Nucleoporin Nup358 Downregulation Tunes the Neuronal Excitability in Mouse Cortical Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Culture
2.2. Constructs
2.3. Electrophysiology
2.3.1. Recordings
2.3.2. Ionic Current Dissection
2.4. Data Analysis
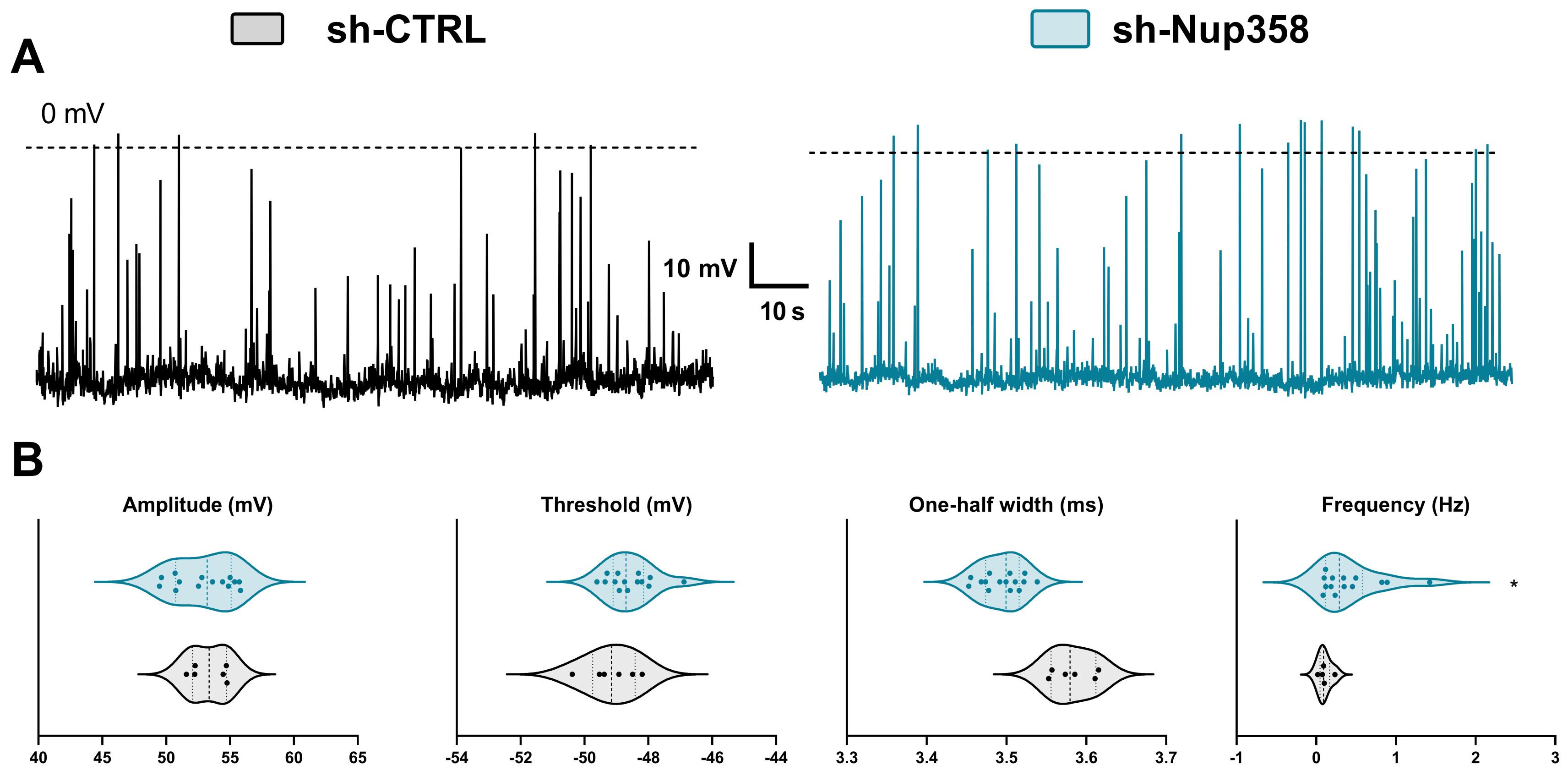
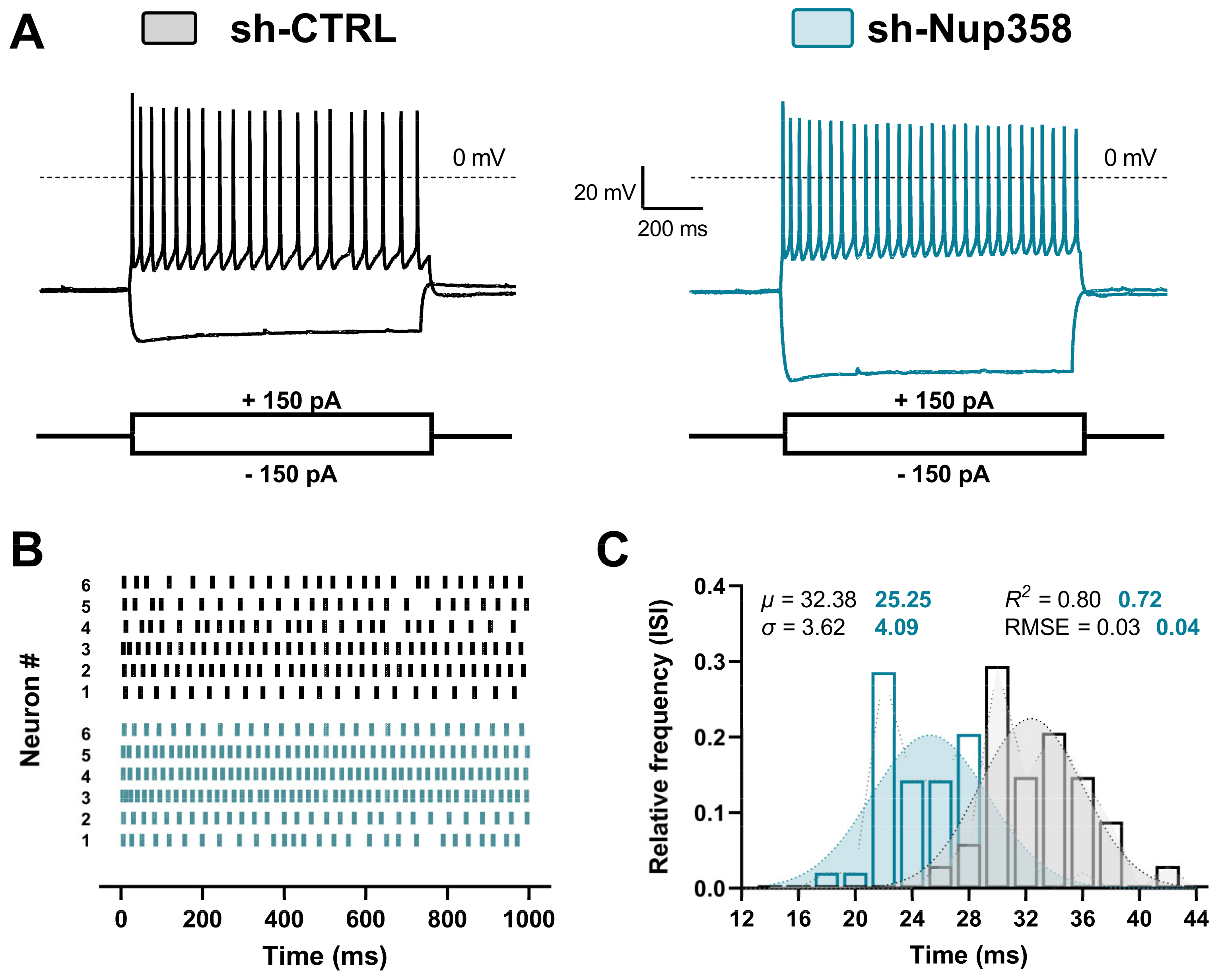
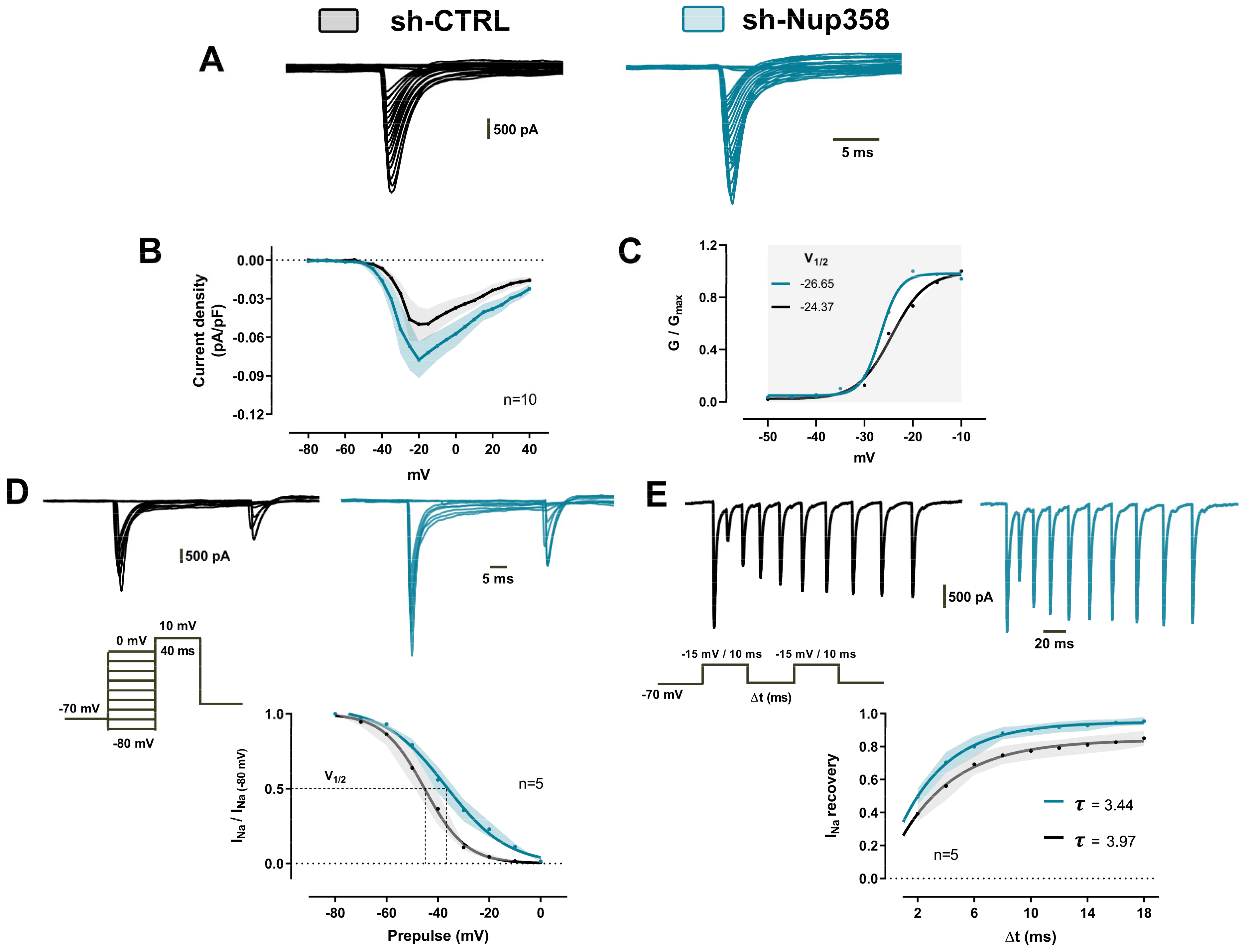
3. Results
4. Discussion
4.1. Nup358 Downregulation and the Neuronal Excitability Alteration
4.2. Nup358 Downregulation: A Promising Window into Ion Channel Pathophysiology in Neurodegenerative Diseases?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, M.; Hurt, E. The nuclear pore complex: Understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 2016, 18, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Medalia, O.; Zwerger, M. Functional architecture of the nuclear pore complex. Annu. Rev. Biophys. 2012, 41, 557–584. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.A. The coming-of-age of nucleocytoplasmic transport in motor neuron disease and neurodegeneration. Cell. Mol. Life Sci. 2019, 76, 2247–2273. [Google Scholar] [CrossRef] [PubMed]
- Coyne, A.N.; Rothstein, J.D. Nuclear pore complexes—A doorway to neural injury in neurodegeneration. Nat. Rev. Neurol. 2022, 18, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Bodoor, K.; Shaikh, S.; Salina, D.; Raharjo, W.H.; Bastos, R.; Lohka, M.; Burke, B. Sequential recruitment of npc proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 1999, 112, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Fahrenkrog, B. dynamics and diverse functions of nuclear pore complex proteins. Nucleus 2012, 3, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Kehlenbach, R.H.; Schirmer, E.C.; Kehlenbach, A.; Fan, F.; Clurman, B.E.; Arnheim, N.; Gerace, L. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol. Cell. Biol. 2000, 20, 5619–5630. [Google Scholar] [CrossRef] [PubMed]
- Rabut, G.; Doye, V.; Ellenberg, J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol. 2004, 6, 1114–1121. [Google Scholar] [CrossRef]
