The End Justifies the Means: Chagas Disease from a Perspective of the Host–Trypanosoma cruzi Interaction
Abstract
1. Introduction
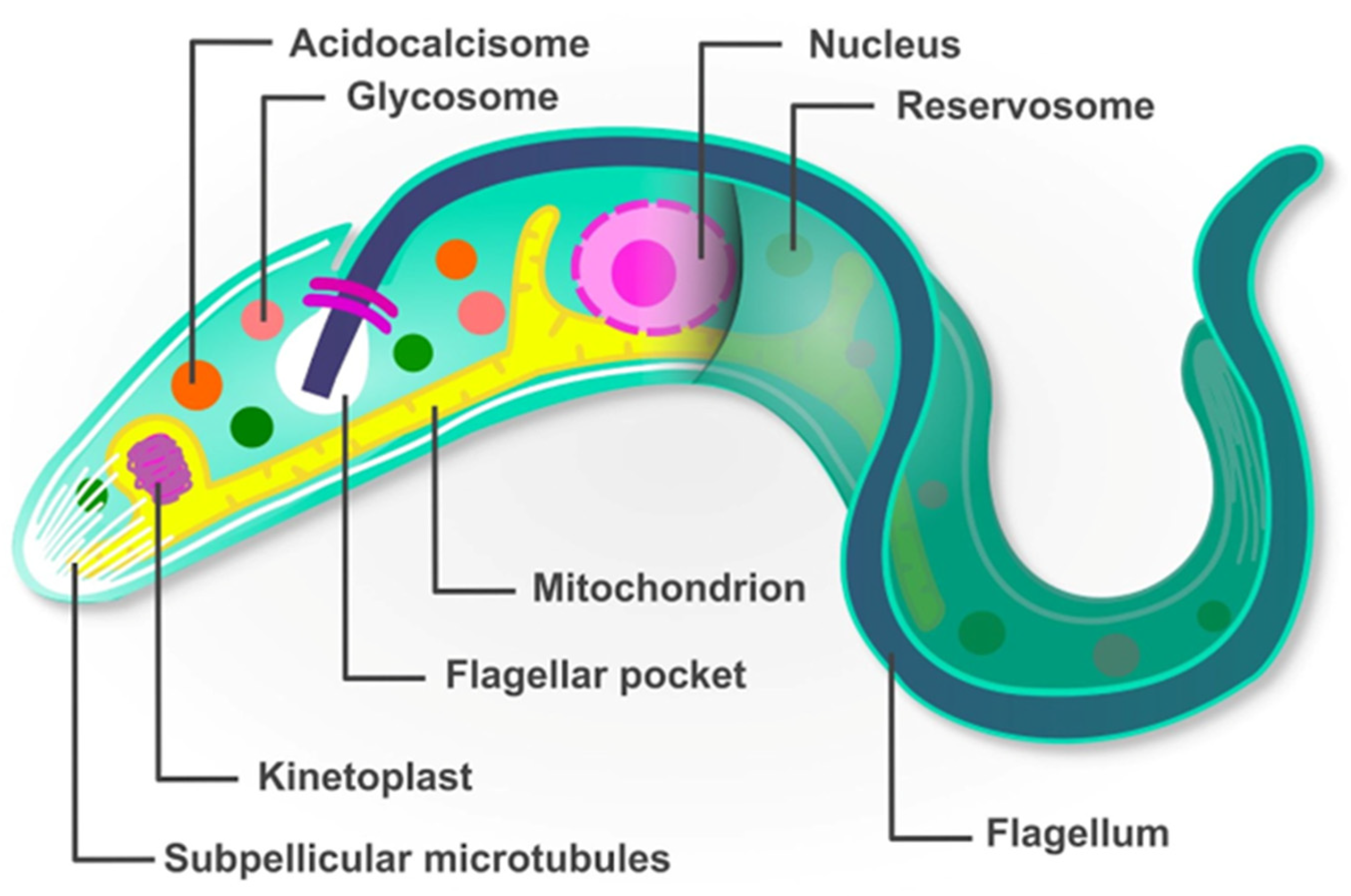
2. Trypanosoma cruzi Has a Peculiar Molecular Biology
2.1. Trans-Sialidases Family
2.2. Mucins Family
2.3. Mucin-Associated Surface Proteins Family (MASPs)
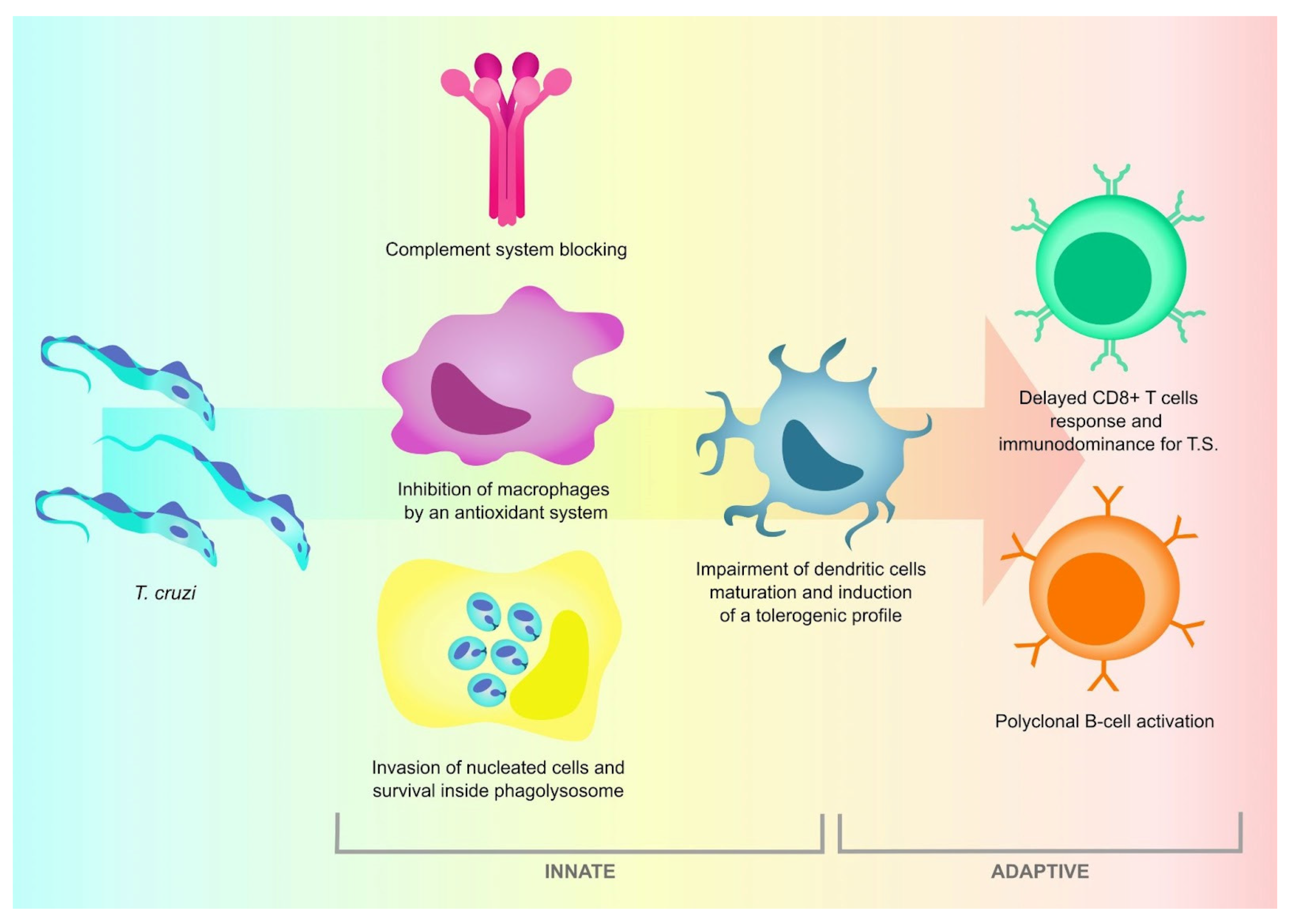
3. The Host Immune Response against T. cruzi Is Effective, but Not Sterilizing
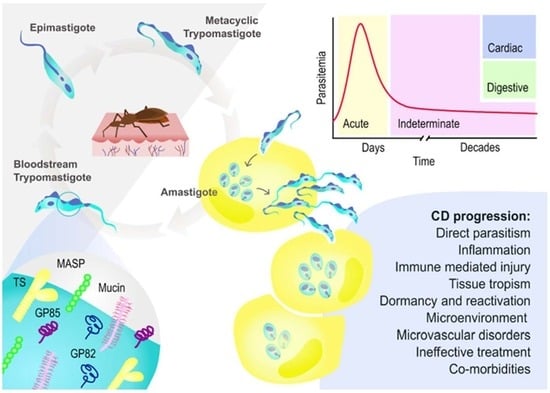
4. The Pathogenesis of Chagas Disease: A Story of Persistence, Tropism and Dormancy
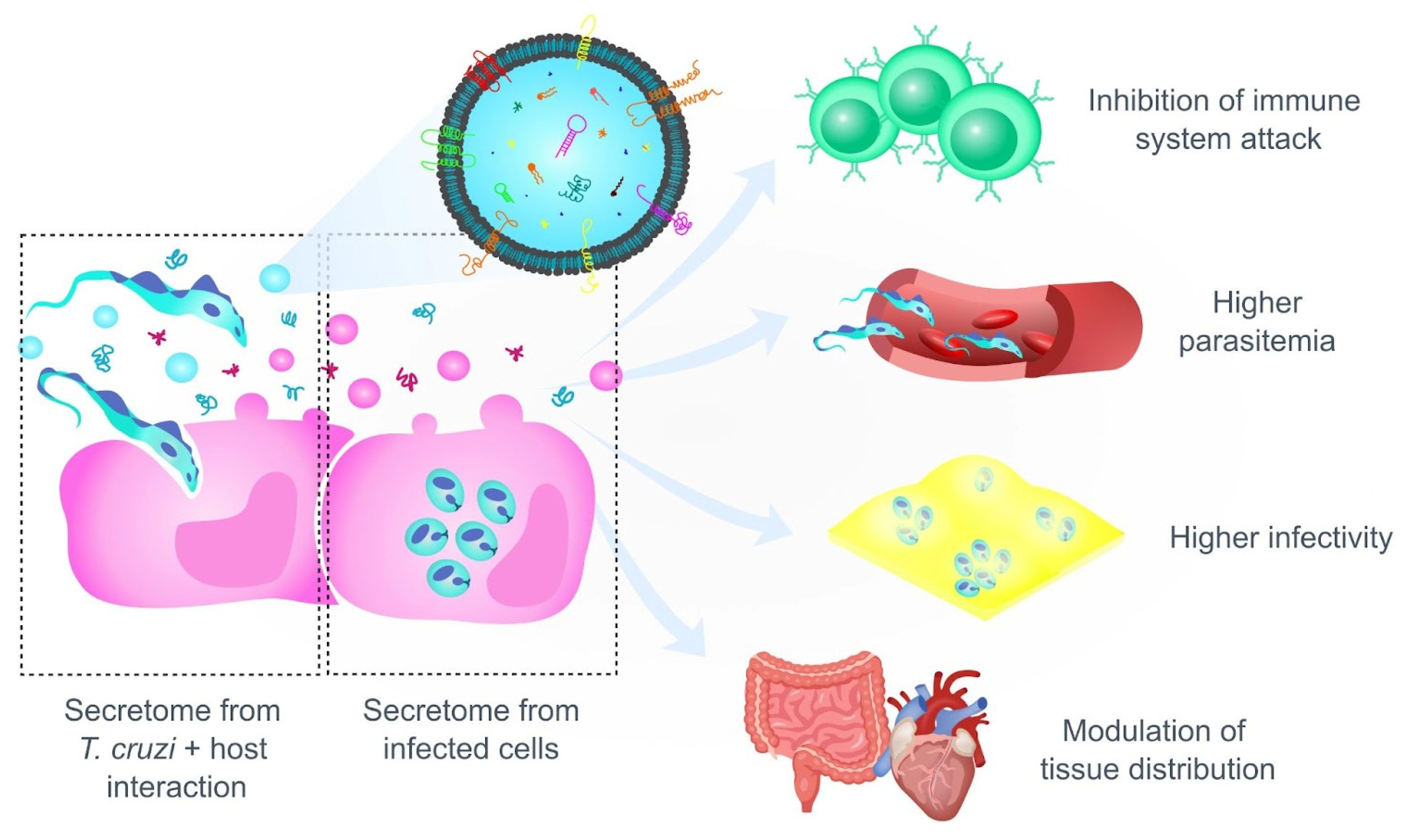
4.1. Trypanosoma cruzi Persistence Is Crucial for the Development of the Pathology
4.2. Preference or Restriction to a Certain Host Tissue? Factors That Determine the Tropism of T. cruzi
5. Perspectives on CD: An Old and Neglected Health Problem
5.1. Migratory Flows to the United States and Europe from Latin American People Have Increased T. cruzi Infection
5.2. Chagas Disease Is Still a Current Health Problem
5.3. Between Neglect and Silence: Key Points for Further Research on CD
- Genetic background of the parasite:
- Complex life cycle:
- Silent path between acute and chronic phase of CD:
- The ineffective treatment for the disease lies in the complexity of the parasite and the lack of public support:
- A neglected disease that reappears, changing the epidemiology and the scenario:
- Higher support and investment to improve public health and research on CD:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral Transmission of Chagas Disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- López-García, A.; Gilabert, J.A. Oral Transmission of Chagas Disease from a One Health Approach: A Systematic Review. Trop. Med. Int. Health 2023, 28, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Silva-dos-Santos, D.; Barreto-de-Albuquerque, J.; Guerra, B.; Moreira, O.C.; Berbert, L.R.; Ramos, M.T.; Mascarenhas, B.A.S.; Britto, C.; Morrot, A.; Serra Villa-Verde, D.M.; et al. Unraveling Chagas Disease Transmission through the Oral Route: Gateways to Trypanosoma cruzi Infection and Target Tissues. PLoS Neglected Trop. Dis. 2017, 11, e0005507. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.M. Chagas Disease: Epidemiology and Barriers to Treatment. Am. J. Med. 2020, 133, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. 2021. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 20 January 2024).
