Application of Biostimulant in Seeds and Soil on Three Chickpea Varieties: Impacts on Germination, Vegetative Development, and Bacterial Facilitation of Nitrogen and Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biostimulant
2.3. Field Trial Design
2.4. Soil
2.5. Bacterial Counts
2.6. Variables Studied
2.7. Statistical Analysis
3. Results
3.1. Germination Percentage GP
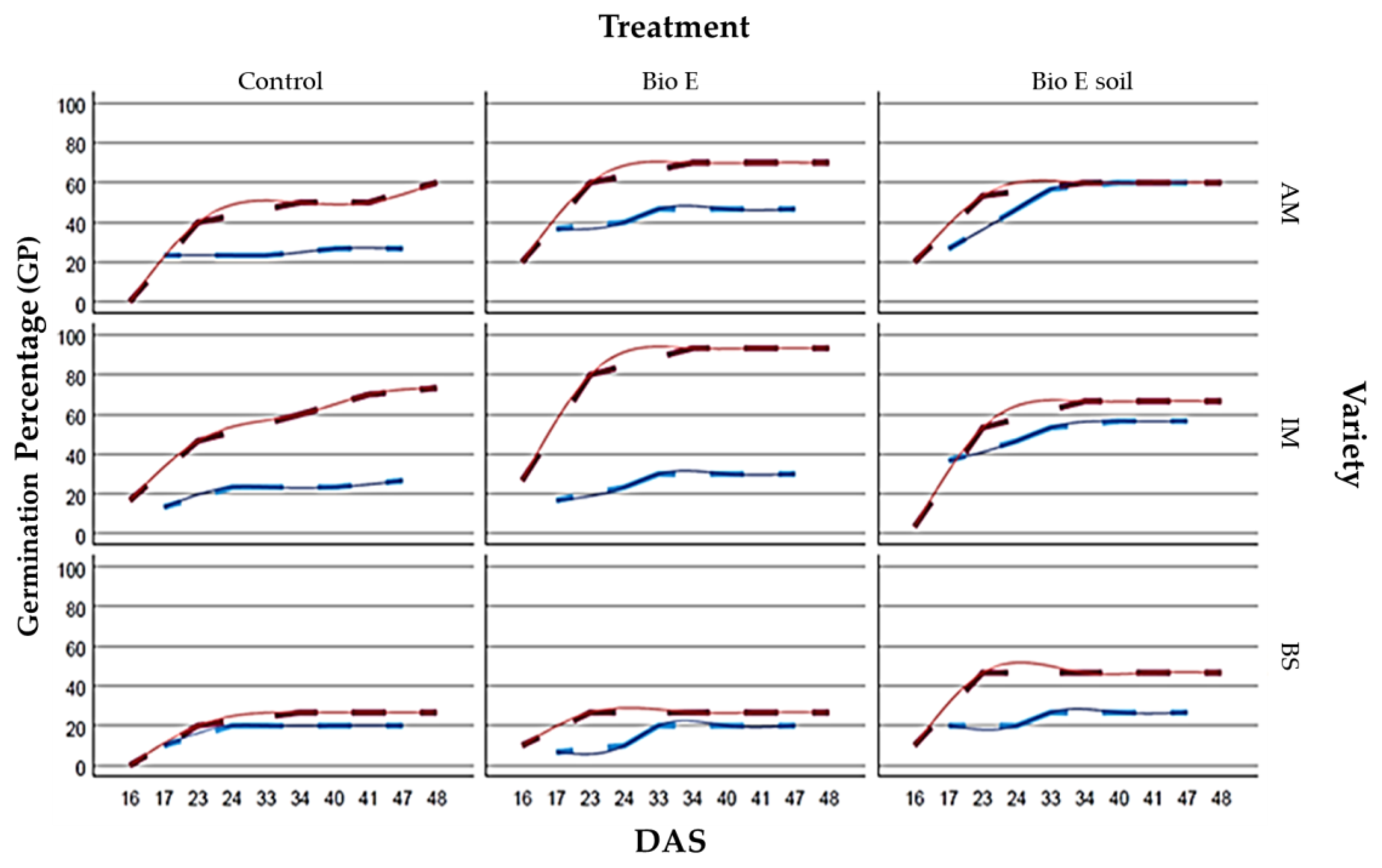
3.1.1. Plot A over Time (DAS)
- 17 DAS: The BioE treatment demonstrates a positive impact on germination, apart from when administered to the seed in the BS variety. The IM variety shows the most effective response to BioE treatment in the soil, while AM exhibits the greatest responsiveness when BioE is applied to the seed.
- 24 DAS: Treatment with BioE promotes germination compared to the control in AM and IM while negatively affecting BS when biostimulant is applied to seeds and having no impact when applied to the soil. In addition, BioE applied to seeds shows a stronger response in AM. The best germination success for both AM and IM is achieved by adding BioE to the soil.
- 33 DAS: Germination has ceased in the control treatment, while seed germination persists in the two treatments with BioE. The detrimental effect of the BS treatment on seed disappears when compared to its control. The three varieties exhibit optimal responses when BioE is added to the soil, with AM showing the best response, closely followed by IM.
- 40 DAS: Very similar to 33 DAS, only continues the germination in AM control and AM e IM when the BioE is on the seed.
- 47 DAS: Germination has ceased in all of the plots and only continues in the AM control.
3.1.2. Plot B over Time
- 16 DAS: The BioE treatment has a positive effect on the GP, except for the case of the IM variety treated with BioE on soil, which displays a lower germination rate than the control. The AM and BS varieties that were not treated do not show any germination. The BioE treatment on seed yields the most efficient response in IM, while both types of biostimulant applications elicit a similar response in AM and BS.
