. 2021, 25, 2290–2296. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Clark, D.A. Anti-TNFalpha therapy in immune-mediated subfertility: State of the art. J. Reprod. Immunol. 2010, 85, 15–24. [Google Scholar] [CrossRef]
- Santiago, K.Y.; Porchia, L.M.; Lopez-Bayghen, E. Endometrial preparation with etanercept increased embryo implantation and live birth rates in women suffering from recurrent implantation failure during IVF. Reprod. Biol. 2021, 21, 100480. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Taranissi, M. Die-off ratio correlates with increased TNF-alpha:IL-10 ratio and decreased IVF success rates correctable with humira. Am. J. Reprod. Immunol. 2012, 68, 428–437. [Google Scholar] [CrossRef]
- Jerzak, M.; Ohams, M.; Gorski, A.; Baranowski, W. Etanercept immunotherapy in women with a history of recurrent reproductive failure. Ginekol. Pol. 2012, 83, 260–264. [Google Scholar]
- Gilardin, L.; Bayry, J.; Kaveri, S.V. Intravenous immunoglobulin as clinical immune-modulating therapy. Can. Med. Assoc. J. 2015, 187, 257–264. [Google Scholar] [CrossRef]
- Kaufman, G.N.; Massoud, A.H.; Dembele, M.; Yona, M.; Piccirillo, C.A.; Mazer, B.D. Induction of regulatory T cells by intravenous immunoglobulin: A bridge between adaptive and innate immunity. Front. Immunol. 2015, 6, 469. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; Ghasemzadeh, A.; Hamdi, K.; Younesi, V.; Nouri, M.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017, 63, 350–359. [Google Scholar] [CrossRef]
- Coulam, C.B.; Krysa, L.W.; Bustillo, M. Intravenous immunoglobulin for in-vitro fertilization failure. Hum. Reprod. 1994, 9, 2265–2269. [Google Scholar] [CrossRef]
- Ho, Y.K.; Chen, H.H.; Huang, C.C.; Lee, C.I.; Lin, P.Y.; Lee, M.S.; Lee, T.H. Peripheral CD56+CD16+ NK Cell Populations in the Early Follicular Phase Are Associated with Successful Clinical Outcomes of Intravenous Immunoglobulin Treatment in Women with Repeated Implantation Failure. Front. Endocrinol. 2019, 10, 937. [Google Scholar] [CrossRef]
- Ramos-Medina, R.; Garcia-Segovia, A.; Gil, J.; Carbone, J.; Aguaron de la Cruz, A.; Seyfferth, A.; Alonso, B.; Alonso, J.; Leon, J.A.; Alecsandru, D.; et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am. J. Reprod. Immunol. 2014, 71, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, L.; Schorsch, M.; Hahn, T. CD3− CD56+ CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG-treatment. Am. J. Reprod. Immunol. 2010, 63, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.B.; Goodman, C. Increased pregnancy rates after IVF/ET with intravenous immunoglobulin treatment in women with elevated circulating C56+ cells. Early Pregnancy 2000, 4, 90–98. [Google Scholar] [PubMed]
- Han, A.R.; Lee, S.K. Immune modulation of i.v. immunoglobulin in women with reproductive failure. Reprod. Med. Biol. 2018, 17, 115–124. [Google Scholar] [CrossRef]
- Saab, W.; Seshadri, S.; Huang, C.; Alsubki, L.; Sung, N.; Kwak-Kim, J. A systemic review of intravenous immunoglobulin G treatment in women with recurrent implantation failures and recurrent pregnancy losses. Am. J. Reprod. Immunol. 2021, 85, e13395. [Google Scholar] [CrossRef]
- Abdolmohammadi-Vahid, S.; Pashazadeh, F.; Pourmoghaddam, Z.; Aghebati-Maleki, L.; Abdollahi-Fard, S.; Yousefi, M. The effectiveness of IVIG therapy in pregnancy and live birth rate of women with recurrent implantation failure (RIF): A systematic review and meta-analysis. J. Reprod. Immunol. 2019, 134–135, 28–33. [Google Scholar] [CrossRef]
- Sapir, T.; Carp, H.; Shoenfeld, Y. Intravenous immunoglobulin (IVIG) as treatment for recurrent pregnancy loss (RPL). Harefuah 2005, 144, 415–420, 453, 454. [Google Scholar]
- Elram, T.; Simon, A.; Israel, S.; Revel, A.; Shveiky, D.; Laufer, N. Treatment of recurrent IVF failure and human leukocyte antigen similarity by intravenous immunoglobulin. Reprod. Biomed. Online 2005, 11, 745–749. [Google Scholar] [CrossRef]
- Bahrami-Asl, Z.; Farzadi, L.; Fattahi, A.; Yousefi, M.; Quinonero, A.; Hakimi, P.; Latifi, Z.; Nejabati, H.R.; Ghasemnejad, T.; Sadigh, A.R.; et al. Tacrolimus Improves the Implantation Rate in Patients with Elevated Th1/2 Helper Cell Ratio and Repeated Implantation Failure (RIF). Geburtshilfe Frauenheilkd 2020, 80, 851–862. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kwak-Kim, J.; Ota, K.; Kuroda, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am. J. Reprod. Immunol. 2015, 73, 353–361. [Google Scholar] [CrossRef]