- Chagas, C. Nova Tripanozomiaze Humana: Estudos Sobre a Morfolojia E O Ciclo Evolutivo Do Schizotrypanum cruzi N. Gen., N. Sp., Ajente Etiolojico de Nova Entidade Morbida Do Homem. Memórias Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- García-Huertas, P.; Cardona-Castro, N. Advances in the Treatment of Chagas Disease: Promising New Drugs, Plants and Targets. Biomed. Pharmacother. 2021, 142, 112020. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob. Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Andrade, S.; Briones, M.; Campbell, D.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.; Machado, C.; et al. A New Consensus for Trypanosoma cruzi Intraspecific Nomenclature: Second Revision Meeting Recommends TcI to TcVI. Memórias Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Marcili, A.; Lima, L.; Cavazzana, M.; Junqueira, A.C.V.; Veludo, H.H.; Silva, F.M.D.; Campaner, M.; Paiva, F.; Nunes, V.L.B.; Teixeira, M.M.G. A New Genotype of Trypanosoma cruzi Associated with Bats Evidenced by Phylogenetic Analyses Using SSU RDNA, Cytochrome B and Histone H2B Genes and Genotyping Based on ITS1 RDNA. Parasitology 2009, 136, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Barnabé, C.; Mobarec, H.I.; Jurado, M.R.; Cortez, J.A.; Brenière, S.F. Reconsideration of the Seven Discrete Typing Units within the Species Trypanosoma cruzi, a New Proposal of Three Reliable Mitochondrial Clades. Infect. Genet. Evol. 2016, 39, 176–186. [Google Scholar] [CrossRef]
- Berná, L.; Pita, S.; Chiribao, M.L.; Parodi-Talice, A.; Alvarez-Valin, F.; Robello, C. Biology of the Trypanosoma cruzi Genome. In Biology of Trypanosoma cruzi; IntechOpen Ebooks; BoD–Books on Demand: Schleswig-Holstein, Germany, 2019. [Google Scholar] [CrossRef]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Gironès, N.; Fresno, M. Trypanosoma cruzi Genome: Organization, Multi-Gene Families, Transcription, and Biological Implications. Genes 2020, 11, 1196. [Google Scholar] [CrossRef]
- Reis-Cunha, J.L.; Pimenta Carvalho, S.A.; Viana Almeida, L.; Coqueiro-dos-Santos, A.; de Almeida Marques, C.; Black, J.; Damasceno, J.; McCulloch, R.; Castanheira Bartholomeu, D.; Charlton Jeffares, D. Aneuploidies Are an Ancestral Feature of Trypanosomatids, and an Ancient Chromosome Duplication Is Maintained in Extant Species. bioRxiv 2023. [Google Scholar] [CrossRef]
- Valeeva, L.R.; Abdulkina, L.R.; Agabekian, I.A.; Shakirov, E. Telomere Biology and Ribosome Biogenesis: Structural and Functional Interconnections. Biochem. Cell Biol. 2023, 101, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Berná, L.; Rodriguez, M.; Chiribao, M.L.; Parodi-Talice, A.; Pita, S.; Rijo, G.; Alvarez-Valin, F.; Robello, C. Expanding an Expanded Genome: Long-Read Sequencing of Trypanosoma cruzi. Microb. Genom. 2018, 4, e000177. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Viraqué, F.; Chiribao, M.L.; Libisch, M.G.; Robello, C. Genome-Wide Chromatin Interaction Map for Trypanosoma cruzi. Nat. Microbiol. 2023, 8, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Clayton, C. Regulation of Gene Expression in Trypanosomatids: Living with Polycistronic Transcription. Open Biol. 2019, 9, 190072. [Google Scholar] [CrossRef] [PubMed]
- Palenchar, J.B.; Bellofatto, V. Gene Transcription in Trypanosomes. Mol. Biochem. Parasitol. 2006, 146, 135–141. [Google Scholar] [CrossRef]
- Araújo, P.R.; Teixeira, S.M. Regulatory Elements Involved in the Post-Transcriptional Control of Stage-Specific Gene Expression in Trypanosoma cruzi: A Review. Memórias Inst. Oswaldo Cruz 2011, 106, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Cross, G.A.M. Effects of 3′ Untranslated and Intergenic Regions on Gene Expression in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1995, 75, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, B.A.A.; Holetz, F.B.; Alves, L.R.; Goldenberg, S. RNA Binding Proteins and Gene Expression Regulation in Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Ozgur, S.; Stoecklin, G. On Track with P-Bodies. Biochem. Soc. Trans. 2010, 38, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lantos, A.B.; Carlevaro, G.; Araoz, B.; Ruiz Diaz, P.; Camara, M.d.l.M.; Buscaglia, C.A.; Bossi, M.; Yu, H.; Chen, X.; Bertozzi, C.R.; et al. Sialic Acid Glycobiology Unveils Trypanosoma cruzi Trypomastigote Membrane Physiology. PLoS Pathog. 2016, 12, e1005559. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.-N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.M.; dos Santos, S.L.; Rodrigues-Luiz, G.F.; Mendes, T.A.O.; Rodrigues, T.S.; Gazzinelli, R.T.; Teixeira, S.M.R.; Fujiwara, R.T.; Bartholomeu, D.C. Genomic Analyses, Gene Expression and Antigenic Profile of the Trans-Sialidase Superfamily of Trypanosoma cruzi Reveal an Undetected Level of Complexity. PLoS ONE 2011, 6, e25914. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Hernández, F.; Rastrojo, A.; Poveda, C.; Gironès, N.; Fresno, M. Genomic Assemblies of Newly Sequenced Trypanosoma cruzi Strains Reveal New Genomic Expansion and Greater Complexity. Sci. Rep. 2018, 8, 14631. [Google Scholar] [CrossRef] [PubMed]
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a Bug and a Hard Place: Trypanosoma cruzi Genetic Diversity and the Clinical Outcomes of Chagas Disease. Expert Rev. Anti-Infect. Ther. 2015, 13, 995–1029. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.R.; Giordano, R.J.; Barbu, E.M.; Torrecilhas, A.C.; Kobayashi, G.S.; Langley, R.R.; Arap, W.; Pasqualini, R.; Colli, W.; Alves, M.J.M. Role of the Gp85/Trans-Sialidases in Trypanosoma cruzi Tissue Tropism: Preferential Binding of a Conserved Peptide Motif to the Vasculature In Vivo. PLoS Neglected Trop. Dis. 2010, 4, e864. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.P.; Souza Onofre, T.; Couto Barbosa, B.; Ramalho Ferreira, É.; Bonfim-Melo, A.; Yoshida, N. Host Cell Protein LAMP-2 Is the Receptor for Trypanosoma cruzi Surface Molecule Gp82 That Mediates Invasion. Cell. Microbiol. 2019, 21, e13003. [Google Scholar] [CrossRef] [PubMed]
- Staquicini, D.I.; Martins, R.M.; Macedo, S.; Sasso, G.R.S.; Atayde, V.D.; Juliano, M.A.; Yoshida, N. Role of GP82 in the Selective Binding to Gastric Mucin during Oral Infection with Trypanosoma cruzi. PLoS Neglected Trop. Dis. 2010, 4, e613. [Google Scholar] [CrossRef]
- Schenkman, S.; Ferguson, M.A.J.; Heise, N.; Cardoso de Almeida, M.L.; Mortara, R.A.; Yoshida, N. Mucin-like Glycoproteins Linked to the Membrane by Glycosylphosphatidylinositol Anchor Are the Major Acceptors of Sialic Acid in a Reaction Catalyzed by Trans-Sialidase in Metacyclic Forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993, 59, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, C.A.; Campo, V.A.; Frasch, A.C.C.; Di Noia, J.M. Trypanosoma cruzi Surface Mucins: Host-Dependent Coat Diversity. Nat. Rev. Microbiol. 2006, 4, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.C.; Gazzinelli, R.T. Proinflammatory Activity of Glycosylphosphatidylinositol Anchors Derived from Trypanosoma cruzi: Structural and Functional Analyses. J. Leukoc. Biol. 2001, 70, 467–477. [Google Scholar] [CrossRef]
- Di Noia, J.M.; D’Orso, I.; Sánchez, D.; Frasch, A.C.C. AU-Rich Elements in the 3′-Untranslated Region of a New Mucin-Type Gene Family of Trypanosoma cruzi Confers MRNA Instability and Modulates Translation Efficiency. J. Biol. Chem. 2000, 275, 10218–10227. [Google Scholar] [CrossRef]