- 23 DAS: Treatment with BioE enhances germination compared to the control in AM and IM but has a lesser impact when applied to the soil. Moreover, BioE applied to seeds elicits a stronger response in AM. Optimal germination success for both AM and IM is achieved by adding BioE to the seed.
- 34 DAS: Germination has ceased in BS treated with BioE, whereas it continues in all other treatments and varieties. AM and IM show optimal responses when BioE is added to the seed, with IM exhibiting the highest response in all treatments, achieving 93.33% germination success.
- 41 DAS: The germination picture is like that of 33 DAS, with the germination continuing in the AM control.
- 48 DAS: Germination has ceased in almost all plots except for the AM and IM control plots, where it continues.
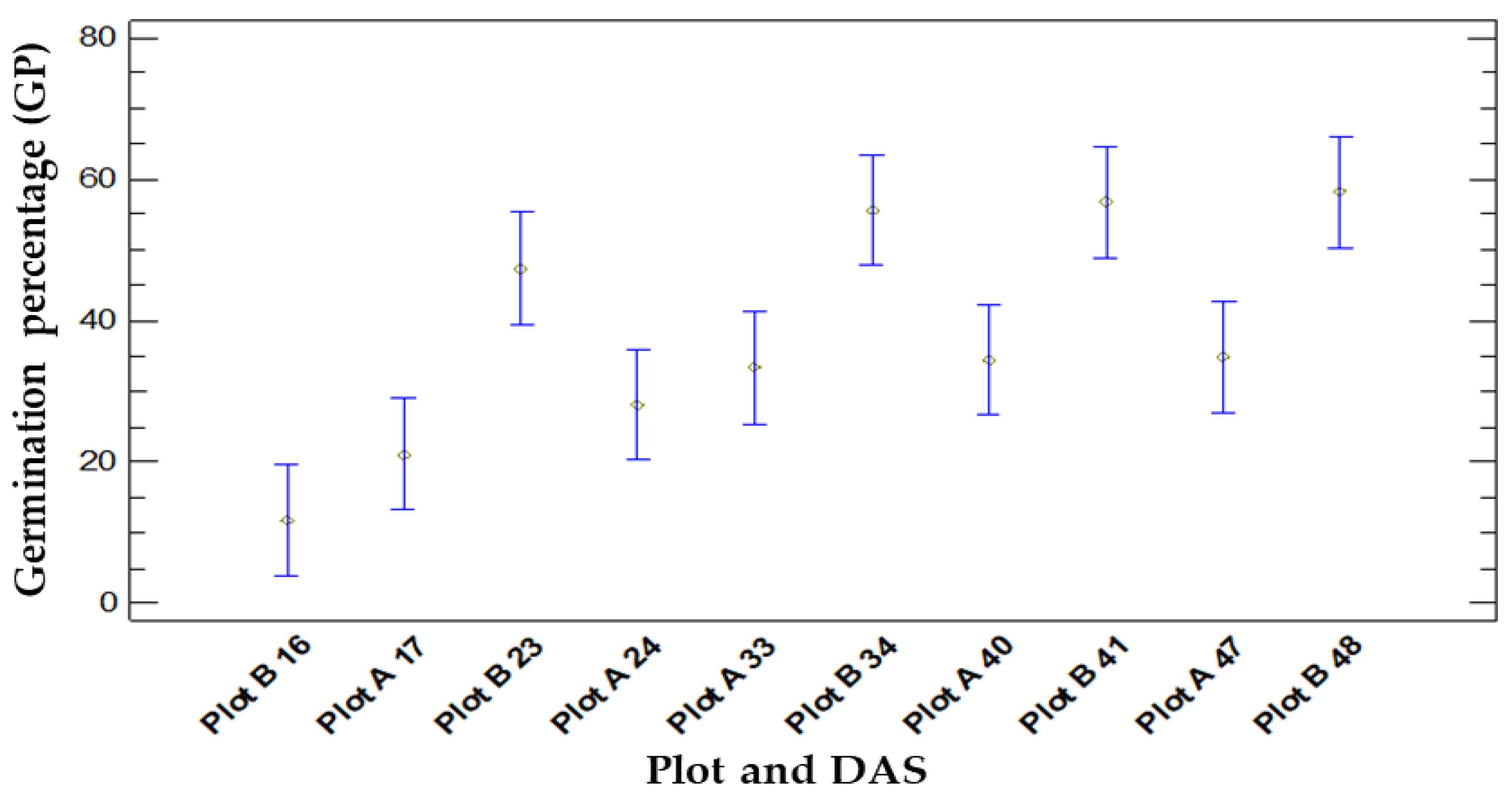
3.1.3. Comparison between Germination and Location (Plot A and B)
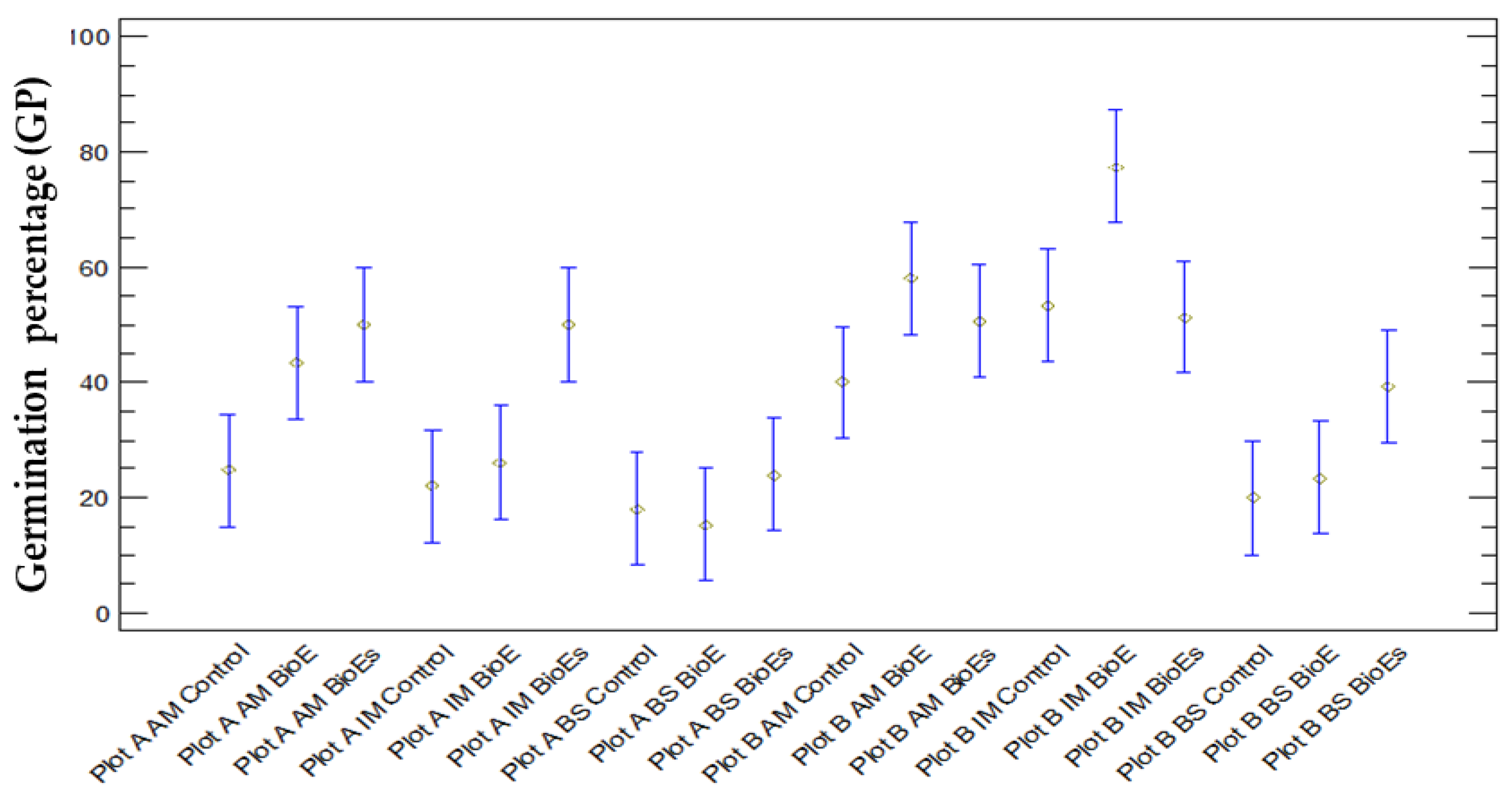
3.1.4. Comparison between GP and Variety (AM, IM, and BS)
3.1.5. Comparison between GP and Treatment
3.1.6. Comparison between GP and Location, Variety, and Treatment
- Plot A:
- The application of a biostimulant treatment on the AM variety reveals a marked enhancement in the GP when compared to untreated controls. This improvement is evident when applied to either the soil or the seed, with BioE showing an average increase from 26.67 to 46.67 and up to 60 in BioE soil, leading to a 20% and 33.33% increase, respectively.
- For IM, the application of biostimulants to the seed was found to have a minimal effect on the GP compared to the untreated control. Nevertheless, the application of BioE to the soil resulted in a significant increase in germination, with the average increasing from 26.66 to 56.66, an increase of 30%.
- In contrast, there was only a small increase in the GP from 20 to 26.66% when BS was applied to the soil in comparison to the control. There was no effect when BS was applied to the seed.
- Plot B:
- For the AM variety, the application of a biostimulant on the seed resulted in an enhanced GP compared to untreated seeds. Specifically, the GP rose by an average of 10%, from 60% in the control group to 70% in the BioE-treated group. Conversely, incorporation of the biostimulant directly into the soil did not cause any noticeable changes, as the GP remained unchanged compared to the control group.
- Treatment of IM with BioE increased the GP compared to the untreated control group, with an average rate of 73.33 increasing to 93.33, resulting in a 20% improvement in germination when the seed was coated with the biostimulant. However, BioE soil had a detrimental effect, as the mean GP dropped to 66.67, resulting in a 6.66% decrease relative to the control group.
- Regarding BS, only soil treatment led to a 20% increase in the GP from 26.67% to 46.67% compared to the control. No significant effect was observed when the biostimulant was applied to the seed (Figure 3).