- Fluhr, H.; Spratte, J.; Heidrich, S.; Ehrhardt, J.; Greinacher, A.; Zygmunt, M. The molecular charge and size of heparins determine their impact on the decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 2011, 17, 354–359. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Kojima, H.; Kanno, Y.; Hashiguchi, M.; Kobata, T. Heparin affects the induction of regulatory T cells independent of anti-coagulant activity and suppresses allogeneic immune responses. Clin. Exp. Immunol. 2020, 202, 119–135. [Google Scholar] [CrossRef]
- Niu, Z.; Zhou, M.; Xia, L.; Zhao, S.; Zhang, A. Uterine cytokine profiles after low-molecular-weight heparin administration are associated with pregnancy outcomes of patients with repeated implantation failure. Front. Endocrinol. 2022, 13, 1008923. [Google Scholar] [CrossRef]
- Spratte, J.; Meyer zu Schwabedissen, H.; Endlich, N.; Zygmunt, M.; Fluhr, H. Heparin inhibits TNF-α signaling in human endometrial stromal cells by interaction with NF-κB. Mol. Hum. Reprod. 2013, 19, 227–236. [Google Scholar] [CrossRef]
- Grandone, E.; Villani, M.; Dentali, F.; Tiscia, G.L.; Colaizzo, D.; Cappucci, F.; Fischetti, L.; Ageno, W.; Margaglione, M. Low-molecular -weight heparin in pregnancies after ART—A retrospective study. Thromb. Res. 2014, 134, 336–339. [Google Scholar] [CrossRef]
- Potdar, N.; Gelbaya, T.A.; Konje, J.C.; Nardo, L.G. Adjunct low-molecular-weight heparin to improve live birth rate after recurrent implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 674–684. [Google Scholar] [CrossRef]
- Elmahashi, M.O.; Elbareg, A.M.; Essadi, F.M.; Ashur, B.M.; Adam, I. Low dose aspirin and low-molecular-weight heparin in the treatment of pregnant Libyan women with recurrent miscarriage. BMC Res. Notes 2014, 7, 23. [Google Scholar] [CrossRef]
- Dias, A.T.B.; Modesto, T.B.; Oliveira, S.A. Effectiveness of the use of Low Molecular Heparin in patients with repetition abortion history: Systematic review and meta-analysis. JBRA Assist. Reprod. 2021, 25, 10–27. [Google Scholar] [CrossRef]
- Siristatidis, C.; Dafopoulos, K.; Salamalekis, G.; Galazios, G.; Christoforidis, N.; Moustakarias, T.; Koutlaki, N.; Bouschanetzis, C.; Loutradis, D.; Drakakis, P. Administration of low-molecular-weight heparin in patients with two or more unsuccessful IVF/ICSI cycles: A multicenter cohort study. Gynecol. Endocrinol. 2018, 34, 747–751. [Google Scholar] [CrossRef]
- Kamel, A.M.; El-Faissal, Y.; Aboulghar, M.; Mansour, R.; Serour, G.I.; Aboulghar, M. Does intrauterine injection of low-molecular-weight heparin improve the clinical pregnancy rate in intracytoplasmic sperm injection? Clin. Exp. Reprod. Med. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Hamdi, K.; Danaii, S.; Farzadi, L.; Abdollahi, S.; Chalabizadeh, A.; Abdollahi Sabet, S. The Role of Heparin in Embryo Implantation in Women with Recurrent Implantation Failure in the Cycles of Assisted Reproductive Techniques (without History of Thrombophilia). J. Fam. Reprod. Health 2015, 9, 59–64. [Google Scholar]
- Akhtar, M.A.; Sur, S.; Raine-Fenning, N.; Jayaprakasan, K.; Thornton, J.; Quenby, S.; Marjoribanks, J. Heparin for assisted reproduction: Summary of a Cochrane review. Fertil. Steril. 2015, 103, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yao, C.; Wei, L.; Lin, Z. The intrauterine perfusion of granulocyte-colony stimulating factor (G-CSF) before frozen-thawed embryo transfer in patients with two or more implantation failures. Hum. Fertil. 2020, 25, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, Q.; Zhang, Y.; Zhou, L.; Lin, J.; Chen, Y.; Qian, X. Treatment of G-CSF in unexplained, repeated implantation failure: A systematic review and meta-analysis. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101866. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.N.C.; Florencio, R.S.; Alves, R.R.F. The role played by granulocyte colony stimulating factor (G-CSF) on women submitted to in vitro fertilization associated with thin endometrium: Systematic review. JBRA Assist. Reprod. 2020, 24, 278–282. [Google Scholar] [CrossRef]