- Yoshida, N. Molecular Basis of Mammalian Cell Invasion by Trypanosoma cruzi. An. Acad. Bras. Ciências 2006, 78, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.d.L.M.; Balouz, V.; Cameán, C.C.; Cori, C.R.; Kashiwagi, G.A.; Gil, S.A.; Macchiaverna, N.P.; Cardinal, M.V.; Guaimas, F.; Lobo, M.M.; et al. Trypanosoma cruzi Surface Mucins Are Involved in the Attachment to the Triatoma infestans Rectal Ampoule. PLoS Neglected Trop. Dis. 2019, 13, e0007418. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeu, D.C.; Cerqueira, G.C.; Leão, A.C.A.; Darocha, W.D.; Pais, F.S.; Macedo, C.; Djikeng, A.; Teixeira, S.M.R.; El-Sayed, N.M. Genomic Organization and Expression Profile of the Mucin-Associated Surface Protein (Masp) Family of the Human Pathogen Trypanosoma cruzi. Nucleic Acids Res. 2009, 37, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- De Pablos, L.M.; González, G.G.; Parada, J.S.; Hidalgo, V.S.; Lozano, I.M.D.; Samblás, M.M.G.; Bustos, T.C.; Osuna, A. Differential Expression and Characterization of a Member of the Mucin-Associated Surface Protein Family Secreted by Trypanosoma cruzi. Infect. Immun. 2011, 79, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, B.; Martínez, I.; Martínez-Velasco, M.L.; Rodríguez-Sosa, M.; González-Canto, A.; Vázquez-Mendoza, A.; Terrazas, L.I. Role of a 49 KDa Trypanosoma cruzi Mucin-Associated Surface Protein (MASP49) during the Infection Process and Identification of a Mammalian Cell Surface Receptor. Pathogens 2023, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- De Pablos, L.M.; Osuna, A. Conserved Regions as Markers of Different Patterns of Expression and Distribution of the Mucin-Associated Surface Proteins of Trypanosoma cruzi. Infect. Immun. 2012, 80, 169–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ermert, D.; Ram, S.; Laabei, M. The Hijackers Guide to Escaping Complement: Lessons Learned from Pathogens. Mol. Immunol. 2019, 114, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Ramirez, M.I. Inefficient Complement System Clearance of Trypanosoma cruzi Metacyclic Trypomastigotes Enables Resistant Strains to Invade Eukaryotic Cells. PLoS ONE 2010, 5, e9721. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.P.; Valck, C.; Sánchez, G.; Gingras, A.R.; Tzima, S.; Molina, M.; Sim, R.B.; Schwaeble, W.J.; Ferreira, A. The Classical Activation Pathway of the Human Complement System Is Specifically Inhibited by Calreticulin from Trypanosoma cruzi. J. Immunol. 2004, 172, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.A.; Bradt, B.; Cooper, N.R.; So, M.M. Characterization of a Trypanosoma cruzi C3 Binding Protein with Functional and Genetic Similarities to the Human Complement Regulatory Protein, Decay-Accelerating Factor. J. Immunol. 1991, 147, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.d.S.; Evans-Osses, I.; Freitas, J.C.; Inal, J.M.; Ramirez, M.I. Complement C2 Receptor Inhibitor Trispanning Confers an Increased Ability to Resist Complement-Mediated Lysis in Trypanosoma cruzi. J. Infect. Dis. 2008, 198, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Ouaissi, M.A.; Velge, P.; Cornette, J.; Kazatchkine, M.D. Gp 58/68, a Parasite Component That Contributes to the Escape of the Trypomastigote Form of T. cruzi from Damage by the Human Alternative Complement Pathway. Immunology 1988, 65, 299–303. [Google Scholar] [PubMed]
- Tambourgi, D.V.; Kipnis, T.L.; da Silva, W.D.; Joiner, K.A.; Sher, A.; Heath, S.; Hall, B.F.; Ogden, G.B. A Partial CDNA Clone of Trypomastigote Decay-Accelerating Factor (T-DAF), a Developmentally Regulated Complement Inhibitor of Trypanosoma cruzi, Has Genetic and Functional Similarities to the Human Complement Inhibitor DAF. Infect. Immun. 1993, 61, 3656–3663. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Toloza, G.; Aguilar-Guzmán, L.; Valck, C.; Menon, S.S.; Ferreira, V.P.; Ferreira, A. Is It Possible to Intervene in the Capacity of Trypanosoma cruzi to Elicit and Evade the Complement System? Front. Immunol. 2021, 12, 789145. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Ansa-Addo, E.; Deolindo, P.; Inal, J.M.; Ramirez, M.I. Trypanosoma cruzi Immune Evasion Mediated by Host Cell-Derived Microvesicles. J. Immunol. 2012, 188, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, M.P.; Ramirez, M.I. Microvesicles Released during the Interaction between Trypanosoma cruzi TcI and TcII Strains and Host Blood Cells Inhibit Complement System and Increase the Infectivity of Metacyclic Forms of Host Cells in a Strain-Independent Process. Pathog. Dis. 2017, 75, ftx077. [Google Scholar] [CrossRef] [PubMed]
- Rossi, I.V.; Nunes, M.A.F.; Sabatke, B.; Ribas, H.T.; Winnischofer, S.M.B.; Ramos, A.S.P.; Inal, J.M.; Ramirez, M.I. An Induced Population of Trypanosoma cruzi Epimastigotes More Resistant to Complement Lysis Promotes a Phenotype with Greater Differentiation, Invasiveness, and Release of Extracellular Vesicles. Front. Cell. Infect. Microbiol. 2022, 12, 1046681. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.M.D.; De Pablos, L.M.; Longhi, S.A.; Zago, M.P.; Schijman, A.G.; Osuna, A. Immune Complexes in Chronic Chagas Disease Patients Are Formed by Exovesicles from Trypanosoma cruzi Carrying the Conserved MASP N-Terminal Region. Sci. Rep. 2017, 7, 44451. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Medei, E.; Bozza, M.T. ROS and Trypanosoma cruzi: Fuel to Infection, Poison to the Heart. PLoS Pathog. 2018, 14, e1006928. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, L.; Peluffo, G.; Alvarez, M.N.; Martínez, A.; Radi, R. Trypanosoma cruzi Antioxidant Enzymes as Virulence Factors in Chagas Disease. Antioxid. Redox Signal. 2013, 19, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Tanowitz, H.B.; Garg, N.J. Pathogenesis of Chronic Chagas Disease: Macrophages, Mitochondria, and Oxidative Stress. Curr. Clin. Microbiol. Rep. 2018, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, K.; Paulino, M.; Salas, C.O.; Zarate-Ramos, J.J.; Vera, B.; Rivera, G. Trypanothione Reductase: A Target for the Development of Anti-Trypanosoma cruzi Drugs. Mini Rev. Med. Chem. 2017, 17, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, L.; Irigoín, F.; Alvarez, M.N.; Peluffo, G.; Taylor, M.C.; Kelly, J.M.; Wilkinson, S.R.; Radi, R. Mitochondrial Superoxide Radicals Mediate Programmed Cell Death in Trypanosoma cruzi: Cytoprotective Action of Mitochondrial Iron Superoxide Dismutase Overexpression. Biochem. J. 2007, 403, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.N.; Peluffo, G.; Piacenza, L.; Radi, R. Intraphagosomal Peroxynitrite as a Macrophage-Derived Cytotoxin against Internalized Trypanosoma cruzi. J. Biol. Chem. 2011, 286, 6627–6640. [Google Scholar] [CrossRef]
- Zago, M.P.; Hosakote, Y.M.; Koo, S.-J.; Dhiman, M.; Piñeyro, M.D.; Parodi-Talice, A.; Basombrio, M.A.; Robello, C.; Garg, N.J. TcI Isolates of Trypanosoma cruzi Exploit the Antioxidant Network for Enhanced Intracellular Survival in Macrophages and Virulence in Mice. Infect. Immun. 2016, 84, 1842–1856. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Fresno, M.; Gironès, N. Comparative Proteomic Analysis of Trypomastigotes from Trypanosoma cruzi Strains with Different Pathogenicity. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 76, 104041. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Prolo, C.; Estrada, D.; Rios, N.; Alvarez, M.N.; Piñeyro, M.D.; Robello, C.; Radi, R.; Piacenza, L. Cytosolic Fe-Superoxide Dismutase Safeguards Trypanosoma cruzi from Macrophage-Derived Superoxide Radical. Proc. Natl. Acad. Sci. USA 2019, 116, 8879–8888. [Google Scholar] [CrossRef] [PubMed]