- Bernad, R.; van der Velde, H.; Fornerod, M.; Pickersgill, H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol. Cell. Biol. 2004, 24, 2373–2384. [Google Scholar] [CrossRef]
- Goldberg, M.W. Nuclear pore complex tethers to the cytoskeleton. Semin. Cell Dev. Biol. 2017, 68, 52–58. [Google Scholar] [CrossRef]
- Vyas, P.; Singh, A.; Murawala, P.; Joseph, J. Nup358 interacts with dishevelled and APKC to regulate neuronal polarity. Biol. Open 2013, 2, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Yoon, D.; Qiu, S.; Danziger, Z.; Grill, W.M.; Wetsel, W.C.; Ferreira, P.A. Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of HnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis. Models Mech. 2017, 10, 559–579. [Google Scholar] [CrossRef]
- Cho, K.; Yoon, D.; Yu, M.; Peachey, N.S.; Ferreira, P.A. Microglial activation in an amyotrophic lateral sclerosis-like model caused by Ranbp2 loss and nucleocytoplasmic transport impairment in retinal ganglion neurons. Cell. Mol. Life Sci. 2019, 76, 3407–3432. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, B.; Roncador, A.; Pischedda, F.; Casini, A.; Thomas, S.; Piccoli, G.; Kiebler, M.; Macchi, P. Ankyrin-G induces nucleoporin Nup358 to associate with the axon initial segment of neurons. J. Cell Sci. 2019, 132, jcs222802. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, H.; Lim, B.C.; Torii, T.; Joshi, A.; Konning, M.; Smith, C.; Palmer, D.J.; Ng, P.; Leterrier, C.; Oses-Prieto, J.A.; et al. Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nat. Comm. 2020, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, S.; Guan, Y.; Shen, Y.; Xu, L. Nucleoporin 50 mediates Kcna4 transcription to regulate cardiac electrical activity. J. Cell Sci. 2021, 134, jcs.256818. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Gao, X.; Tang, Q.; Huang, L.; Gao, S.; Yu, S.; Huang, J.; Li, J.; Zhou, D.; Zhang, Y.; et al. Nucleoporin 107 facilitates the nuclear export of Scn5a mRNA to regulate cardiac bioelectricity. J. Cell. Mol. Med. 2019, 23, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garduño, A.M.; Juarez-Hernandez, L.J.; Polanco, M.J.; Tosatto, L.; Michelatti, D.; Arosio, D.; Basso, M.; Pennuto, M.; Musio, C. Altered ionic currents and amelioration by IGF-1 and PACAP in motoneuron-derived cells modelling SBMA. Biophys. Chem. 2017, 229, 68–76. [Google Scholar] [CrossRef]
- Martínez-Rojas, V.A.; Jiménez-Garduño, A.M.; Michelatti, D.; Tosatto, L.; Marchioretto, M.; Arosio, D.; Basso, M.; Pennuto, M.; Musio, C. ClC-2-like chloride current alterations in a cell model of spinal and bulbar muscular atrophy, a polyglutamine disease. J. Mol. Neurosci. 2021, 71, 662–674. [Google Scholar] [CrossRef]
- Martínez-Rojas, V.A.; Arosio, D.; Pennuto, M.; Musio, C. Clenbuterol-sensitive delayed outward potassium currents in a cell model of spinal and bulbar muscular atrophy. Pflügers Arch. Eur. J. Physiol. 2021, 473, 1213–1227. [Google Scholar] [CrossRef]
- Zona, C.; Pieri, M.; Carunchio, I. Voltage-dependent sodium channels in spinal cord motor neurons display rapid recovery from fast inactivation in a mouse model of amyotrophic lateral sclerosis. J. Neurophysiol. 2006, 96, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; He, J.; Zhou, R.; Lorenzo, D.; Babcock, H.P.; Bennett, V.; Zhuang, X. Developmental mechanism of the periodic membrane skeleton in axons. Elife 2014, 3, e04581. [Google Scholar] [CrossRef]
- Hutten, S.; Dormann, D. Nucleocytoplasmic transport defects in neurodegeneration—Cause or consequence? Semin. Cell Dev. Biol. 2020, 99, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ionic Channels of Excitable Membrane, 3rd ed.; Oxford University Press: Oxford, UK, 2001; pp. 1–814. [Google Scholar]
