Natural Sources of Therapeutic Agents Used in Skin Conditions
Abstract
1. Introduction
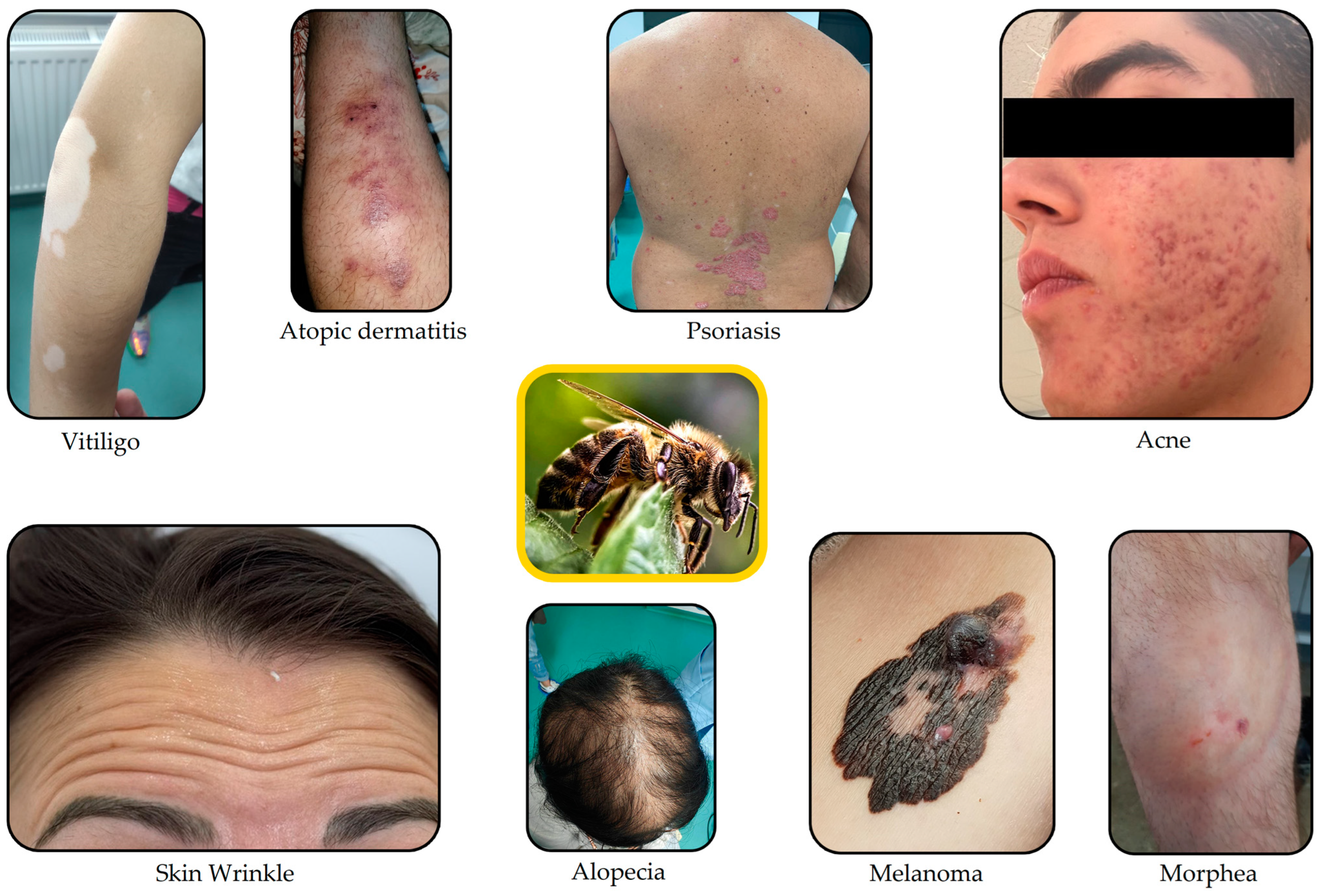
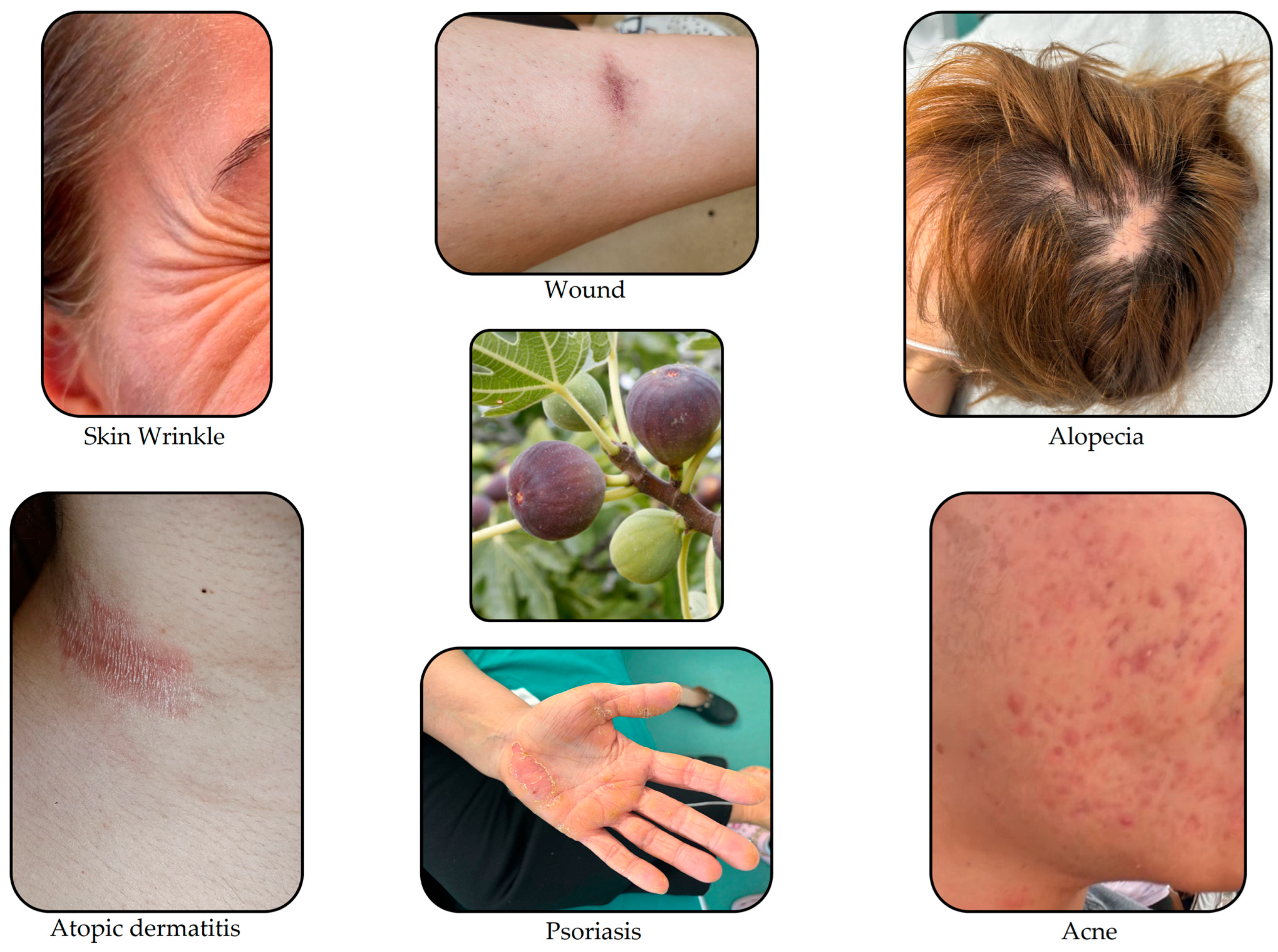
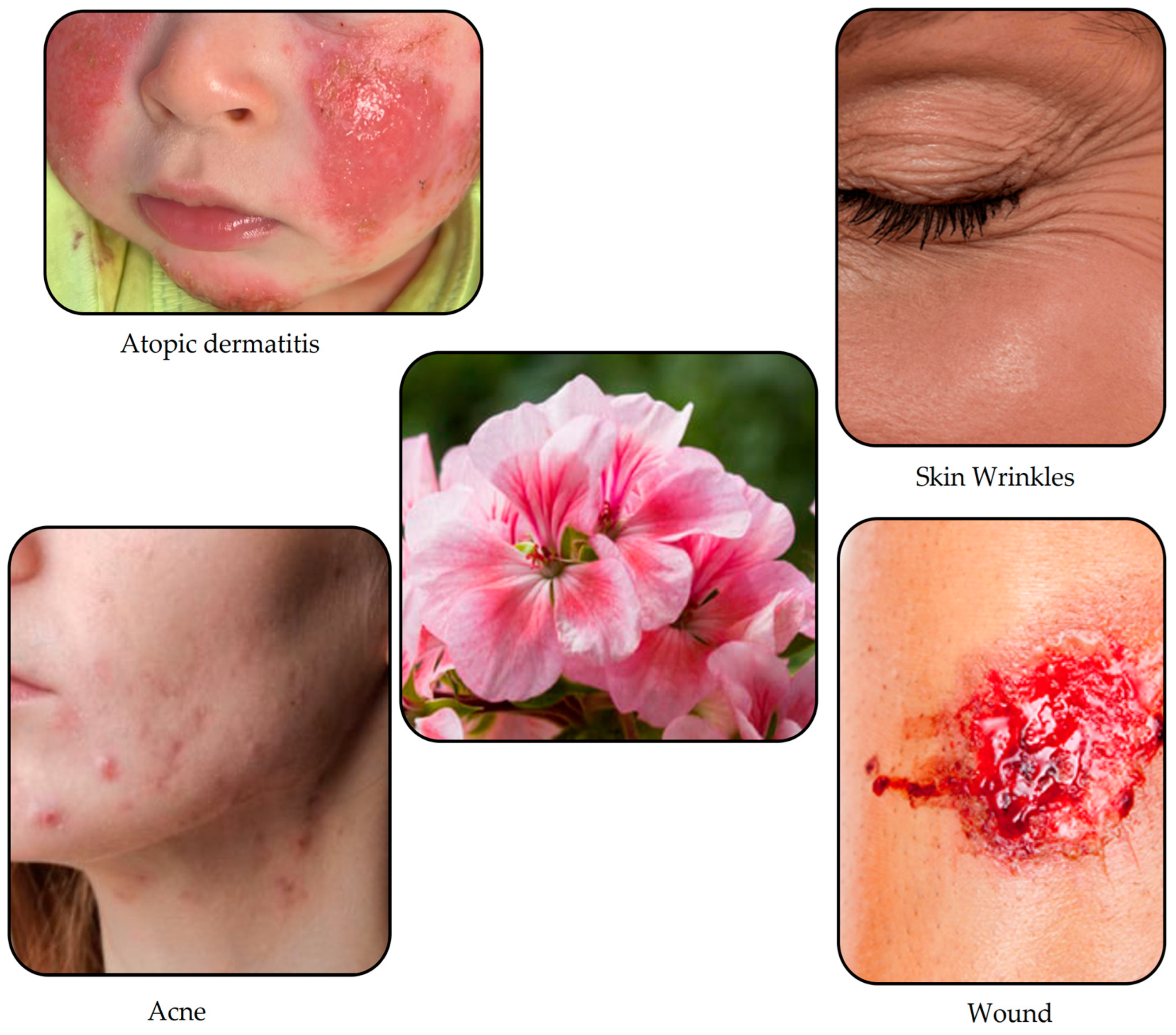
Skin Conditions That Can Be Treated with Therapeutic Agents from Natural Sources
2. Bee Venom
3. Ficus carica
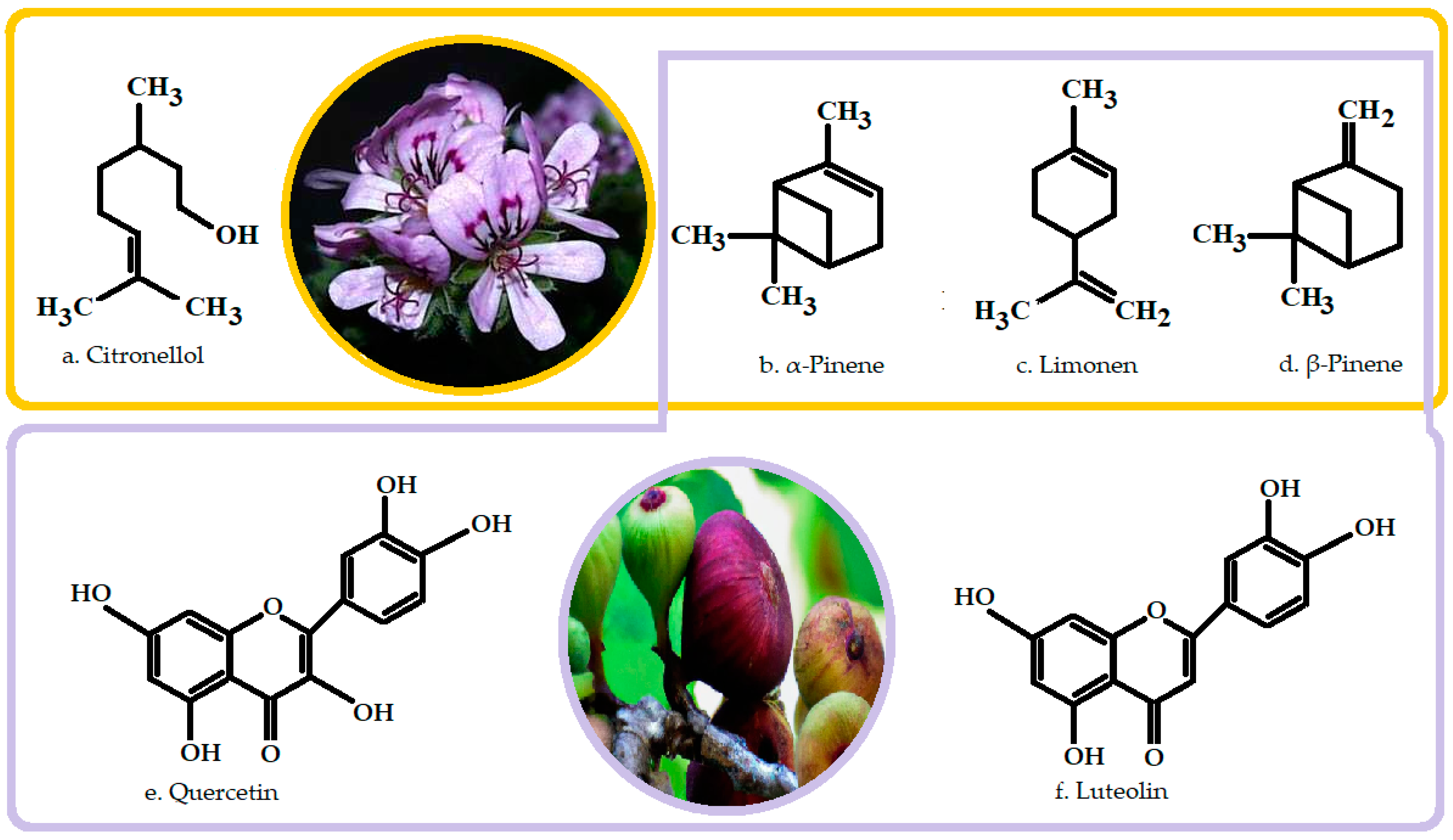
4. Geranium Essential Oil
5. Scientific Evidence of the Beneficial Effects of Bee Venom, Ficus carica, and Geranium Essential Oil for Skin Care and Treatment
5.1. Atopic Dermatitis
5.1.1. Bee Venom in Atopic Dermatitis
5.1.2. Ficus carica in Atopic Dermatitis
5.1.3. Geranium Essential Oil in Dermatitis
5.2. Acne
5.2.1. Bee Venom in Acne
5.2.2. Ficus carica in Acne
5.2.3. Geranium Essential Oil in Acne
5.3. Psoriasis
5.3.1. Bee Venom in Psoriasis
5.3.2. Ficus carica in Psoriazis
5.4. Wound Healing
5.4.1. Bee Venom in Wound Treatment
5.4.2. Ficus carica in Wound Treatment
5.4.3. Geranium Essential Oil in Wound Treatment
5.5. Alopecia
5.5.1. Bee Venom in Alopecia
5.5.2. Ficus carica in Alopecia
5.6. Wrinkles
5.6.1. Bee Venom for Wrinkles
5.6.2. Ficus carica for Wrinkles
5.6.3. Geranium Essential Oil for Wrinkles
5.7. Melanoma
Bee Venom in Melanoma
5.8. Melasma
Ficus carica in Melasma
5.9. Morphea
Bee Venom in Morphea
5.10. Vitiligo
Bee Venom in Vitiligo
5.11. Fungal Infections
5.11.1. Bee Venom in Fungal Infections
5.11.2. Geranium Essential Oil in Fungal Infections
6. Safety Profile and Challenges in BV, FC, and GEO Use
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Dos Santos Szewczyk, K. Dermatological Management of Aged Skin. Cosmetics 2023, 10, 55. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Devel Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Kassab, Y.W.; Muhamad, S.A.; Aldahoul, H.; Mohammed, I.; Paneerselvam, G.; Ayad, M. The impact of skin disorders on patients’ quality of life in Malaysia. J. Clin. Intensive Care Med. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulou, C.; Christopoulou, S.D.; Hahalis, P.; Kotsalou, C.; Lamari, F.N.; Vantarakis, A. Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity. Pathogens 2021, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Oh, M.J.; Lee, D.H.; Lee, Y.S.; Lee, J.; Kim, D.H.; Choi, C.H.; Song, M.J.; Song, H.S.; Hong, J.T. Anti-inflammatory effect of bee venom in phthalic anhydride-induced atopic dermatitis animal model. Inflammopharmacology 2020, 28, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.S.; LaNatra, N.; Stiller, M.J. Nsaids in dermatologic therapy: Review and preview. J. Cutan. Med. Surg. 2002, 6, 449–459. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Hele, D.J. Soft steroids: A new approach to the treatment of inflammatory airways diseases. Pulm. Pharmacol. Ther. 2003, 16, 321–325. [Google Scholar] [CrossRef]
- Bin, L.; Leung, D.Y.M. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52–65. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Giavina-Bianchi, P. Systemic treatment for severe atopic dermatitis. Arch. Immunol. Ther. Exp. 2019, 67, 69–78. [Google Scholar] [CrossRef]
- Singh, R.; Heron, C.E.; Ghamrawi, R.I.; Strowd, L.C.; Feldman, S.R. Emerging role of janus kinase inhibitors for the treatment of atopic dermatitis. ImmunoTargets Ther. 2020, 9, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xie, H.; Cheng, L.; Li, J. Clinical characteristics and epidermal barrier function of papulopustular rosacea: A comparison study with acne vulgaris. Pak. J. Med. Sci. 2016, 32, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Jappe, U. Pathological mechanisms of acne with special emphasis on Propionibacterium acnes and related therapy. Acta Derm. Venereol. 2003, 83, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Leccia, M.T.; Auffret, N.; Poli, F.; Claudel, J.P.; Corvec, S.; Dreno, B. Topical acne treatments in Europe and the issue of antimicrobial resistance. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Kaczmarkiewicz, A.; Stachowiak, N.; Sionkowska, A. Evaluation of Sebostatic Activity of Juniperus communis Fruit Oil and Pelargonium graveolens Oil Compared to Niacinamide. Cosmetics 2017, 4, 36. [Google Scholar] [CrossRef]