- Mesías, A.C.; Sasoni, N.; Arias, D.G.; Brandán, C.P.; Orban, O.C.; Kunick, C.; Robello, C.; Comini, M.A.; Garg, N.J.; Zago, M.P. Trypanothione Synthetase Confers Growth, Survival Advantage and Resistance to Anti-Protozoal Drugs in Trypanosoma cruzi. Free Radic. Biol. Med. 2019, 130, 23–34. [Google Scholar] [CrossRef]
- Van Overtvelt, L.; Vanderheyde, N.; Verhasselt, V.; Ismaili, J.; De Vos, L.; Goldman, M.; Willems, F.; Vray, B. Trypanosoma cruzi Infects Human Dendritic Cells and Prevents Their Maturation: Inhibition of Cytokines, HLA-DR, and Costimulatory Molecules. Infect. Immun. 1999, 67, 4033–4040. [Google Scholar] [CrossRef] [PubMed]
- Poncini, C.V.; Soto, C.D.A.; Batalla, E.; Solana, M.E.; Cappa, S.M.G. Trypanosoma cruzi Induces Regulatory Dendritic Cells In Vitro. Infect. Immun. 2008, 76, 2633–2641. [Google Scholar] [CrossRef]
- Soto, C.D.A.; Mirkin, G.A.; Solana, M.E.; Cappa, S.M.G. Trypanosoma cruzi Infection Modulates in Vivo Expression of Major Histocompatibility Complex Class II Molecules on Antigen-Presenting Cells and T-Cell Stimulatory Activity of Dendritic Cells in a Strain-Dependent Manner. Infect. Immun. 2003, 71, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.D.A.; Solana, M.E.; Poncini, C.V.; Pino-Martinez, A.M.; Tekiel, V.; González-Cappa, S.M. Dendritic Cells Devoid of IL-10 Induce Protective Immunity against the Protozoan Parasite Trypanosoma cruzi. Vaccine 2010, 28, 7407–7413. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.C.; Ward, A.; Olmo, F.; Jayawardhana, S.; Francisco, A.F.; Lewis, M.D.; Kelly, J.M. Intracellular DNA Replication and Differentiation of Trypanosoma cruzi Is Asynchronous within Individual Host Cells In Vivo at All Stages of Infection. PLoS Neglected Trop. Dis. 2020, 14, e0008007. [Google Scholar] [CrossRef] [PubMed]
- Pinge-Filho, P. Can Extracellular Vesicles Produced during Infection by Trypanosoma cruzi Function as Damage-Associated Molecular Patterns in the Host? Med. Hypotheses 2021, 155, 110667. [Google Scholar] [CrossRef] [PubMed]
- Cerbán, F.M.; Stempin, C.C.; Volpini, X.; Carrera Silva, E.A.; Gea, S.; Motran, C.C. Signaling Pathways That Regulate Trypanosoma cruzi Infection and Immune Response. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165707. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L.; Grusby, M.J.; Postan, M.; Glimcher, L.H. Trypanosoma cruzi Infection in MHC-Deficient Mice: Further Evidence for the Role of Both Class I- and Class II-Restricted T Cells in Immune Resistance and Disease. Int. Immunol. 1996, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, M.E.; Bakhiet, M.; Olsson, T.; Kristensson, K.; Mak, T.; Wigzell, H.; Orn, A. Differential Susceptibilities of Mice Genomically Deleted of CD4 and CD8 to Infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 1993, 61, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Castelo-Branco, M.T.L.; Lannes-Vieira, J.; Gattass, C.R. Trypanosoma cruzi: Protective Response of Vaccinated Mice Is Mediated by CD8+ Cells, Prevents Signs of Polyclonal T Lymphocyte Activation, and Allows Restoration of a Resting Immune State after Challenge. Exp. Parasitol. 1999, 91, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.V.A.; Araujo Furlan, C.L.; Fiocca Vernengo, F.; Montes, C.L.; Gruppi, A. Understanding CD8+ T Cell Immunity to Trypanosoma cruzi and How to Improve It. Trends Parasitol. 2019, 35, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Piccin, M.P.; Guillermo, L.V.C.; Vellozo, N.S.; Filardy, A.A.; Pereira-Marques, S.T.; Rigoni, T.S.; Pereira-Manfro, W.F.; DosReis, G.A.; Lopes, M.F. Apoptotic CD8 T-Lymphocytes Disable Macrophage-Mediated Immunity to Trypanosoma cruzi Infection. Cell Death Dis. 2016, 7, e2232. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.L.; Weatherly, D.B.; Laucella, S.A.; Cabinian, M.A.; Crim, M.T.; Sullivan, S.; Heiges, M.; Craven, S.H.; Rosenberg, C.S.; Collins, M.H.; et al. CD8+ T-Cell Responses to Trypanosoma cruzi Are Highly Focused on Strain-Variant Trans-Sialidase Epitopes. PLoS Pathog. 2006, 2, e77. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.M.; Bustamante, J.M.; Tarleton, R.L. CD8+ T Cells in Trypanosoma cruzi Infection. Curr. Opin. Immunol. 2009, 21, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tzelepis, F.; de Alencar, B.C.G.; Penido, M.L.O.; Claser, C.; Machado, A.V.; Bruna-Romero, O.; Gazzinelli, R.T.; Rodrigues, M.M. Infection with Trypanosoma cruzi Restricts the Repertoire of Parasite-Specific CD8+ T Cells Leading to Immunodominance. J. Immunol. 2008, 180, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Carter, D.; Reed, S.G. Immunological Dominance of Trypanosoma cruzi Tandem Repeat Proteins. Infect. Immun. 2008, 76, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- Cazzulo, J.J.; Frasch, A.C. SAPA/Trans-Sialidase and Cruzipain: Two Antigens from Trypanosoma cruzi Contain Immunodominant but Enzymatically Inactive Domains. FASEB J. 1992, 6, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, C.S.; Martin, D.L.; Tarleton, R.L. CD8+ T Cells Specific for Immunodominant Trans-Sialidase Epitopes Contribute to Control of Trypanosoma cruzi Infection but Are Not Required for Resistance. J. Immunol. 2010, 185, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Pitcovsky, T.A.; Buscaglia, C.A.; Mucci, J.; Campetella, O. A Functional Network of Intramolecular Cross-Reacting Epitopes Delays the Elicitation of Neutralizing Antibodies to Trypanosoma cruzi Trans-Sialidase. J. Infect. Dis. 2002, 186, 397–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bermejo, D.A.; Vesely, M.C.A.; Khan, M.; Rodríguez, E.V.A.; Montes, C.L.; Merino, M.C.; Toellner, K.M.; Mohr, E.; Taylor, D.; Cunningham, A.F.; et al. Trypanosoma cruzi Infection Induces a Massive Extrafollicular and Follicular Splenic B-Cell Response Which Is a High Source of Non-Parasite-Specific Antibodies. Immunology 2010, 132, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L. CD8+ T Cells in Trypanosoma cruzi Infection. Semin. Immunopathol. 2015, 37, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Minoprio, P.; Burlen, O.; Pereira, P.; Guilbert, B.; Andrade, L.; Hontebeyrie-Joskowicz, M.; Coutinho, A. Most B Cells in Acute Trypanosoma cruzi Infection Lack Parasite Specificity. Scand. J. Immunol. 1988, 28, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.A.; Guyach, S.E.; Norris, K.A. Specific Humoral Immunity versus Polyclonal B Cell Activation in Trypanosoma cruzi Infection of Susceptible and Resistant Mice. PLoS Neglected Trop. Dis. 2010, 4, e733. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.L.; Zuñiga, E.I.; Vazquez, J.; Arce, C.; Gruppi, A. Trypanosoma cruzi Mitochondrial Malate Dehydrogenase Triggers Polyclonal B-Cell Activation. Clin. Exp. Immunol. 2002, 127, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C. The Case for the Development of a Chagas Disease Vaccine: Why? How? When? Trop. Med. Infect. Dis. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Beaumier, C.M.; Gillespie, P.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of Vaccine Research and Development of Vaccines for Leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, L.E.; Marcus, R.; Novick, G.; Sosa-Estani, S.; Ralston, K.; Zaidel, E.J.; Forsyth, C.; Ribeiro, A.L.P.; Mendoza, I.; Falconi, M.L.; et al. WHF IASC Roadmap on Chagas Disease. Glob. Heart 2020, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Cançado, J.R. Long Term Evaluation of Etiological Treatment of Chagas Disease with Benznidazole. Rev. Inst. Med. Trop. São Paulo 2002, 44, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Costa, E.A.P.; Victória, C.; Fortaleza, C.M.C.B. Predictors of Development of Cardiac and Digestive Disorders among Patients with Indeterminate Chronic Chagas Disease. PLoS Neglected Trop. Dis. 2021, 15, e0009680. [Google Scholar] [CrossRef] [PubMed]
