Mild Traumatic Brain Injury as a Risk Factor for Parkinsonism, Tics, and Akathisia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Review: Narrative Summary
3.1.1. Parkinsonism
3.1.2. Tic Disorder
3.1.3. Akathisia
3.1.4. Meta-Analysis
3.1.5. DLBs, Tics, and Akathisia
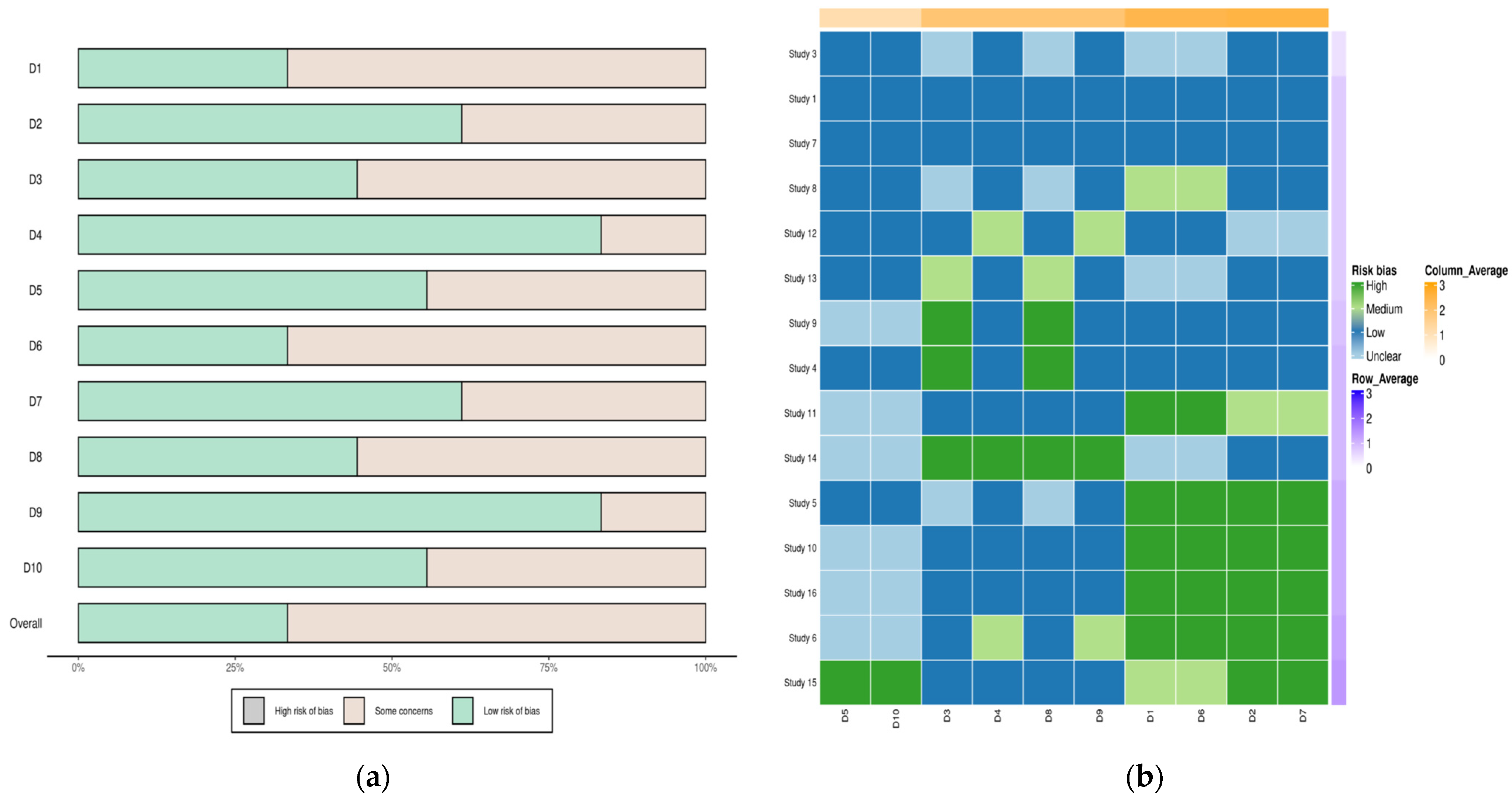
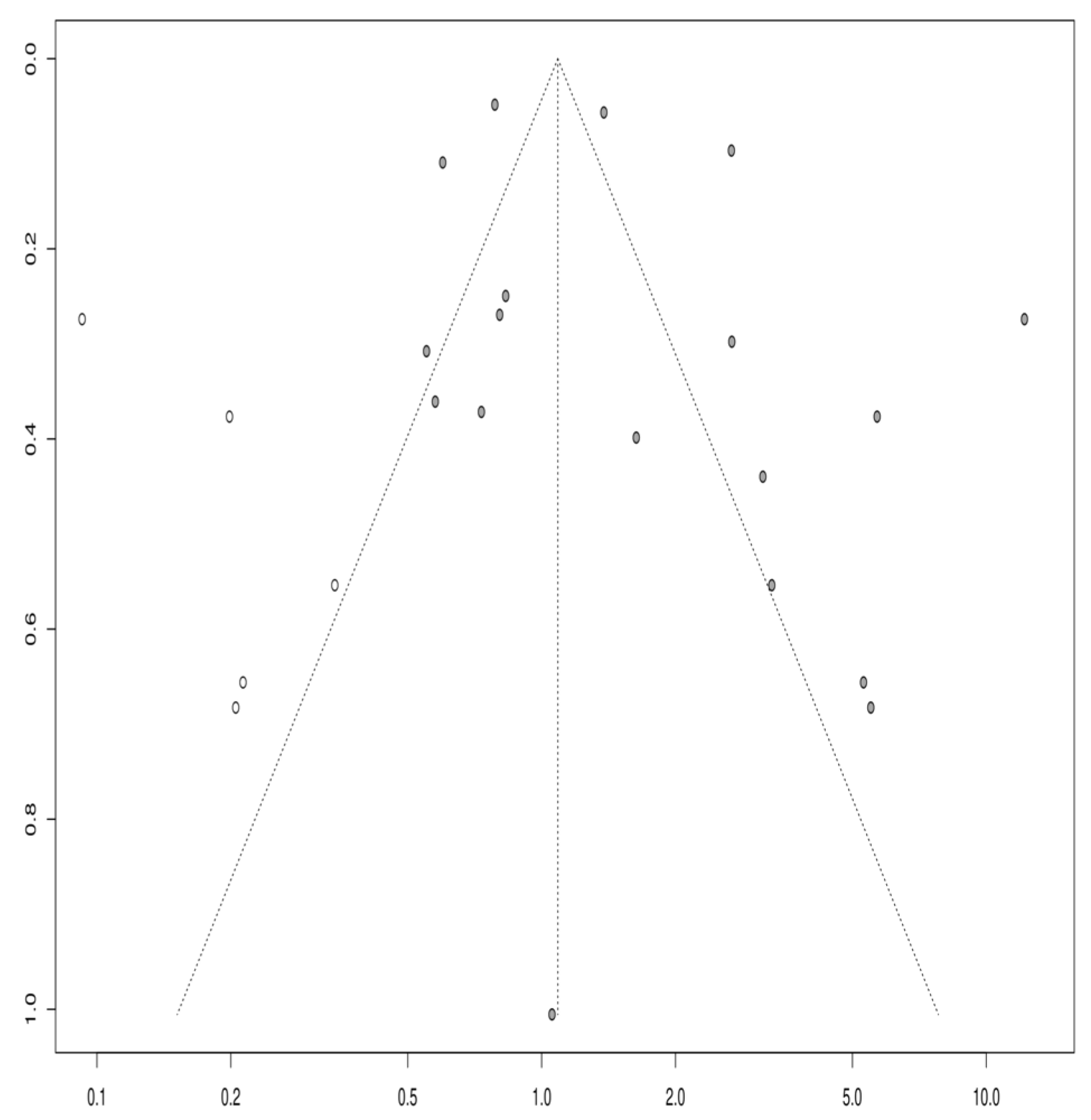
3.1.6. Publication Bias
4. Discussion
5. Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. JNS 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.M.; Baloyannis, S. Post-concussion syndrome and chronic traumatic encephalopathy: Narrative review on the neuropathology, neuroimaging and fluid biomarkers. Diagnostics 2022, 18, 740. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Petridis, F.; Chatzikonstantinou, S.; Kazis, D. Alpha-synuclein levels in the differential diagnosis of Lewy bodies dementia and other neurodegenerative disorders: A meta-analysis. Alzheimer Dis. Assoc. Disord. 2020, 34, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Goldman, S.; Tanner, C.M.; Yaffe, K. Traumatic brain injury in later life increases risk for P arkinson disease. Ann. Neurol. 2015, 77, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Seidler, A.; Hellenbrand, W.; Robra, B.P.; Vieregge, P.; Nischan, P.; Joerg, J.; Schneider, E. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: A case-control study in Germany. Neurology 1996, 46, 1275. [Google Scholar] [CrossRef] [PubMed]
- Schiehser, D.M.; Filoteo, J.V.; Litvan, I.; Pirogovsky-Turk, E.; Lessig, S.L.; Song, D.S. Cognitive functioning in individuals with Parkinson’s disease and traumatic brain injury: A longitudinal study. Park. Relat. Disord. 2016, 30, 58–61. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall: Boca Raton, FL, USA; CRC Press: London, UK, 2021. [Google Scholar]