- Nicolescu, A.C.; Bucur, Ș.; Giurcăneanu, C.; Gheucă-Solovăstru, L.; Constantin, T.; Furtunescu, F.; Ancuța, I.; Constantin, M.M. Prevalence and Characteristics of Psoriasis in Romania-First Study in Overall Population. J. Pers. Med. 2021, 11, 523. [Google Scholar] [CrossRef]
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Nwabudike, L.C.; Tatu, A.L. Magistral Prescription With Silver Nitrate and Peru Balsam in Difficult-to-Heal Diabetic Foot Ulcers. Am. J. Ther. 2018, 25, e679–e680. [Google Scholar] [CrossRef]
- Nwabudike, L.C.; Tatu, A.L. Reply to Gambichler T et al. Altered epigenetic pathways and cell cycle dysregulation in healthy appearing skin of patients with koebnerized squamous cell carcinomas following skin surgery. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e3–e4. [Google Scholar] [CrossRef]
- Huang, K.P.; Mullangi, S.; Guo, Y.; Qureshi, A.A. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the united states. JAMA Dermatol. 2013, 149, 789–794. [Google Scholar] [CrossRef]
- Kezic, S.; Novak, N.; Jakasa, I.; Jungersted, J.M.; Simon, M.; Brandner, J.M. Skin barrier in atopic dermatitis. Front. Biosci. 2014, 19, 542–556. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.C.; Miot, L.D.; Miot, H.A. Melasma: A clinical and epidemiological review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef]
- Sarkar, R.; Arora, P.; Garg, V.K.; Sonthalia, S.; Gokhale, N. Melasma update. Indian Dermatol. Online J. 2014, 5, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Papara, C.; De Luca, D.A.; Bieber, K.; Vorobyev, A.; Ludwig, R.J. Morphea: The 2023 update. Front. Med. 2023, 10, 1108623. [Google Scholar] [CrossRef]
- Bastonini, E.; Bellei, B.; Filoni, A.; Kovacs, D.; Iacovelli, P.; Picardo, M. Involvement of non-melanocytic skin cells in vitiligo. Exp. Dermatol. 2019, 28, 667–673. [Google Scholar] [CrossRef]
- Roberts, G.H.L.; Santorico, S.A.; Spritz, R.A. The genetic architecture of vitiligo. Pigment. Cell Melanoma Res. 2019, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.A. Dermatopathology and the Diagnosis of Fungal Infections. Br. J. Biomed. Sci. 2023, 80, 11314. [Google Scholar] [CrossRef]
- Majtan, J.; Bucekova, M.; Jesenak, M. Natural Products and Skin Diseases. Molecules 2021, 26, 4489. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First Characterization of The Venom from Apis mellifera syriaca, A honey bee from the Middle East Region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.J.; Park, E.; Asandei, A.; Choi, J.Y.; Lee, S.C.; Seo, C.H.; Luchian, T.; Park, Y. Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci. Rep. 2020, 10, 10145. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.; Zalewski, A.; Zambrowski, G.; Swiecicka, I. Chemical Composition and Antimicrobial Properties of Honey Bee Venom. Molecules 2023, 28, 4135. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. Stud. Nat. Prod. Chem. 2018, 60, 459–484. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Sherman, R.A.; Gileva, O.S.; Kim, C.M.H. Biotherapy—History, Principles and Practice; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400765849. [Google Scholar]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y. Bee venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- Sİg, A.K.; Güney, M.; Özlem, Ö.Z.; Hüseyin, Ş.A.N. Bee venom: A medical perspective. Turk. J. Clin. Lab. 2019, 10, 414–421. [Google Scholar] [CrossRef]
- Jang, S.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Badawi, J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins 2021, 13, 337. [Google Scholar] [CrossRef]
- Iouraouine, E.M.; Soraia, F.; Saïd, B.; Harandou, M.; Maria, G.C.; Miguel, V.-B. Analytical methods for honeybee venom characterization. J. Adv. Pharm. Technol. Res. 2022, 13, 154–160. [Google Scholar]