- Linhares-Lacerda, L.; Granato, A.; Gomes-Neto, J.F.; Conde, L.; Freire-De-Lima, L.; de Freitas, E.O.; Freire-De-Lima, C.G.; Barroso, S.P.C.; Guerra, R.J.d.A.; Pedrosa, R.C.; et al. Circulating Plasma MicroRNA-208a as Potential Biomarker of Chronic Indeterminate Phase of Chagas Disease. Front. Microbiol. 2018, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2019, 46, 100507. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Fortes Francisco, A.; Taylor, M.C.; Burrell-Saward, H.; McLatchie, A.P.; Miles, M.A.; Kelly, J.M. Bioluminescence Imaging of Chronic Trypanosoma cruzi Infections Reveals Tissue-Specific Parasite Dynamics and Heart Disease in the Absence of Locally Persistent Infection. Cell. Microbiol. 2014, 16, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Laugier, L.; Frade, A.F.; Ferreira, F.M.; Baron, M.A.; Teixeira, P.C.; Cabantous, S.; Ferreira, L.R.P.; Louis, L.; Rigaud, V.O.C.; Gaiotto, F.A.; et al. Whole-Genome Cardiac DNA Methylation Fingerprint and Gene Expression Analysis Provide New Insights in the Pathogenesis of Chronic Chagas Disease Cardiomyopathy. Clin. Infect. Dis. 2017, 65, 1103–1111. [Google Scholar] [CrossRef]
- Arantes, R.M.; Marche, H.H.; Bahia, M.T.; Cunha, F.Q.; Rossi, M.A.; Silva, J.S. Interferon-γ-Induced Nitric Oxide Causes Intrinsic Intestinal Denervation in Trypanosoma cruzi-Infected Mice. Am. J. Pathol. 2004, 164, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, A.B.M.; Arantes, R.M.E.; Vago, A.R.; Lemos, E.M.; Adad, S.J.; Correa-Oliveira, R.; Reis, D.D. Comparative Study of the Presence of Trypanosoma cruzi KDNA, Inflammation and Denervation in Chagasic Patients with and without Megaesophagus. Parasitology 2005, 131, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.F.; Cangussú, S.D.; Duz, A.L.C.; Cartelle, C.T.; de Lourdes Noviello, M.; Veloso, V.M.; Bahia, M.T.; Almeida-Leite, C.M.; Arantes, R.M.E. Enteric Neuronal Damage, Intramuscular Denervation and Smooth Muscle Phenotype Changes as Mechanisms of Chagasic Megacolon: Evidence from a Long-Term Murine Model of Tripanosoma cruzi Infection. PLoS ONE 2016, 11, e0153038. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.D.; Lisboa, A.d.S.; Fujiwara, R.T.; de Freitas, M.A.R.; Adad, S.J.; Oliveira, R.C.; Reis, D.D.; da Silveira, A.B.M. Characterization of Enteroglial Cells and Denervation Process in Chagasic Patients with and without Megaesophagus. Hum. Pathol. 2010, 41, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.M.; Sant’Ana, D.M.G.; Araújo, E.J.A.; Toledo, M.J.O.; Gomes, M.L.; de Araújo, S.M. Neuronal Changes Caused by Trypanosoma cruzi: An Experimental Model. An. Acad. Bras. Cienc. 2011, 83, 545–555. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.-I.; Tripathi, A.; Vargas, F.; Knight, R.; Dorrestein, P.C.; Siqueira-Neto, J.L. Experimental Chagas Disease-Induced Perturbations of the Fecal Microbiome and Metabolome. PLoS Neglected Trop. Dis. 2018, 12, e0006344. [Google Scholar] [CrossRef] [PubMed]
- Hossain, E.; Khanam, S.; Dean, D.A.; Wu, C.; Lostracco-Johnson, S.; Thomas, D.; Kane, S.S.; Parab, A.R.; Flores, K.; Katemauswa, M.; et al. Mapping of Host-Parasite-Microbiome Interactions Reveals Metabolic Determinants of Tropism and Tolerance in Chagas Disease. Sci. Adv. 2020, 6, eaaz2015. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhis, W.C.; Eisen, H. Fl-160. A Surface Antigen of Trypanosoma cruzi That Mimics Mammalian Nervous Tissue. J. Exp. Med. 1989, 169, 641–652. [Google Scholar] [CrossRef]
- Cunha-Neto, E.; Coelho, V.; Guilherme, L.; Fiorelli, A.; Stolf, N.; Kalil, J. Autoimmunity in Chagas’ Disease. Identification of Cardiac Myosin-B13 Trypanosoma cruzi Protein Crossreactive T Cell Clones in Heart Lesions of a Chronic Chagas’ Cardiomyopathy Patient. J. Clin. Investig. 1996, 98, 1709–1712. [Google Scholar] [CrossRef]
- Lewis, M.D.; Kelly, J.M. Putting Infection Dynamics at the Heart of Chagas Disease. Trends Parasitol. 2016, 32, 899–911. [Google Scholar] [CrossRef]
- Cruz, J.S.; Roman-Campos, D.; Monti-Rocha, R.; Machado, F.S.; Santos-Miranda, A.; Sales-Junior, P.A.; Campos, P.P. Altered Cardiomyocyte Function and Trypanosoma cruzi Persistence in Chagas Disease. Am. J. Trop. Med. Hyg. 2016, 94, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Vago, A.R.; Andrade, L.O.; Leite, A.A.; Reis, D.D.; Macedo, A.M.; Adad, S.J.; Tostes, S.; Moreira, M.d.C.V.; Filho, G.B.; Pena, S.D. Genetic Characterization of Trypanosoma cruzi Directly from Tissues of Patients with Chronic Chagas Disease. Am. J. Pathol. 2000, 156, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Brener, Z. Tissue Tropism of Different Trypanosoma cruzi Strains. J. Parasitol. 1978, 64, 475. [Google Scholar] [CrossRef] [PubMed]
- Vera-Cruz, J.M.; Magallón-Gastelum, E.; Grijalva, G.; Rincón, A.R.; Ramos-García, C.; Armendáriz-Borunda, J. Molecular Diagnosis of Chagas’ Disease and Use of an Animal Model to Study Parasite Tropism. Parasitol. Res. 2003, 89, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Machado, S.; Chiari, E.; Pena, S.D.J.; Macedo, A.M. Differential Tissue Distribution of Diverse Clones of Trypanosoma cruzi in Infected Mice. Mol. Biochem. Parasitol. 1999, 100, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.O.; Galvao, C.; Meirelles, M.d.N.S.L.; Chiari, E.; Pena, S.D.J.; Macedo, A.M. Differential Tissue Tropism of Trypanosoma cruzi Strains: An in Vitro Study. Mem. Inst. Oswaldo Cruz 2010, 105, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.J.; Vago, A.R.; Chiari, E.; Meira, F.C.A.; Galvão, L.M.C.; Machado, C.R.S. Trypanosoma cruzi: Mixture of Two Populations Can Modify Virulence and Tissue Tropism in Rat. Exp. Parasitol. 2003, 104, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Machado, S.; Chiari, E.; Pena, S.D.J.; Macedo, A.M. Trypanosoma cruzi: Role of Host Genetic Background in the Differential Tissue Distribution of Parasite Clonal Populations. Exp. Parasitol. 2002, 100, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Francisco, A.F.; Taylor, M.S.; Jayawardhana, S.; Kelly, J.M. Host and Parasite Genetics Shape a Link between Trypanosoma cruzi Infection Dynamics and Chronic Cardiomyopathy. Cell. Microbiol. 2016, 18, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.I.; Lewis, M.D.; Taylor, M.C.; Kelly, J.M. Incomplete Recruitment of Protective T Cells Is Associated with Trypanosoma cruzi Persistence in the Mouse Colon. Infect. Immun. 2021, 90, e0038221. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, F.; Desruisseaux, M.S.; Machado, F.S.; Upadhya, R.; Zhao, D.; Schwartz, G.J.; Teixeira, M.M.; Albanese, C.; Lisanti, M.P.; Chua, S.C.; et al. Response of Adipose Tissue to Early Infection with Trypanosoma cruzi (Brazil Strain). J. Infect. Dis. 2012, 205, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Nagajyothi; Mukherjee, S.; de Almeida, C.J.G.; Jelicks, L.A.; Schubert, W.; Lin, Y.; Jayabalan, D.S.; Zhao, D.; Braunstein, V.L.; et al. The Adipocyte as an Important Target Cell for Trypanosoma cruzi Infection. J. Biol. Chem. 2005, 280, 24085–24094. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.M.; Segatto, M.; Menezes, Z.; Macedo, A.M.; Gelape, C.; Andrade, L.d.O.; Nagajyothi, F.; Scherer, P.E.; Teixeira, M.M.; Tanowitz, H.B. Evidence for Trypanosoma cruzi in Adipose Tissue in Human Chronic Chagas Disease. Microbes Infect. 2011, 13, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- González, F.B.; Villar, S.R.; Toneatto, J.; Pacini, M.F.; Márquez, J.; D’attilio, L.; Bottasso, O.A.; Piwien-Pilipuk, G.; Pérez, A.R. Immune Response Triggered by Trypanosoma cruzi Infection Strikes Adipose Tissue Homeostasis Altering Lipid Storage, Enzyme Profile and Adipokine Expression. Med. Microbiol. Immunol. 2018, 208, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.I.; Lewis, M.D.; Khan, A.A.; McCann, C.J.; Francisco, A.F.; Jayawardhana, S.; Taylor, M.C.; Kelly, J.M. In Vivo Analysis of Trypanosoma cruzi Persistence Foci at Single-Cell Resolution. mBio 2020, 11, e01242-20. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.C.; Kelly, J.M. Iron Metabolism in Trypanosomatids, and Its Crucial Role in Infection. Parasitology 2010, 137, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Cortez, M.; Flannery, A.R.; Tam, C.; Mortara, R.A.; Andrews, N.W. Trypanosoma cruzi Subverts the Sphingomyelinase-Mediated Plasma Membrane Repair Pathway for Cell Invasion. J. Exp. Med. 2011, 208, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; da Silveira, J.F.; Almeida, I.C. Proteomic Analysis of Trypanosoma cruzi Secretome: Characterization of Two Populations of Extracellular Vesicles and Soluble Proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, E.; Yashunsky, D.V.; Nohara, L.L.; Torrecilhas, A.C.; Nikolaev, A.V.; Almeida, I.C. GPIomics: Global Analysis of Glycosylphosphatidylinositol-Anchored Molecules of Trypanosoma cruzi. Mol. Syst. Biol. 2009, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Chaturvedi, A. Mining SNPs in Extracellular Vesicular Transcriptome of Trypanosoma cruzi: A Step Closer to Early Diagnosis of Neglected Chagas Disease. PeerJ 2016, 4, e2693. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, K.S.; Vasconcellos, C.I.; Soares, R.P.; Mendes, M.T.; Ellis, C.C.; Aguilera-Flores, M.; de Almeida, I.C.; Schenkman, S.; Iwai, L.K.; Torrecilhas, A.C. Proteomic Analysis Reveals Different Composition of Extracellular Vesicles Released by Two Trypanosoma cruzi Strains Associated with Their Distinct Interaction with Host Cells. J. Extracell. Vesicles 2018, 7, 1463779. [Google Scholar] [CrossRef] [PubMed]
- Prescilla-Ledezma, A.; Linares, F.; Ortega-Muñoz, M.; Moreira, L.R.; Jódar-Reyes, A.B.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F.; Osuna, A. Molecular Recognition of Surface Trans-Sialidases in Extracellular Vesicles of the Parasite Trypanosoma cruzi Using Atomic Force Microscopy (AFM). Int. J. Mol. Sci. 2022, 23, 7193. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Silva, M.R.; das Neves, R.F.C.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular Vesicles Shed by Trypanosoma cruzi Are Linked to Small RNA Pathways, Life Cycle Regulation, and Susceptibility to Infection of Mammalian Cells. Parasitol. Res. 2013, 113, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.R.; Serrano, F.R.; Osuna, A. Extracellular Vesicles of Trypanosoma cruzi Tissue-Culture Cell-Derived Trypomastigotes: Induction of Physiological Changes in Non-Parasitized Culture Cells. PLoS Neglected Trop. Dis. 2019, 13, e0007163. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.T.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; e Silva, N.C.; Abrahamsohn, I.d.A.; Colli, W.; Alves, M.J.M. Trypanosoma cruzi: Parasite Shed Vesicles Increase Heart Parasitism and Generate an Intense Inflammatory Response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bautista-López, N.L.; Ndao, M.; Camargo, F.V.; Nara, T.; Annoura, T.; Hardie, D.B.; Borchers, C.H.; Jardim, A. Characterization and Diagnostic Application of Trypanosoma cruzi Trypomastigote Excreted-Secreted Antigens Shed in Extracellular Vesicles Released from Infected Mammalian Cells. J. Clin. Microbiol. 2017, 55, 744–758. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Lima, F.M.; Ruiz, J.C.; Almeida, I.C.; da Silveira, J.F. Characterization of the Small RNA Content of Trypanosoma cruzi Extracellular Vesicles. Mol. Biochem. Parasitol. 2014, 193, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Calero, T.; Garcia-Silva, R.; Pena, A.; Robello, C.; Persson, H.; Rovira, C.; Naya, H.; Cayota, A. Profiling of Small RNA Cargo of Extracellular Vesicles Shed by Trypanosoma cruzi Reveals a Specific Extracellular Signature. Mol. Biochem. Parasitol. 2015, 199, 19–28. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Koo, S.-J.; Gupta, S.; Liang, L.Y.; Bahar, B.; Silla, L.; Nuñez-Burgos, J.; Barrientos, N.; Zago, M.P.; Garg, N.J. Gene Expression Profiling and Functional Characterization of Macrophages in Response to Circulatory Microparticles Produced during Trypanosoma cruzi Infection and Chagas Disease. J. Innate Immun. 2016, 9, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, O.A.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; et al. Vesicles from Different Trypanosoma cruzi Strains Trigger Differential Innate and Chronic Immune Responses. J. Extracell. Vesicles 2015, 4, 28734. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.C.; Ancarola, M.E.; Volpato-Rossi, I.; Marcilla, A.; Ramirez, M.I.; Rosenzvit, M.C.; Cucher, M.; Poncini, C.V. Extracellular Vesicles from Trypanosoma cruzi-Dendritic Cell Interaction Show Modulatory Properties and Confer Resistance to Lethal Infection as a Cell-Free Based Therapy Strategy. Front. Cell. Infect. Microbiol. 2022, 12, 980817. [Google Scholar] [CrossRef] [PubMed]
- Aline, F.; Bout, D.; Amigorena, S.; Roingeard, P.; Dimier-Poisson, I. Toxoplasma gondii Antigen-Pulsed-Dendritic Cell-Derived Exosomes Induce a Protective Immune Response against T. gondii Infection. Infect. Immun. 2004, 72, 4127–4137. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Kim, E.D.; Song, H.; Chai, J.Y.; Seo, K.Y. Immunogenicity of Exosomes from Dendritic Cells Stimulated with Toxoplasma gondii Lysates in Ocularly Immunized Mice. Korean J. Parasitol. 2020, 58, 185–189. [Google Scholar] [CrossRef]
- del Cacho, E.; Gallego, M.; Lee, S.H.; Lillehoj, H.S.; Quilez, J.; Lillehoj, E.P.; Sánchez-Acedo, C. Induction of Protective Immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina Infections Using Dendritic Cell-Derived Exosomes. Infect. Immun. 2012, 80, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.K.; Berzel, S.; Fajardo-Moser, M.; Remer, K.A.; Moll, H. Fragments of Antigen-Loaded Dendritic Cells (DC) and DC-Derived Exosomes Induce Protective Immunity against Leishmania major. Vaccine 2010, 28, 5785–5793. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas Disease (American Trypanosomiasis). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 20 January 2024).