- Schlahsa, L.; Jaimes, Y.; Blasczyk, R.; Figueiredo, C. Granulocyte-colony-stimulatory factor: A strong inhibitor of natural killer cell function. Transfusion 2011, 51, 293–305. [Google Scholar] [CrossRef]
- Fu, L.L.; Xu, Y.; Yan, J.; Zhang, X.Y.; Li, D.D.; Zheng, L.W. Efficacy of granulocyte colony-stimulating factor for infertility undergoing IVF: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 34. [Google Scholar] [CrossRef]
- Hou, Z.; Jiang, F.; Yang, J.; Liu, Y.; Zha, H.; Yang, X.; Bie, J.; Meng, Y. What is the impact of granulocyte colony-stimulating factor (G-CSF) in subcutaneous injection or intrauterine infusion and during both the fresh and frozen embryo transfer cycles on recurrent implantation failure: A systematic review and meta-analysis? Reprod. Biol. Endocrinol. 2021, 19, 125. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Sun, Y.X.; Shen, X.Y.; Jiang, Y.; Liu, J.Y. Effect of intrauterine perfusion of granular leukocyte-colony stimulating factor on the outcome of frozen embryo transfer. World J. Clin. Cases 2021, 9, 9038–9049. [Google Scholar] [CrossRef]
- Kalem, Z.; Namli Kalem, M.; Bakirarar, B.; Kent, E.; Makrigiannakis, A.; Gurgan, T. Intrauterine G-CSF Administration in Recurrent Implantation Failure (RIF): An Rct. Sci. Rep. 2020, 10, 5139. [Google Scholar] [CrossRef]
- Kamath, M.S.; Kirubakaran, R.; Sunkara, S.K. Granulocyte-colony stimulating factor administration for subfertile women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2020, 1, CD013226. [Google Scholar] [CrossRef]
- Craciunas, L.; Tsampras, N.; Raine-Fenning, N.; Coomarasamy, A. Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2018, 10, CD011537. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Wang, W.; Qu, Q.; Zhang, N.; Wang, X.; Fang, J.; Ma, Z.; Hao, C. Intrauterine administration of human chorionic gonadotropin improves the live birth rates of patients with repeated implantation failure in frozen-thawed blastocyst transfer cycles by increasing the percentage of peripheral regulatory T cells. Arch. Gynecol. Obstet. 2019, 299, 1165–1172. [Google Scholar] [CrossRef]
- Pourmoghadam, Z.; Soltani-Zangbar, M.S.; Sheikhansari, G.; Azizi, R.; Eghbal-Fard, S.; Mohammadi, H.; Siahmansouri, H.; Aghebati-Maleki, L.; Danaii, S.; Mehdizadeh, A.; et al. Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. J. Reprod. Immunol. 2020, 142, 103182. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Cheng, Y.; Zhou, D.; Yin, T.; Xu, W.; Yu, N.; Yang, J. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 2017, 119, 15–22. [Google Scholar] [CrossRef]
- Conforti, A.; Longobardi, S.; Carbone, L.; Iorio, G.G.; Cariati, F.; Campitiello, M.R.; Strina, I.; Palese, M.; D’Hooghe, T.; Alviggi, C. Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12193. [Google Scholar] [CrossRef]
- Robertson, S.A.; Guerin, L.R.; Bromfield, J.J.; Branson, K.M.; Ahlstrom, A.C.; Care, A.S. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol. Reprod. 2009, 80, 1036–1045. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 2007, 13, 491–501. [Google Scholar] [CrossRef]
- Gunther, V.; Alkatout, I.; Meyerholz, L.; Maass, N.; Gorg, S.; von Otte, S.; Ziemann, M. Live Birth Rates after Active Immunization with Partner Lymphocytes. Biomedicines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Agrawal, S.; Pandey, M.K.; Mandal, S.; Mishra, L.; Agarwal, S. Humoral immune response to an allogenic foetus in normal fertile women and recurrent aborters. BMC Pregnancy Childbirth 2002, 2, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stope, M.B.; Mustea, A.; Sänger, N.; Einenkel, R. Immune Cell Functionality during Decidualization and Potential Clinical Application. Life 2023, 13, 1097. https://doi.org/10.3390/life13051097
Stope MB, Mustea A, Sänger N, Einenkel R. Immune Cell Functionality during Decidualization and Potential Clinical Application. Life. 2023; 13(5):1097. https://doi.org/10.3390/life13051097
Chicago/Turabian StyleStope, Matthias B., Alexander Mustea, Nicole Sänger, and Rebekka Einenkel. 2023. "Immune Cell Functionality during Decidualization and Potential Clinical Application" Life 13, no. 5: 1097. https://doi.org/10.3390/life13051097
APA StyleStope, M. B., Mustea, A., Sänger, N., & Einenkel, R. (2023). Immune Cell Functionality during Decidualization and Potential Clinical Application. Life, 13(5), 1097. https://doi.org/10.3390/life13051097