- Schmidt, J.O. Clinical consequences of toxic envenomations by Hymenoptera. Toxicon 2018, 150, 96–104. [Google Scholar] [CrossRef]
- Owen, M.D.; Pfaff, L.A. Melittin synthesis in the venom system of the honey bee (Apis mellifera L.). Toxicon 1995, 33, 1181–1188. [Google Scholar] [CrossRef]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.; Abd El-Wahed, A.; Gao, R.; Guo, Z.; Tahir, H.E.; Zhao, C.; Du, M.; Farag, M.A.; Musharraf, S.G.; et al. Honeybee products: An updated review of neurological actions. Trends Food Sci. Technol. 2020, 101, 17–27. [Google Scholar] [CrossRef]
- Kim, M.; Han, C.H. Pharmacopuncture for stroke survivors: A systematic review of randomized controlled trials in South Korea. Complement. Ther. Clin. Pract. 2020, 40, 101179. [Google Scholar] [CrossRef]
- El-Seedi, H.; Abd El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Park, J.; Kwon, O.; An, H.J.; Park, K.K. Antifungal Effects of Bee Venom Components on Trichophyton rubrum: A Novel Approach of Bee Venom Study for Possible Emerging Antifungal Agent. Ann. Dermatol. 2018, 30, 202–210. [Google Scholar] [CrossRef]
- Rangachari, B.; Jeong Hwa, K.; Mi-Na, J.; Chenglian, X.; Jin Kyu, P.; Jae Kwon, L. Bee wax coated water-soluble fraction of bee venom improved altered glucose homeostasis in streptozotocin-induced diabetic rats. J. Tradit. Chin. Med. 2019, 39, 842–852. [Google Scholar] [PubMed]
- Zhou, J.; Wan, C.; Cheng, J.; Huang, H.; Lovell, J.F.; Jin, H. Delivery Strategies for Melittin-Based Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 17158–17173. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Lee, S.; Choi, D.B.; Shin, D.; Kim, J.; Yang, H.; Bae, H. Bee Venom Phospholipase A2 Ameliorates Atherosclerosis by Modulating Regulatory T Cells. Toxins 2020, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Keum, D.J.; Kwak, J.W.; Chung, H.-S.; Bae, H. Bee Venom Phospholipase A2 Protects against Acetaminophen-Induced Acute Liver Injury by Modulating Regulatory T Cells and IL-10 in Mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rahim, A.H.; Abd-El-Moneim, O.M.; Abd El-Kader, H.A.; Abd El Raouf, A. Inhibitory effect of bee venom against potassium bromate causing genetic toxicity and biochemical alterations in mice. J. Arab. Soc. Med. Res. 2018, 13, 89–98. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A.; Cristea, V.C. Demodex folliculorum associated Bacillus pumilus in lesional areas in rosacea. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Alazragi, R.S.; Salem, N.A. Potential Therapeutic effect of Bee Venom on Cisplatin-Induced Hepatotoxicity. J. Pharm. Res. Int. 2021, 33, 200–210. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nwabudike, L.C. Reply to: Kubiak K et al. Endosymbiosis and its significance in dermatology. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e346–e347. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother Res. 2021, 35, 743–750. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Niedbała, G.; Alqarni, M.; Zirintunda, G.; Ssempijja, F.; Musinguzi, S.P.; Usman, I.M.; Matama, K.; Hetta, H.F.; Mbiydzenyuy, N.E.; et al. Bee Venom-A Potential Complementary Medicine Candidate for SARS-CoV-2 Infections. Front. Public Health 2020, 8, 594458. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nadasdy, T.; Bujoreanu, F.C. Familial clustering of COVID-19 skin manifestations. Dermatol. Ther. 2020, 33, e14181. [Google Scholar] [CrossRef] [PubMed]