- Phelps, A.; Alosco, M.L.; Baucom, Z.; Hartlage, K.; Palmisano, J.N.; Weuve, J.; Stern, R.A. Association of playing college American football with long-term health outcomes and mortality. JAMA Netw. Open 2022, 5, e228775. [Google Scholar] [CrossRef]
- Gardner, R.C.; Byers, A.L.; Barnes, D.E.; Li, Y.; Boscardin, J.; Yaffe, K. Mild TBI and risk of Parkinson disease: A chronic effects of neurotrauma consortium study. Neurology 2018, 90, e1771–e1779. [Google Scholar] [CrossRef]
- Taylor, C.A.; Saint-Hilaire, M.H.; Cupples, L.A.; Thomas, C.A.; Burchard, A.E.; Feldman, R.G.; Myers, R.H. Environmental, medical, and family history risk factors for Parkinson’s disease: A New England-based case control study. Am. J. Med. Genet. 1999, 88, 742–749. [Google Scholar] [CrossRef]
- Goldman, S.M.; Tanner, C.M.; Oakes, D.; Bhudhikanok, G.S.; Gupta, A.; Langston, J.W. Head injury and Parkinson’s disease risk in twins. Ann. Neurol. 2006, 60, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Lo, S.K.; See, L.C.; Chen, H.Z.; Chen, R.S.; Weng, Y.H.; Lu, C.S. Environmental risk factors of young onset Parkinson’s disease: A case-control study. Clin. Neurol. Neurosurg. 2002, 104, 328–333. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.J.; LeCouteur, D.G.; Green, A.C.; Brayne, C.; Johnson, A.G.; Chan, D.; Pond, S.M. The epidemiology of Parkinson’s disease in an Australian population. Neuroepidemiology 1998, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kuopio, A.M.; Marttila, R.J.; Helenius, H.; Rinne, U.K. Environmental risk factors in Parkinson’s disease. Mov. Disord. 1999, 14, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Baldereschi, M.; Di Carlo, A.; Vanni, P.; Ghetti, A.; Carbonin, P.; Amaducci, L.; Inzitari, D.; Italian Longitudinal Study on Aging Working Group. Lifestyle-related risk factors for Parkinson’s disease: A population-based study. Acta Neurol. Scand. 2003, 108, 239–244. [Google Scholar] [CrossRef]