- Leone, L.; Colussi, C.; Gironi, K.; Longo, V.; Fusco, S.; Li Puma, D.D.; D’Ascenzo, M.; Grassi, C. Altered Nup153 expression impairs the function of cultured hippocampal neural stem cells isolated from a mouse model of alzheimer’s disease. Mol. Neurobiol. 2019, 56, 5934–5949. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Searle, K.; Webb, M.; Yi, H.; Ferreira, P.A. Ranbp2 haploinsufficiency mediates distinct cellular and biochemical phenotypes in brain and retinal dopaminergic and glia cells elicited by the parkinsonian neurotoxin, 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP). Cell. Mol. Life Sci. 2012, 69, 3511–3527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawa, Y.; Rasband, M.N. Endogenously expressed Ranbp2 is not at the axon initial segment. J. Cell Sci. 2021, 134, jcs256180. [Google Scholar] [CrossRef] [PubMed]
- Grubb, M.S.; Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010, 465, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Stoler, O.; Scheller, A.; Khrapunsky, Y.; Goebbels, S.; Kirchhoff, F.; Gutnick, M.J.; Wolf, F.; Fleidervish, I.A. Role of sodium channel subtype in action potential generation by neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 2018, 115, E7184–E7192. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.; Li, L.; He, Q.; Li, M.; Shu, Y. Biophysical properties of somatic and axonal voltage-gated sodium channels in midbrain dopaminergic neurons. Front. Cell. Neurosci. 2019, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hetzer, M.W. Nuclear pore complex maintenance and implications for age-related diseases. Trends Cell Biol. 2022, 32, 216–227. [Google Scholar] [CrossRef]
- Sheffield, L.G.; Miskiewicz, H.B.; Tannenbaum, L.B.; Mirra, S.S. Nuclear pore complex proteins in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Fallini, C.; Khalil, B.; Smith, C.L.; Rossoll, W. Traffic jam at the nuclear pore: All roads lead to nucleocytoplasmic transport defects in ALS/FTD. Neurobiol. Dis. 2020, 140, 104835. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lusk, C.P. Emerging connections between nuclear pore complex homeostasis and ALS. Int. J. Mol. Sci. 2022, 23, 1329. [Google Scholar] [CrossRef] [PubMed]