- El-Wahed, A.A.A.; Khalifa, S.A.M.; Elashal, M.H.; Musharraf, S.G.; Saeed, A.; Khatib, A.; Tahir, H.E.; Zou, X.; Naggar, Y.A.; Mehmood, A.; et al. Cosmetic Applications of Bee Venom. Toxins 2021, 13, 810. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial activity and antibiotic-enhancing effects of honeybee venom against methicillin-resistant Staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.Y.; Lee, G. Potential Therapeutic Applications of Bee Venom on Skin Disease and Its Mechanisms: A Literature Review. Toxins 2019, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Jodidio, M.; Schwartz, R.A. Bee venom: Apitherapy and more. Ital. J. Dermatol. Venerol. 2024, 159, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Shirasawa, K.; Nogata, H.; Hirata, C.; Tashiro, K.; Habu, T.; Kim, S.; Himeno, S.; Kuhara, S.; Ikegami, H.; et al. Identification of RAN1 orthologue associated with sex determination through whole genome sequencing analysis in fig (Ficus carica L.). Sci. Rep. 2017, 7, 41124. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Ali, B.; Mujeeb, M.; Aeri, V.; Mir, S.R.; Faiyazuddin, M.; Shakeel, F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Nat. Prod. Res. 2012, 26, 460–465. [Google Scholar] [CrossRef]
- Saraswathi, J.; Venkatesh, K.; Nirmala Baburao, N.B.; Hilal, M.H.; Rani, A.R. Phytopharmacological importance of Pelargonium species. J. Med. Plants Res. 2011, 5, 2587–2598. Available online: https://www.researchgate.net/publication/282387522 (accessed on 4 July 2011).
- Roman, S.; Voaides, C.; Babeanu, N. Exploring the Sustainable Exploitation of Bioactive Compounds in Pelargonium sp.: Beyond a Fragrant Plant. Plants 2023, 12, 4123. [Google Scholar] [CrossRef] [PubMed]
- Sangchart, P.; Panyatip, P.; Damrongrungruang, T.; Priprem, A.; Mahakunakorn, P.; Puthongking, P. Anti-Inflammatory Comparison of Melatonin and Its Bromobenzoylamide Derivatives in Lipopolysaccharide (LPS)-Induced RAW 264.7 Cells and Croton Oil-Induced Mice Ear Edema. Molecules 2021, 26, 4285. [Google Scholar] [CrossRef]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Gu, H.M.; Jeon, M.J.; Han, S.M.; Pak, S.C.; Lee, C.K.; Park, I.S.; et al. Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br. J. Pharmacol. 2018, 175, 4310–4324. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Sung, W.J.; Han, S.M.; Pak, S.C.; Kim, M.K.; et al. Beneficial effects of melittin on ovalbumin-induced atopic dermatitis in mouse. Sci. Rep. 2017, 7, 17679. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Lee, S.J.; Park, J.Y.; Park, K.D.; Han, S.M.; Kim, M.K.; et al. Apamin inhibits TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via suppressions of the NF-κB signaling pathway and STAT in human keratinocytes. Pharmacol. Rep. 2017, 69, 1030–1035. [Google Scholar] [CrossRef]
- Gu, H.; Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Han, S.M.; Leem, J.; Park, K.K. Therapeutic effects of bee venom on experimental atopic dermatitis. Mol. Med. Rep. 2018, 18, 3711–3718. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.W.; Kim, H. Chung DKBee Venom Alleviates Atopic Dermatitis Symptoms through the Upregulation of Decay-Accelerating Factor (DAF/CD55). Toxins 2019, 11, 239. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, W.R.; An, H.J.; Kim, J.Y.; Chung, H.; Han, S.M.; Lee, M.L.; Lee, K.G.; Pak, S.C.; Park, K.K. Bee venom ameliorates compound 48/80-induced atopic dermatitis-related symptoms. Int. J. Clin. Exp. Pathol. 2013, 6, 2896–2903. [Google Scholar]
- Niculet, E.; Chioncel, V.; Elisei, A.; Miulescu, M.; Olimpia, D.B.; Nwabudike, L.; Craescu, M.; Draganescu, M.; Bujoreanu, F.; Marinescu, E.; et al. Multifactorial expression of IL 6 with update on COVID 19 and the therapeutic strategies of its blockade (Review). Exp. Ther. Med. 2021, 21, 3. [Google Scholar] [CrossRef]
- Jung, K.H.; Baek, H.; Kang, M.; Kim, N.; Lee, S.Y.; Bae, H. Bee Venom Phospholipase A2 Ameliorates House Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mice. Toxins 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- You, C.E.; Moon, S.H.; Lee, K.H.; Kim, K.H.; Park, C.W.; Seo, S.J.; Cho, S.H. Effects of Emollient Containing Bee Venom on Atopic Dermatitis: A Double-Blinded, Randomized, Base-Controlled, Multicenter Study of 136 Patients. Ann. Dermatol. 2016, 28, 593–599. [Google Scholar] [CrossRef]