3.2. Vegetative Development
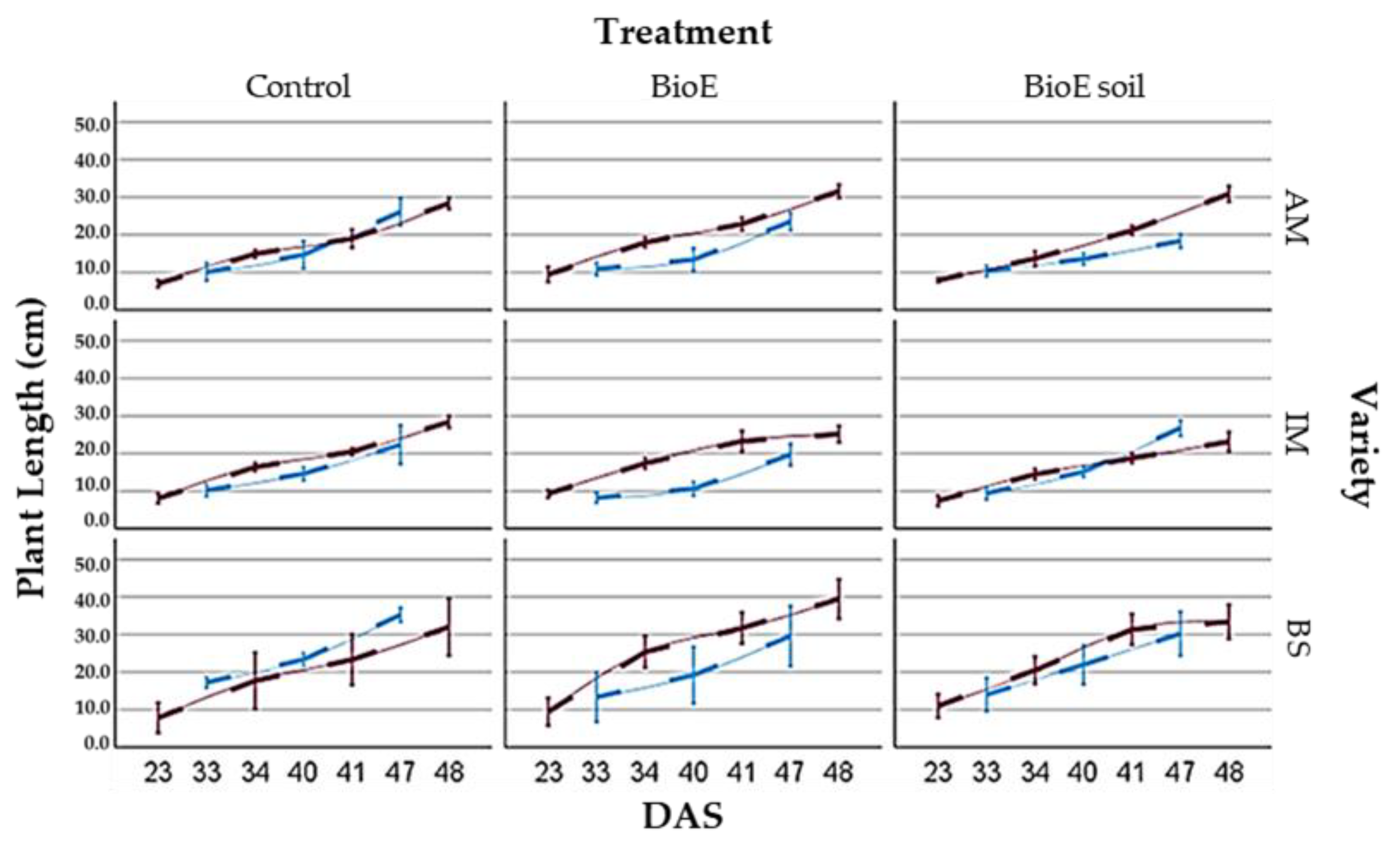
3.2.1. Average Plant Length
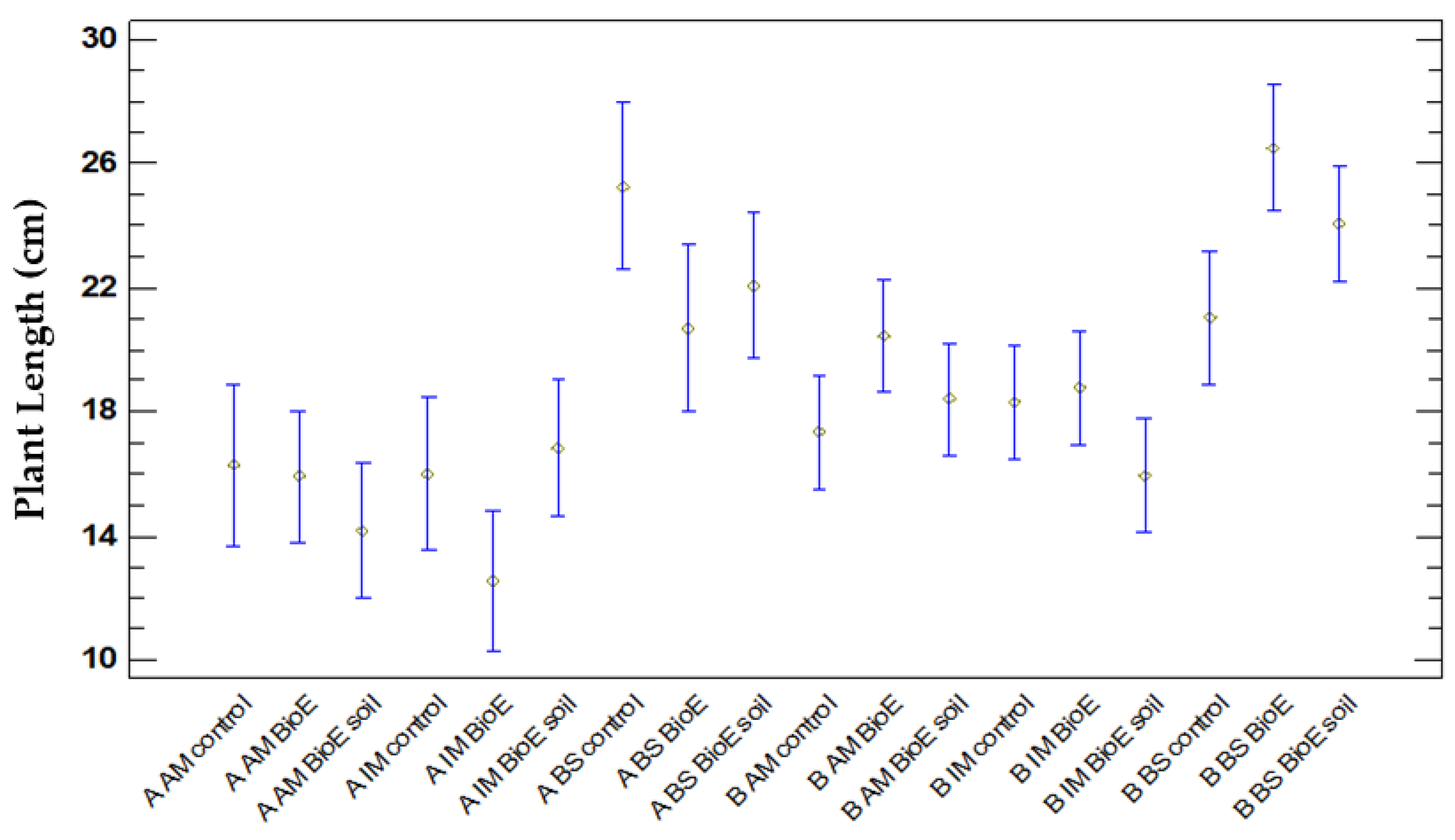
Comparison of Plant Length and Location (Plot A, Plot B)
Comparison of Plant Length (cm) and Variety
Comparison of Plant Length (cm) and Biostimulant Treatment
Comparison of Plant Lengths concerning Location Treatment and Variety
3.2.2. Average Number of Nodes
Comparison over Time (DAS)
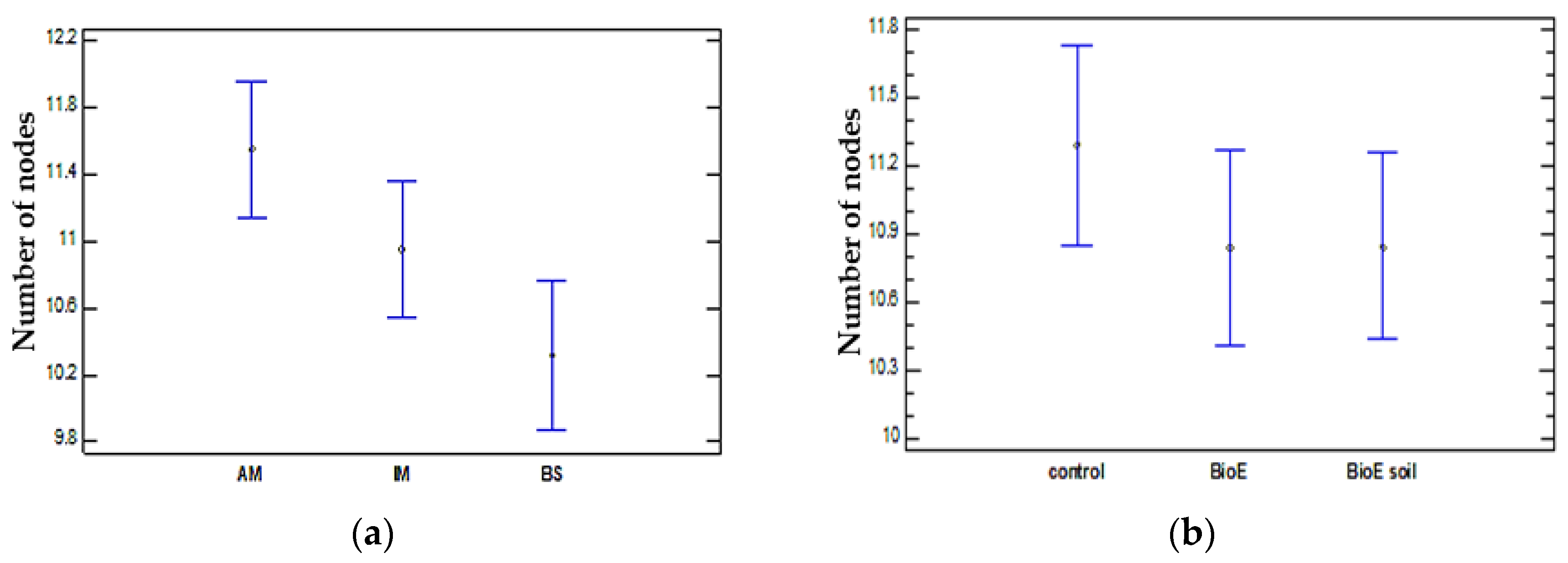
Comparison of Number of Nodes and Location
Number of Nodes and Variety Comparison
Comparison of Number of Nodes and Biostimulant Treatment (Control, BioE, and BioE Soil)
Comparison of Number of Nodes concerning Location, Treatment, and Variety
3.3. Soil
4. Discussion
4.1. Germination Success
4.2. Vegetative Development
4.2.1. Plant Length
4.2.2. Number of Nodes
4.3. Soil
5. Conclusions
6. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parra, L.; Mostaza-Colado, D.; Sánchez Hernández, J.; Marín Peira, J.; Mauri, P.V. Evaluation of the Biostimulant Effect on Germination of Chickpea seeds. In Proceedings of the 26th International Congress on Project Management and Engineering, Terrassa, Spain, 5–8 July 2022; p. 13. [Google Scholar]
- Gómez, E.; Mostaza-Colado, D.; Martínez Molina, M.; Sánchez Hernández, J.; Muñoz García, P.; Marín, J.; Mauri, P.V. Nueva Metodología de Cultivo de Garbanzo (Cicer arietinum L.) para la Adaptación al Cambio Climático. el Empleo de Bioestimulantes. In Proceedings of the XII Congreso Ibérico de Agroingeniería, Sevilla, Spain, Comunicación Oral. 4–6 September 2023. [Google Scholar]
- Commission Regulation. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. OJ L. 32002R0178 COUNCIL. EP; 1 February 2002. Available online: https://www.informea.org/en/legislation/regulation-ec-no-1782002-european-parliament-and-council-laying-down-general-principles#:~:text=(EC)%20No.-.178%2F2002%20of%20the%20European%20Parliament%20and%20of%20the%20Council.in%20matters%20of%20food%20safety (accessed on 5 September 2023).