- Fichtman, B.; Harel, T.; Biran, N.; Zagairy, F.; Applegate, C.D.; Salzberg, Y.; Gilboa, T.; Salah, S.; Shaag, A.; Simanovsky, N.; et al. Pathogenic variants in NUP214 cause “plugged” nuclear pore channels and acute febrile encephalopathy. Am. J. Hum. Genet. 2019, 105, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.R.; Frosch, M.P.; Hyman, B.T. Altered localization of nucleoporin 98 in primary tauopathies. Brain Commun. 2022, 5, fcac334. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shakkottai, V.G. Targeting Ion channels and Purkinje neuron intrinsic membrane excitability as a therapeutic strategy for cerebellar ataxia. Life 2023, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. New paradigms in pharmaceutical development. Life 2022, 12, 1433. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Naidoo, V.; Martínez-Iglesias, O.; Corzo, L.; Cacabelos, N.; Pego, R.; Carril, J.C. Personalized management and treatment of Alzheimer’s disease. Life 2022, 12, 460. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rojas, V.A.; Pischedda, F.; Romero-Maldonado, I.; Khalaf, B.; Piccoli, G.; Macchi, P.; Musio, C. Nucleoporin Nup358 Downregulation Tunes the Neuronal Excitability in Mouse Cortical Neurons. Life 2023, 13, 1791. https://doi.org/10.3390/life13091791
Martínez-Rojas VA, Pischedda F, Romero-Maldonado I, Khalaf B, Piccoli G, Macchi P, Musio C. Nucleoporin Nup358 Downregulation Tunes the Neuronal Excitability in Mouse Cortical Neurons. Life. 2023; 13(9):1791. https://doi.org/10.3390/life13091791
Chicago/Turabian StyleMartínez-Rojas, Vladimir A., Francesca Pischedda, Isabel Romero-Maldonado, Bouchra Khalaf, Giovanni Piccoli, Paolo Macchi, and Carlo Musio. 2023. "Nucleoporin Nup358 Downregulation Tunes the Neuronal Excitability in Mouse Cortical Neurons" Life 13, no. 9: 1791. https://doi.org/10.3390/life13091791
APA StyleMartínez-Rojas, V. A., Pischedda, F., Romero-Maldonado, I., Khalaf, B., Piccoli, G., Macchi, P., & Musio, C. (2023). Nucleoporin Nup358 Downregulation Tunes the Neuronal Excitability in Mouse Cortical Neurons. Life, 13(9), 1791. https://doi.org/10.3390/life13091791