- Abbasi, S.; Kamalinejad, M.; Babaie, D.; Shams, S.; Sadr, Z.; Gheysari, M.; Askari, V.R.; Rakhshandeh, H. A new topical treatment of atopic dermatitis in pediatric patients based on Ficus carica L. (Fig): A randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 35, 85–91. [Google Scholar] [CrossRef]
- Sabzghabaee, A.M.; Shirdare, Z.; Ebadian, B.; Aslani, A.; Ghannadi, A. Clinical evaluation of the essential oil of Pelargonium graveolens for the treatment of denture stomatitis. Dent. Res. J. 2011, 8, S105–S108. [Google Scholar]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Mekarnia, M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med. 2013, 8, 22520. [Google Scholar] [CrossRef]
- Han, S.; Lee, K.; Yeo, J.; Baek, H.; Park, K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J. Med. Plants Res. 2010, 4, 459–464. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef]
- Dumitriu Buzia, O.; Manole Palivan, C.C.; Bezman, V.; Topor, G.; Tatu, A.L.; Kamel, E.; Ionuta, G. Antibacterial action of certain tretinoin and benzoyl peroxide liposomes. Case study. Roum. J. Oral Rehabil. 2020, 12, 272–280. [Google Scholar]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef]
- An, H.J.; Lee, W.R.; Kim, K.H.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Lee, K.G.; Lee, C.K.; Park, K.K. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014, 34, 1341–1348. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Pak, S.C. Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. J. Integr. Med. 2013, 11, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Pak, S.C.; Nicholls, Y.M.; Macfarlane, N. Evaluation of anti-acne property of purified bee venom serum in humans. J. Cosmet. Dermatol. 2016, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Tatu, A.L.; Elisei, A.M.; Bezman, V.; Diaconu, C.; Buzia, O.D. Liposomes, Formulation and Pharmacotechnical Assessment of Anti-Acne Preparations. Rev. Chim. 2019, 70, 425–430. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Aulifa, D.L.; Mustarichie, R.; Hendriani, R.; Erika, I.; Herawati, M.J.A.T. Anti-acne cream of leaves extract of fig (ficus carica l.) From ciwidey district, indonesia, against propionibacterium acnes and staphylococcus epidermidis. Int. J. Appl. Pharm. 2023, 15, 145–148. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Raboh, F.A.A.; Ramzy, N.E.; Shaaban, D.M.; Khader, D.Y. Bee venom and propolis as new treatment modality in patients with localized plaque psoriases. Int. Res. J. Med. Med. Sci. 2013, 1, 27–33. Available online: https://www.researchgate.net/publication/280774799 (accessed on 1 February 2013).
- Eltaher, S.; Mohammed, G.F.; Younes, S.; Elakhras, A. Efficacy of the apitherapy in the treatment of recalcitrant localized plaque psoriasis and evaluation of tumor necrosis factor-alpha (tnf-alpha) serum level: A double-blind randomized clinical trial. J. Dermatol. Treat. 2015, 26, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Song, X.; Tan, L.; Guo, C.; Wang, M.; Liu, H.; Cao, Z.; Li, Y.; Peng, C. A Review of the Pharmacological Properties of Psoralen. Front. Pharmacol. 2020, 11, 571535. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, M.Y. In Vitro and In Vivo Anti-Psoriasis Activity of Ficus carica Fruit Extracts via JAK-STAT Modulation. Life 2023, 13, 1671. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Komosinska-Vassev, K.; Rzepecka-Stojko, A.; Olczyk, P. Bee Venom in Wound Healing. Molecules 2020, 26, 148. [Google Scholar] [CrossRef]