- Colglazier, W. Sustainable development agenda: 2030. Science 2015, 349, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Barrios, M.A.; Estrada, J.A.S.E.; González, M.T.R.; Barrios, P.A. Rendimiento de garbanzo en función del tipo de suelo y niveles de nitrógeno. Rev. Mex. Cienc. Agrícolas 2015, 2, 295–299. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 5 September 2023).
- Rouphael, Y.; Colla, G. Plant Biostimulants: Rationale. State of the Art and Evolution. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccollo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant Activity of Phosphate in Horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.M.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant Activity of Silicon in Horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascalea, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Mauri Ablanque, P.V.; Vergara García, G.; Simorte Sánchez de Rivera, T. Mejora del Garbanzo. Agric. Rev. Agropecu. Y Ganad. 2001, 828, 428–431. [Google Scholar]
- Gabilondo, R.; Sánchez, J.; Gandía, M.L.; Montero-Muñoz, I.; Mauri, P.V.; Marín, J.; Muñoz, P.; Mostaza-Colado, D. Rhizobacteria-Based Biostimulant Mixture Effect on Chickpea (Cicer arietinum L.) Greenhouse and Cultivation Assays. Int. J. Plant Soil Sci. 2023, 35, 47–61. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107, 1–32. [Google Scholar]
- Prieto, K.R.; Echaide-Aquino, F.; Huerta-Robles, A.; Valério, H.P.; Macedo-Raygoza, G.; Prado, F.M.; Medeiros, M.H.G.; Brito, H.F.; da Silva, I.G.N.; Cunha Felinto, M.C.F.; et al. Endophytic Bacteria and Rare Earth Elements; Promising Candidates for Nutrient use Efficiency in Plants. In Plant Macronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–306. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Fu, X. Integrated Effects of Co-Inoculation with Phosphate-Solubilizing Bacteria and N2-Fixing Bacteria on Microbial Population and Soil Amendment under C Deficiency. Int. J. Environ. Res. Public Health 2019, 16, 2442. [Google Scholar] [CrossRef]
- Rajwar, A.; Sahgal, M.; Johri, B.N. Legume–Rhizobia Symbiosis and Interactions in Agroecosystems. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N., Ed.; Springer: New Delhi, India, 2013; pp. 233–265. [Google Scholar]
- Tien, T.M.; Gaskins, M.H.; Hubbell, D. Plant Growth Substances Produced by Azospirillum Brasilense and their Effect on the Growth of Pearl Millet (Pennisetum americanum L.). Appl. Environ. Microbiol. 1979, 37, 1016–1024. [Google Scholar] [CrossRef]
- Gabilondo, R.; Sánchez, J.; Muñoz, P.; Montero-Muñoz, I.; Mauri, P.V.; Marín, J.; Mostaza-Colado, D. Evaluation of Biostimulatory Activity of Commercial Formulations on Three Varieties of Chickpea. Agriculture 2023, 13, 474. [Google Scholar] [CrossRef]
- Nunes da Silva, M.; Medeiros, J.S.; Santos, C.S.; Vasconcelos, M.W. Low Water Provision Differently Affects Plant Growth and seed Mineral Profile of Kabuli and Desi Chickpeas (Cicer arietinum). In Proceedings of the 31st International Horticulture Congress, Angers, France, 14–20 August 2022. [Google Scholar]
- Toker, C. A Note on the Evolution of Kabuli Chickpeas as Shown by Induced Mutations in Cicer Reticulatum Ladizinsky. Genet. Resour. Crop Evol. 2009, 56, 7–12. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and Seed Yield of Cool Season Grain Legumes in Mediterranean Environments of South-Western Australia. Aust. J. Agric. Res. 1999, 50, 375. [Google Scholar] [CrossRef]
- Mostaza-Colado, D.; Gabilondo, R.; Muñoz, P.; Marín, J.; Mauri, P.V. Nueva Variedad de Garbanzo más Digestiva; La Revista Profesional de Sanidad Vegetal; Phytoma España: Valencia, Spain, 2023; Volume 347, p. 134. ISSN 1131-8988. [Google Scholar]
- INIA. El Encín Suelo y Clima; Departamento de Ecología—C.R.I.D.A 06 (TAJO)—INIA: Madrid, Spain, 1977. [Google Scholar]