- de Oliveira Junior, W.A.; Gomez i Prat, J.; Albajar-Viñas, P.; Carrazzone, C.; Kropf, S.P.; Dehousse, A.; Camargo, A.M.d.A.; Anselmi, M.; Barba, M.C.P.; Guiu, I.C.; et al. How People Affected by Chagas Disease Have Struggled with Their Negligence: History, Associative Movement and World Chagas Disease Day. Memórias Inst. Oswaldo Cruz 2022, 117, e220066. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Junior, A.N.; de Sousa, A.S. The Continuous Challenge of Chagas Disease Treatment: Bridging Evidence-Based Guidelines, Access to Healthcare, and Human Rights. Rev. Soc. Bras. Med. Trop. 2017, 50, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-De-La-Fuente, A.L.; Yadón, Z.E. A Scientometric Evaluation of the Chagas Disease Implementation Research Programme of the PAHO and TDR. PLoS Neglected Trop. Dis. 2013, 7, e2445. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.; Brum-Soares, L.; Reis, R.; Cubides, J.-C. Chagas Disease: Review of Needs, Neglect, and Obstacles to Treatment Access in Latin America. Rev. Soc. Bras. Med. Trop. 2017, 50, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Abras, A.; Ballart, C.; Fernández-Arévalo, A.; Pinazo, M.-J.; Gascón, J.; Muñoz, C.; Gállego, M. Worldwide Control and Management of Chagas Disease in a New Era of Globalization: A Close Look at Congenital Trypanosoma cruzi Infection. Clin. Microbiol. Rev. 2022, 35, e0015221. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Reguero, L.; Subirà, C.; Blázquez-Pérez, A.; Requena-Méndez, A. Estimating Chagas Disease Prevalence and Number of Underdiagnosed, and Undertreated Individuals in Spain. Travel Med. Infect. Dis. 2022, 47, 102284. [Google Scholar] [CrossRef] [PubMed]
- Colombo, V.; Giacomelli, A.; Casazza, G.; Galimberti, L.; Bonazzetti, C.; Sabaini, F.; Ridolfo, A.L.; Antinori, S. Trypanosoma cruzi Infection in Latin American Pregnant Women Living Outside Endemic Countries and Frequency of Congenital Transmission: A Systematic Review and Meta-Analysis. J. Travel Med. 2020, 28, taaa170. [Google Scholar] [CrossRef] [PubMed]
- Flores-Chavez, M.; Fernandez, B.; Puente, S.; Torres, P.; Rodriguez, M.; Monedero, C.; Cruz, I.; Garate, T.; Canavate, C. Transfusional Chagas Disease: Parasitological and Serological Monitoring of an Infected Recipient and Blood Donor. Clin. Infect. Dis. 2008, 46, e44–e47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Centre for Disease Prevention and Control. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants within the EU/EEA; European Centre for Disease Prevention and Control: Solna, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-screening-and-vaccination-infectious-diseases-newly (accessed on 20 January 2024).
- Soriano-Arandes, A.; Angheben, A.; Serre-Delcor, N.; Treviño-Maruri, B.; Gómez i Prat, J.; Jackson, Y. Control and Management of Congenital Chagas Disease in Europe and Other Non-Endemic Countries: Current Policies and Practices. Trop. Med. Int. Health 2016, 21, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanz, M.; Crespillo-Andújar, C.; Chamorro-Tojeiro, S.; Monge-Maillo, B.; Perez-Molina, J.A.; Norman, F.F. Chagas Disease in Europe. Trop. Med. Infect. Dis. 2023, 8, 513. [Google Scholar] [CrossRef]
- Gonzaga, B.M.; Ferreira, R.R.; Coelho, L.L.; Carvalho, A.C.; Garzoni, L.R.; Araujo-Jorge, T.C. Clinical Trials for Chagas Disease: Etiological and Pathophysiological Treatment. Front. Microbiol. 2023, 14, 1295017. [Google Scholar] [CrossRef] [PubMed]
- Morilla, M.J.; Romero, E.L. Nanomedicines against Chagas Disease: An Update on Therapeutics, Prophylaxis and Diagnosis. Nanomedicine 2015, 10, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Cristovão-Silva, A.C.; Brelaz-De-Castro, M.C.A.; Leite, A.C.L.; Pereira, V.R.A.; Hernandes, M.Z. Chagas Disease Treatment and Rational Drug Discovery: A Challenge That Remains. Front. Pharmacol. 2019, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.C.; Medeiros, T.S.; Pereira, E.L.A.; da Silva, J.F.O.; Oliveira, J.W.F.; Fernandes-Pedrosa, M.F.; da Silva, M.d.S.; da Silva-Júnior, A.A. From Benznidazole to New Drugs: Nanotechnology Contribution in Chagas Disease. Int. J. Mol. Sci. 2023, 24, 13778. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M. Drug Discovery for Chagas Disease: A Viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Silva, A.M.; de Caland, L.B.; Oliveira, A.L.C.d.S.L.; de Araújo-Júnior, R.F.; Fernandes-Pedrosa, M.F.; Cornélio, A.M.; da Silva-Júnior, A.A. Designing Structural Features of Novel Benznidazole-Loaded Cationic Nanoparticles for Inducing Slow Drug Release and Improvement of Biological Efficacy. Mater. Sci. Eng. C 2017, 78, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.d.O.; Saraiva, J.; de Albuquerque, S.; Marchetti, J.M. Solid Dispersion of Ursolic Acid in Gelucire 50/13: A Strategy to Enhance Drug Release and Trypanocidal Activity. AAPS Pharm. Sci. Tech. 2012, 13, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.I.d.S.; Fernandes, A.G.d.A.; Silva, J.J.N.; Andricopulo, A.D.; Lemos, S.S.; Lang, E.S.; Abram, U.; Deflon, V.M. Dithiocarbazate Complexes with the [M(PPh3)]2+ (M=Pd or Pt) Moiety: Synthesis, Characterization and Anti-Trypanosoma cruzi Activity. J. Inorg. Biochem. 2010, 104, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Islan, G.A.; Durán, M.; Cacicedo, M.L.; Nakazato, G.; Kobayashi, R.K.; Martinez, D.S.; Castro, G.R.; Durán, N. Nanopharmaceuticals as a Solution to Neglected Diseases: Is It Possible? Acta Trop. 2017, 170, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Quezada, C.Q.; Azevedo, C.S.; Charneau, S.; Santana, J.M.; Chorilli, M.; Carneiro, M.B.; Bastos, I.M.D. Advances in Nanocarriers as Drug Delivery Systems in Chagas Disease. Int. J. Nanomed. 2019, 14, 6407–6424. [Google Scholar] [CrossRef] [PubMed]
- Arrua, E.C.; Hartwig, O.; Loretz, B.; Murgia, X.; Ho, D.-K.; Bastiat, G.; Lehr, C.-M.; Salomon, C.J. Formulation of Benznidazole-Lipid Nanocapsules: Drug Release, Permeability, Biocompatibility, and Stability Studies. Int. J. Pharm. 2023, 642, 123120. [Google Scholar] [CrossRef] [PubMed]
- Nhavene, E.P.F.; da Silva, W.M.; Junior, R.R.T.; Gastelois, P.L.; Venâncio, T.; Nascimento, R.; Batista, R.J.C.; Machado, C.R.; Macedo, W.A.d.A.; de Sousa, E.M.B. Chitosan Grafted into Mesoporous Silica Nanoparticles as Benznidazol Carrier for Chagas Diseases Treatment. Microporous Mesoporous Mater. 2018, 272, 265–275. [Google Scholar] [CrossRef]
- Oliveira, A.C.d.J.; Silva, E.B.; de Oliveira, T.C.; Ribeiro, F.d.O.S.; Nadvorny, D.; Oliveira, J.W.d.F.; Borrego-Sánchez, A.; Rodrigues, K.A.d.F.; Silva, M.S.; Rolim-Neto, P.J.; et al. pH-Responsive Phthalate Cashew Gum Nanoparticles for Improving Drugs Delivery and Anti-Trypanosoma cruzi Efficacy. Int. J. Biol. Macromol. 2023, 230, 123272. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.Z.L.; de Araújo, C.N.; Cardoso, I.C.C.; Mangabeira, K.S.d.S.; Rocha, A.P.; Charneau, S.; Santana, J.M.; Motta, F.N.; Bastos, I.M.D. Metacyclogenesis as the Starting Point of Chagas Disease. Int. J. Mol. Sci. 2023, 25, 117. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, I.V.; de Souza, D.A.S.; Ramirez, M.I. The End Justifies the Means: Chagas Disease from a Perspective of the Host–Trypanosoma cruzi Interaction. Life 2024, 14, 488. https://doi.org/10.3390/life14040488
Rossi IV, de Souza DAS, Ramirez MI. The End Justifies the Means: Chagas Disease from a Perspective of the Host–Trypanosoma cruzi Interaction. Life. 2024; 14(4):488. https://doi.org/10.3390/life14040488
Chicago/Turabian StyleRossi, Izadora Volpato, Denise Andréa Silva de Souza, and Marcel Ivan Ramirez. 2024. "The End Justifies the Means: Chagas Disease from a Perspective of the Host–Trypanosoma cruzi Interaction" Life 14, no. 4: 488. https://doi.org/10.3390/life14040488
APA StyleRossi, I. V., de Souza, D. A. S., & Ramirez, M. I. (2024). The End Justifies the Means: Chagas Disease from a Perspective of the Host–Trypanosoma cruzi Interaction. Life, 14(4), 488. https://doi.org/10.3390/life14040488