- Han, S.; Lee, K.; Yeo, J.; Kim, W.; Park, K. Biological effects of treatment of an animal skin wound with honeybee (Apis mellifera L.) venom. J. Plast Reconstr. Aesthet. Surg. 2011, 64, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Badr, G.; Badr, B.M.; Allam, A.; Ghamdi, A.A.; Al-Wadaan, M.A.; Al-Waili, N.S. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing nrf2, ang-1, and tie-2 signaling. Mol. Immunol. 2018, 103, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Abdel-Raheem, I.T. Accelerated wound healing and anti-inflammatory effects of physically cross-linked polyvinyl alcohol-chitosan hydrogel containing honey bee venom in diabetic rats. Arch. Pharm. Res. 2014, 37, 1016–1031. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.; Hozzein, W.N.; Badr, B.M.; Al Ghamdi, A.; Saad Eldien, H.M.; Garraud, O. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. J. Cell. Physiol. 2016, 231, 159–2171. [Google Scholar] [CrossRef]
- Sumathra, M.; Rajan, M.; Amarnath Praphakar, R.; Marraiki, N.; Elgorban, A.M. In Vivo Assessment of a Hydroxyapatite/κ-Carrageenan-Maleic Anhydride-Casein/Doxorubicin Composite-Coated Titanium Bone Implant. ACS Biomater. Sci. Eng. 2020, 6, 1650–1662. [Google Scholar] [CrossRef]
- Feng, J.; Niu, Y.; Zhang, Y.; Zuo, H.; Wang, S.; Liu, X. Ficus carica extract impregnated amphiphilic polymer scaffold for diabetic wound tissue regenerations. Artif. Cells Nanomed. Biotechnol. 2021, 49, 219–229. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Poznańska-Kurowska, K.; Kaszuba, A.; Kowalczyk, E. The antibacterial activity of geranium oil against Gram-negative bacteria isolated from difficult-to-heal wounds. Burns 2014, 40, 1046–1051. [Google Scholar] [CrossRef]
- Hosking, A.M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019, 5, 72–89. [Google Scholar] [CrossRef]
- Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. [Google Scholar] [CrossRef]
- Park, S.; Erdogan, S.; Hwang, D.; Hwang, S.; Han, E.H.; Lim, Y.H. Bee venom promotes hair growth in association with inhibiting 5alpha-reductase expression. Biol. Pharm. Bull. 2016, 39, 1060–1068. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, T.Y.; Goo, B.; Park, Y. Bee Venom Stimulates Growth Factor Release from Adipose-Derived Stem Cells to Promote Hair Growth. Toxins 2024, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Keresztessy, Z.; Bodnár, M.; Ber, E.; Hajdu, I.; Zhang, M.; Hartmann, J.F.; Minko, T.; Borbély, J. Self-assembling chitosan/poly-γ-glutamic acid nanoparticles for targeted drug delivery. Colloid Polym. Sci. 2009, 287, 759–765. [Google Scholar] [CrossRef]
- Hajdu, I.; Bodnár, M.; Filipcsei, G.; Hartmann, J.F.; Daróczi, L.; Zrínyi, M.; Borbély, J. Nanoparticles prepared by self-assembly of chitosan and poly-γ-glutamic acid. Colloid Polym. Sci. 2008, 286, 343–350. [Google Scholar] [CrossRef]
- Lee, H.J.; Kwon, H.K.; Kim, H.S.; Kim, M.I.; Park, H.J. Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules. Int. J. Mol. Sci. 2019, 20, 3447. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, G.G.; Park, K.K. Skin Sensitization Study of Bee Venom (Apis mellifera L.) in Guinea Pigs. Toxicol. Res. 2012, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Hong, I.P.; Woo, S.O.; Chun, S.N.; Park, K.K.; Nicholls, Y.M.; Pak, S.C. The beneficial effects of honeybee-venom serum on facial wrinkles in humans. Clin. Interv. Aging 2015, 10, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Akhtar, N.; Ali, A. Effects of Cream Containing Ficus carica L. Fruit Extract on Skin Parameters: In vivo Evaluation. Indian J. Pharm. Sci. 2014, 76, 560–564. [Google Scholar] [PubMed]
- Lohani, A.; Verma, A.; Hema, G.; Pathak, K. Topical Delivery of Geranium/Calendula Essential Oil-Entrapped Ethanolic Lipid Vesicular Cream to Combat Skin Aging. Biomed Res. Int. 2021, 2021, 4593759. [Google Scholar] [CrossRef]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef]
- Tu, W.C.; Wu, C.C.; Hsieh, H.L.; Chen, C.Y.; Hsu, S.L. Honeybee venom induces calcium-dependent but caspase-independent apoptotic cell death in human melanoma A2058 cells. Toxicon 2008, 52, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Salma Shamsi, Y.; Nikhat, S.; Manjhi, M.; Akhtar, M.W.; Ahmad, S. Clinical evaluation of a topical Unani pharmacopoeial formulation Tila-e-Kalf in the management of melasma (Kalf): A randomized controlled clinical trial. Avicenna J. Phytomed. 2023, 13, 255–264. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.H. Bee venom acupuncture for circumscribed morphea in a patient with systemic sclerosis: A case report. Medicine 2018, 97, e13404. [Google Scholar] [CrossRef] [PubMed]