- Aquilanti, L.; Favilli, F.; Clemeti, F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol. Biochem. 2004, 36, 1475–1483. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Woomer, P.L. Most Probable Number Counts. In Methods of Soil Analysis. Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Ed.; Soil Science Society American: Madison, WI, USA, 1994; pp. 59–79. [Google Scholar]
- Conde, J.M. Nuevas Líneas Avanzadas de Garbanzo y Estudio Agro y Económico de la Variedad “Blanco Sinaloa 92” en Varias Parcelas de la Campiña de Sevilla. Tesis de Grado en Ingeniería Agrícola. Escuela Técnica Superior de Ingeniería Agronómica; Universidad de Sevilla: Sevilla, Spain, 2016. [Google Scholar]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef]
- Patil, N.S.; Karadge, B.A. Effect of Carbonates on seed germination and seedling growth in Portulaca oleracea L. J. Adv. Sci. Res. 2014, 5, 5–7. [Google Scholar]
- Gómez-Garza, R.M. Blanco Sinaloa-92: New Variety of Chickpea for the Northwest of Mexico; Foll. Téc. Campo Exp. Culiacán INIFAP: Culiacán Sinaloa, México, 1993; Volume 13, p. 12. [Google Scholar]
- Postgate, J.R.; Hunter, J.R. The survival of starved bacteria. Microbiology 1962, 29, 233–263. [Google Scholar] [CrossRef]
- Ramzan, M.; Ullah, M.A.; Khaliq, M.; Abbas, M.; Parveen, Z.; Irshad, M. Efficiency of Nitrogen Fixing. Nodulation Formation. Phosphate Solubilizing Bacteria and plant Growth Promoting Rhizobacteria on Yield of Chickpea. J. Agric. Res. 2023, 61, 43–50. [Google Scholar] [CrossRef]
- Zamljen, T.; Lojen, S.; Zupanc, V.; Slatnar, A. Determination of the yield, enzymatic and metabolic response of two Capsicum spp. cultivars to deficit irrigation and fertilization using the stable isotope 15N. Chem. Biol. Technol. Agric. 2023, 10, 129. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, S.; Liu, L.L. Studies on Phosphorus Solubilizing Activity of a Strain of Phosphobacteria Isolated from Chestnut Type Soil in China. Bioresour. Technol. 2008, 99, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.A.B. Growth Optimization of Potassium Solubilizing Bacteria Isolated from Biofertilizer; Faculty of Chemical & Natural Resources Engineering, University Malaysia Pahang: Pekan, Malaysia, 2009. [Google Scholar]
- Verma, A.; Patidar, Y.; Vaishampayan, A. Isolation and Purification of Potassium Solubilizing Bacteria from Different Regions of India and its Effect on Crop’s Yield. Indian J. Microbiol. Res. 2016, 3, 483–488. [Google Scholar] [CrossRef]





| Plot A | Plot B | ||
|---|---|---|---|
| Parameter | Value | Parameter | Value |
| pH | 8.4 | pH | 7.2 |
| C.E. (dS/m) | 0.19 | C.E. (dS/m) | 0.14 |
| Carbonates (%) | 6.05 | Carbonates (%) | 0.00 |
| N (%) | 0.12 | N (%) | 0.12 |
| SOM (%) | 2.02 | SOM (%) | 2.02 |
| P (mg/kg) | 1.4 | P (mg/kg) | 67.9 |
| Ca (mg/kg) | 5159 | Ca (mg/kg) | 2113 |
| Mg (mg/kg) | 411 | Mg (mg/kg) | 415 |
| Na (mg/kg) | 34 | Na (mg/kg) | 105 |
| K (mg/kg) | 119 | K (mg/kg) | 227 |
| Germination Percentage (GP) | Plot A | Plot B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety | Treatment | 17 DAS | 24 DAS | 33 DAS | 40 DAS | 47 DAS | 16 DAS | 23 DAS | 34 DAS | 41 DAS | 48 DAS |
| AM | Control | 23.33 | 23.33 | 23.33 | 26.67 | 26.67 | 0.00 | 40.00 | 50.00 | 50.00 | 60.00 |
| BioE | 36.67 | 40.00 | 46.67 | 46.67 | 46.67 | 20.00 | 60.00 | 70.00 | 70.00 | 70.00 | |
| BioE soil | 26.67 | 46.67 | 56.67 | 60.00 | 60.00 | 20.00 | 53.33 | 60.00 | 60.00 | 60.00 | |
| IM | Control | 13.33 | 23.33 | 23.33 | 23.33 | 26.67 | 16.67 | 46.67 | 60.00 | 70.00 | 73.33 |
| BioE | 16.67 | 23.33 | 30.00 | 30.00 | 30.00 | 26.67 | 80.00 | 93.33 | 93.33 | 93.33 | |
| BioE soil | 36.67 | 46.67 | 53.33 | 56.67 | 56.67 | 3.33 | 53.33 | 66.67 | 66.67 | 66.67 | |
| BS | Control | 10.00 | 20.00 | 20.00 | 20.00 | 20.00 | 0.00 | 20.00 | 26.67 | 26.67 | 26.67 |
| BioE | 6.67 | 10.00 | 20.00 | 20.00 | 20.00 | 10.00 | 26.67 | 26.67 | 26.67 | 26.67 | |