- Smargiassi, A.; Mutti, A.; De Rosa, A.; De Palma, G.; Negrotti, A.; Calzetti, S. A case-control study of occupational and environmental risk factors for Parkinson’s disease in the Emilia-Romagna region of Italy. Neurotoxicology 1998, 19, 709–712. [Google Scholar]
- Bower, J.H.; Maraganore, D.M.; Peterson, B.J.; McDonnell, S.K.; Ahlskog, J.E.; Rocca, W.A. Head trauma preceding PD: A case-control study. Neurology 2003, 60, 1610–1615. [Google Scholar] [CrossRef]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Counsell, C.; Mozzoni, P.; et al. Environmental risk factors for Parkinson’s disease and parkinsonism: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 666–672. [Google Scholar] [CrossRef]
- Rugbjerg, K.; Ritz, B.; Korbo, L.; Martinussen, N.; Olsen, J.H. Risk of Parkinson’s Disease after Hospital Contact for Head Injury: Population Based Case-Control Study. BMJ 2008, 337, 34–36. [Google Scholar] [CrossRef]
- Sanyal, J.; Chakraborty, D.P.; Sarkar, B.; Banerjee, T.K.; Mukherjee, S.C.; Ray, B.C.; Rao, V.R. Environmental and familial risk factors of Parkinsons disease: Case-control study. Can. J. Neurol. Sci. 2010, 37, 637–642. [Google Scholar] [CrossRef]
- De Michele, G.; Filla, A.; Volpe, G.; De Marco, V.; Gogliettino, A.; Ambrosio, G.; Campanella, G. Environmental and genetic risk factors in Parkinson’s disease: A case–control study in southern Italy. Mov. Disord. 1996, 11, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Martyn, C.N.; Osmond, C. Parkinson’s disease and the environment in early life. J. Neurol. Sci. 1995, 132, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Mielke, M.M.; Turcano, P.; Ahlskog, J.E.; Bower, J.H.; Savica, R. Traumatic Brain Injury Preceding Clinically Diagnosed α-Synucleinopathies: A Case-Control Study. Neurology 2020, 