- Mihăilă, B.; Dinică, R.M.; Tatu, A.L.; Buzia, O.D. New insights in vitiligo treatments using bioactive compounds from Piper nigrum. Exp. Ther. Med. 2019, 17, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, N.H.; Koo, B.S.; Lee, H.J.; Lee, A.Y. Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity, and migration. Exp. Mol. Med. 2007, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Jacobs, S.E.; Pentland, A.P. Spla2-x stimulates cutaneous melanocyte dendricity and pigmentation through a lysophosphatidylcholine-dependent mechanism. J. Investig. Dermatol. 2006, 126, 855–861. [Google Scholar] [CrossRef]
- Maeda, K.; Tomita, Y.; Naganuma, M.; Tagami, H. Phospholipases induce melanogenesis in organ-cultured skin. Photochem. Photobiol. 1996, 64, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.R.; Kim, J.J.; Park, G.S.; Oh, S.M.; Han, C.S.; Lee, M.Y. The antifungal activity of bee venom against dermatophytes. J. Appl. Biol. Chem. 2012, 55, 7–11. [Google Scholar] [CrossRef]
- Lee, S.B. Antifungal activity of bee venom and sweet bee venom against clinically isolated candida albicans. J. Pharmacopunct. 2016, 19, 45–50. [Google Scholar] [CrossRef]
- Jaradat, N.; Hawash, M.; Qadi, M.; Abualhasan, M.; Odetallah, A.; Qasim, G.; Awayssa, R.; Akkawi, A.; Abdullah, I.; Al-Maharik, N. Chemical Markers and Pharmacological Characters of Pelargonium graveolens Essential Oil from Palestine. Molecules 2022, 27, 5721. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Langrand, J.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Significance of Arbuscular Mycorrhizal Fungi in Mitigating Abiotic Environmental Stress in Medicinal and Aromatic Plants: A Review. Foods 2022, 11, 2591. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, F.; Jutel, M.; Biló, B.M.; Birnbaum, J.; Muller, U. Prevention and treatment of hymenoptera venom allergy: Guidelines for clinical practice. Allergy 2005, 60, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, M.-S.; Lee, J.-Y.; Yeom, S.-R.; Kwon, Y.-D.; Kim, D.-W. The case report of anaphylaxis after treated with bee-venom acupuncture. J. Korean Med. Rehabil. 2015, 25, 175–182. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Shafaghat, F.; Zwiener, R.D. Immunology of bee venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I.; El Rabey, H.A.; Almutairi, F.M.; Tayel, A.A.; Al-Duais, M.A.; Zidan, N.S.; Sakran, M.I. Effectual Anticancer Potentiality of Loaded Bee Venom onto Fungal Chitosan Nanoparticles. Int. J. Polym. Sci. 2020, 2020, 2785304. [Google Scholar] [CrossRef]
- Bala, I.; Hariharan, S.; Kumar, M.N. PLGA nanoparticles in drug delivery: The state of the art. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Kim, J.-H.; Jeon, J.-W.; Park, J.-K.; Lee, B.-J.; Suh, G.-H.; Cho, C.-W. Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles. Molecules 2015, 20, 15072–15083. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
| Nr. | Skin Diseases | Bee Venom | ||
|---|---|---|---|---|
| Human Studies | Anial Studies | In Vitro Studies | ||
| 1. | Atopic dermatitis (DA) | Double-blind, randomized, multicentre study with q36 patients [83] | Mouse models with 1-chloro-2,4-dinitrobenzene-induced DA [75,79] Mice models with ovalbumin-induced DA [76,78] Mice models with 48/80-induced DA symptoms [80] Mice models with DA induced by Dermatophagoides farinae extract [82] | TNF-α/IFN-γ stimulated human keratinocytes [75] TNF-α/IFN-γ stimulated human keratinocytes [77] |
| 2. | Acne | Double-blind, controlled study of 12 patients [92] Prospective, non-comparative study with 30 subjects [93] | Intradermal injection of P. acnes into mouse ears to cause inflammation [88,91] | Production of inflammatory cytokines (IL-8) and tumor necrosis (TNF-α) was examined in THP-1 cells [87] Effect of melittin on inflammatory cytokine production in heat-destroyed P.acnes-treated keratinocytes [88] |