| BioE soil | 20.00 | 20.00 | 26.67 | 26.67 | 26.67 | 10.00 | 46.67 | 46.67 | 46.67 | 46.67 | |
| Plant Length (cm) | Plot A | Plot B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33 DAS | 40 DAS | 47 DAS | 23 DAS | 33 DAS | 41 DAS | 48 DAS | |||||||||
| Variety | Treatment | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| AM | Control | 10.1 | 2.6 | 14.0 | 4.3 | 26.1 | 3.5 | 7.0 | 1.4 | 14.9 | 1.6 | 19.0 | 3.4 | 28.4 | 2.2 |
| BioE | 10.9 | 2.4 | 13.4 | 4.3 | 23.5 | 3.3 | 9.5 | 2.8 | 18.0 | 1.9 | 22.9 | 2.4 | 31.6 | 2.5 | |
| BioE soil | 10.4 | 2.0 | 13.8 | 2.2 | 18.4 | 2.6 | 7.9 | 0.8 | 13.7 | 2.8 | 21.2 | 1.8 | 30.9 | 2.9 | |
| IM | Control | 10.2 | 1.7 | 14.6 | 2.0 | 22.3 | 6.2 | 8.1 | 1.9 | 16.2 | 1.3 | 20.5 | 1.5 | 28.4 | 2.2 |
| BioE | 8.2 | 1.7 | 10.6 | 2.4 | 19.7 | 3.5 | 9.3 | 1.5 | 17.4 | 2.1 | 23.3 | 3.9 | 25.1 | 3.1 | |
| BioE soil | 9.4 | 2.4 | 15.1 | 2.1 | 26.8 | 2.8 | 7.4 | 2.0 | 14.5 | 2.1 | 18.7 | 1.9 | 23.2 | 3.7 | |
| BS | Control | 17.3 | 1.4 | 23.4 | 1.7 | 35.2 | 1.8 | 7.9 | 3.9 | 17.7 | 9.0 | 23.3 | 8.1 | 32.0 | 9.1 |
| BioE | 13.4 | 6.3 | 19.2 | 7.2 | 29.6 | 7.7 | 9.5 | 4.4 | 25.4 | 5.0 | 31.7 | 5.0 | 39.4 | 6.3 | |
| BioE soil | 14.0 | 5.4 | 22.0 | 6.3 | 30.2 | 7.1 | 11.0 | 4.4 | 20.5 | 5.1 | 31.4 | 5.8 | 33.4 | 6.4 | |
| Number of Nodes | Plot A | Plot B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33 DAS | 40 DAS | 23 DAS | 33 DAS | 41 DAS | 48 DAS | ||||||||
| Variety | Treatment | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| AM | Control | 10.14 | 1.35 | 11.88 | 2.85 | 5.10 | 1.10 | 11.50 | 0.97 | 13.50 | 1.84 | 18.80 | 1.32 |
| BioE | 9.50 | 1.08 | 11.00 | 3.16 | 5.60 | 1.26 | 10.90 | 1.29 | 12.90 | 1.29 | 16.70 | 1.34 | |
| BioE soil | 9.00 | 1.49 | 11.80 | 1.32 | 6.10 | 0.57 | 10.20 | 2.04 | 14.20 | 1.12 | 18.70 | 1.79 | |
| IM | Control | 10.00 | 1.15 | 12.00 | 0.82 | 6.20 | 0.79 | 11.60 | 0.70 | 13.80 | 0.79 | 16.30 | 1.70 |
| BioE | 8.11 | 2.15 | 9.78 | 1.92 | 5.80 | 1.32 | 11.50 | 1.18 | 13.00 | 0.67 | 14.60 | 2.50 | |
| BioE soil | 8.80 | 1.75 | 11.80 | 1.03 | 5.00 | 1.05 | 9.70 | 1.42 | 12.10 | 1.29 | 15.90 | 1.73 | |
| BS | Control | 9.67 | 0.82 | 11.83 | 1.17 | 4.50 | 2.26 | 8.13 | 4.09 | 11.25 | 3.01 | 13.25 | 3.33 |
| BioE | 7.83 | 1.94 | 10.33 | 2.42 | 5.13 | 2.10 | 10.88 | 2.42 | 12.88 | 1.55 | 16.50 | 1.41 | |
| BioE soil | 8.50 | 2.78 | 11.75 | 2.76 | 5.90 | 1.52 | 9.70 | 2.16 | 12.10 | 2.85 | 13.70 | 2.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, E.; Alonso, A.; Sánchez, J.; Muñoz, P.; Marín, J.; Mostaza-Colado, D.; Mauri, P.V. Application of Biostimulant in Seeds and Soil on Three Chickpea Varieties: Impacts on Germination, Vegetative Development, and Bacterial Facilitation of Nitrogen and Phosphorus. Life 2024, 14, 148. https://doi.org/10.3390/life14010148
Gómez E, Alonso A, Sánchez J, Muñoz P, Marín J, Mostaza-Colado D, Mauri PV. Application of Biostimulant in Seeds and Soil on Three Chickpea Varieties: Impacts on Germination, Vegetative Development, and Bacterial Facilitation of Nitrogen and Phosphorus. Life. 2024; 14(1):148. https://doi.org/10.3390/life14010148
Chicago/Turabian StyleGómez, Elisa, Alejandro Alonso, Jorge Sánchez, Pedro Muñoz, José Marín, David Mostaza-Colado, and Pedro V. Mauri. 2024. "Application of Biostimulant in Seeds and Soil on Three Chickpea Varieties: Impacts on Germination, Vegetative Development, and Bacterial Facilitation of Nitrogen and Phosphorus" Life 14, no. 1: 148. https://doi.org/10.3390/life14010148
APA StyleGómez, E., Alonso, A., Sánchez, J., Muñoz, P., Marín, J., Mostaza-Colado, D., & Mauri, P. V. (2024). Application of Biostimulant in Seeds and Soil on Three Chickpea Varieties: Impacts on Germination, Vegetative Development, and Bacterial Facilitation of Nitrogen and Phosphorus. Life, 14(1), 148. https://doi.org/10.3390/life14010148