94, e764–e773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Schaffert, J.; Lobue, C.; Womack, K.B.; Hart, J.; Cullum, C.M. Traumatic Brain Injury and Age of Onset of Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2018, 66, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, N.; Nair, K.P.S.; Romanoski, C.; Singh, R.; Venketswara, G. Tics after traumatic brain injury. Brain Inj. 2011, 25, 629–633. [Google Scholar] [CrossRef]
- Desai, A.; Nierenberg, D.W.; Duhaime, A.C. Akathisia after mild traumatic head injury: Case report. J. Neurosurg. Pediatr. 2010, 5, 460–464. [Google Scholar] [CrossRef]
- Johnson, L.; Williams, G.; Sherrington, C.; Pilli, K.; Chagpar, S.; Auchettl, A.; Hassett, L. The effect of physical activity on health outcomes in people with moderate-to-severe traumatic brain injury: A rapid systematic review with meta-analysis. BMC Public Health 2023, 23, 63. [Google Scholar] [CrossRef]
- Werneck, A.L.; Alvarenga, H. Genetics, drugs and environmental factors in Parkinson’s disease. A case-control study. Arq. Neuropsiquiatr. 1999, 57, 347–355. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Romila, L.; Ciobica, A.; Burlui, V.; Kamal, F.Z.; Mavroudis, I. Mild Traumatic Brain Injury as a Risk Factor for Parkinsonism, Tics, and Akathisia: A Systematic Review and Meta-Analysis. Life 2024, 14, 32. https://doi.org/10.3390/life14010032
Khan N, Romila L, Ciobica A, Burlui V, Kamal FZ, Mavroudis I. Mild Traumatic Brain Injury as a Risk Factor for Parkinsonism, Tics, and Akathisia: A Systematic Review and Meta-Analysis. Life. 2024; 14(1):32. https://doi.org/10.3390/life14010032
Chicago/Turabian StyleKhan, Nashaba, Laura Romila, Alin Ciobica, Vasile Burlui, Fatima Zahra Kamal, and Ioannis Mavroudis. 2024. "Mild Traumatic Brain Injury as a Risk Factor for Parkinsonism, Tics, and Akathisia: A Systematic Review and Meta-Analysis" Life 14, no. 1: 32. https://doi.org/10.3390/life14010032