| 3. | Psoriasis | 48 patients, randomized to four different treatment groups [96] Randomized double-blind study with 50 patients [97] | ||
| 4. | Wound | Mice models with dorsal wounds [102] Type I diabetic mouse models with diabetic wounds [103,105] Diabetic rat models with wounds [104] | ||
| 5. | Alopecia | Dorsal skin of female C57BL/6 mice [111] BV-treated, adipose-derived stem cells were injected subcutaneously into mice [112] | ||
| 6. | Wrinkles | Clinical study with 22 female volunteers [117] | Assessment of skin sensitization to BV in guinea pigs and rats [116] | |
| 7. | Melanoma | There are no human studies at present | Perfluorocarbon nanoparticles loaded with melittin for mouse melanoma B16F10 [123] | B16F10, A375SM, and SKMEL28 melanoma cells [121] Human melanoma cells A2058 [122] |
| 8. | Morphea | Clinical study; patients of 64 years; acupuncture [125] | ||
| 9. | Vitiligo | Human melanocytes [127] Guinea pig skins grown in organs [129] | ||
| 10. | Fungal infection | Forty-eight plates inoculated with Trichophyton rubrum [51] Ten clinical isolates of C. albicans that were cultured from blood and vagina via the disc diffusion method [131] | ||
| Nr. | Skin Diseases | Ficuscarica | ||
|---|---|---|---|---|
| Human Studies | Animal Studies | In Vitro Studies | ||
| 1. | Atopic dermatitis | The randomized, placebo-controlled clinical trial involved 45 children aged from 4 months to 14 years [84] | ||
| 2. | Acne | Volunteer group for irritation test and preference test [95] | ||
| 3. | Psoriasis | Mouse model of IMQ-induced psoriasis [100] | LPS-stimulated RAW 264.7 cells [100] | |
| 4. | Wound | Human wound cells (WS1) were cultured in Eagle Environment Modificat Dulbecco [107] | ||
| 5. | Alopecia | Telogen-stage C57BL/6N mouse models [115] | HaCaT cells (5 × 103 cells/well); normal human keratinocytes [115] | |
| 6. | Wrinkles | Randomized, open-label, single-blind, placebo-controlled trial with 21 women (age 45–65 years) [118] Simple, blind, and comparison study with 11 Asian men [119] | ||
| 7. | Melasma | Randomized controlled clinical trial; 65 patients diagnosed with melasma; women and men over 18 years of age [124] | ||
| Nr. | Skin Diseases | Geranium Essential Oil | ||
|---|---|---|---|---|
| Human Studies | Animal Studies | In Vitro Studies | ||
| 1. | Atopic dermatitis | Croton-oil-induced ear edema in mouse models [86] | ||
| 2. | Acne | Evaluation of sebostatic activity on 3 women and 3 men [15] | ||
| 3. | Wound | Samples of Gram-negative clinical strains were isolated from swabs from serious injuries of 63 patients [108] | ||
| 4. | Wrinkles | Skin irritation study in rat models [120] | Collagenase inhibition test [120] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Tatu, A.L.; Cocoș, D.I.; Nwabudike, L.C.; Chirilov, A.M.; Stefan, C.S.; Earar, K.; Dumitriu Buzia, O. Natural Sources of Therapeutic Agents Used in Skin Conditions. Life 2024, 14, 492. https://doi.org/10.3390/life14040492
Dinu M, Tatu AL, Cocoș DI, Nwabudike LC, Chirilov AM, Stefan CS, Earar K, Dumitriu Buzia O. Natural Sources of Therapeutic Agents Used in Skin Conditions. Life. 2024; 14(4):492. https://doi.org/10.3390/life14040492
Chicago/Turabian StyleDinu, Monica, Alin Laurențiu Tatu, Dorin Ioan Cocoș, Lawrence Chukwudi Nwabudike, Ana Maria Chirilov, Claudia Simona Stefan, Kamel Earar, and Olimpia Dumitriu Buzia. 2024. "Natural Sources of Therapeutic Agents Used in Skin Conditions" Life 14, no. 4: 492. https://doi.org/10.3390/life14040492
APA StyleDinu, M., Tatu, A. L., Cocoș, D. I., Nwabudike, L. C., Chirilov, A. M., Stefan, C. S., Earar, K., & Dumitriu Buzia, O. (2024). Natural Sources of Therapeutic Agents Used in Skin Conditions. Life, 14(4), 492. https://doi.org/10.3390/life14040492









