New Equations for the Estimation of the Age of the Formation of the Harris Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Bone Growth Equation Development and Selection
2.3. Age at HL Calculation Tool Development
2.4. Bland–Altman Comparison of Method Outputs
3. Results
3.1. Bone Growth Curve Selection
3.2. Age at HL Calculation Tool Development
3.3. New Method and Byers’ Method Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, O.; Piontek, J. Does the occurrence of Harris lines affect the morphology of human long bones? HOMO—J. Comp. Hum. Biol. 2002, 52, 254–276. [Google Scholar] [CrossRef]
- Chauveau, A.; Augias, A.; Froment, A.; Beuret, F.; Charlier, P. Radio-Scannographic Correlation of Harris Lines in Adults: Forensic and Anthropological Perspectives. Eur. J. Forensic Sci. 2016, 3, 21–28. [Google Scholar] [CrossRef]
- Miszkiewicz, J.J. Histology of a Harris line in a human distal tibia. J. Bone Miner. Metab. 2015, 33, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.J.M.J.; Dąbrowski, P. How to calculate the age at formation of Harris lines? A step-by-step review of current methods and a proposal for modifications to Byers’ formulas. Archaeol. Anthropol. Sci. 2019, 11, 1169–1185. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Gannon, N.P. Park-Harris Lines. J. Am. Acad. Orthop. Surg. 2022, 30, e1483–e1494. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.B.; Hoppa, R.D. Brief communication: A re-evaluation of the impact of radiographic orientation on the identification and interpretation of Harris lines. Am. J. Phys. Anthropol. 2015, 156, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R. New and Emerging Prospects for the Paleopathological Study of Starvation. Pathways 2020, 1, 66–83. [Google Scholar] [CrossRef]
- Nowak, O. Linie Harrisa jako miernik reakcji morfologicznej na warunki życia: Interpretacjie, kontrowersje, propozycje badawcze. Przegląd Antropol. 1996, 59, 77–86. [Google Scholar] [CrossRef]
- González-Reimers, E.; Pérez-Ramírez, A.; Santolaria-Fernández, F.; Rodríguez-Rodríguez, E.; Martínez-Riera, A.; Durán-Castellón, M.d.C.; Alemán-Valls, M.R.; Gaspar, M.R. Association of Harris lines and shorter stature with ethanol consumption during growth. Alcohol 2007, 41, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Jerszyńska, B.; Nowak, O. Harris Lines in subadult and adult skeletons from the medieval cemetery in Cedynia, Poland. Var. Evol. 2001, 9, 33–43. [Google Scholar]
- Nowakowski, D. Frequency of appearance of transverse (Harris) lines reflects living conditions of the Pleistocene bear-Ursus ingressus-(Sudety Mts., Poland). PLoS ONE 2018, 13, e0196342. [Google Scholar] [CrossRef] [PubMed]
- MacChiarelli, R.; Bondioli, L.; Censi, L.; Hernaez, M.K.; Salvadei, L.; Sperduti, A. Intra- and interobserver concordance in scoring Harris lines: A test on bone sections and radiographs. Am. J. Phys. Anthropol. 1994, 95, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Primeau, C.; Jakobsen, L.S.; Lynnerup, N. CT imaging vs. traditional radiographic imaging for evaluating Harris Lines in tibiae. Anthropol. Anz. 2016, 73, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, A.; Psonak, D. The affinity of the Harris lines to bone massiveness. Anthropol. Anz. 2020, 78, 207. [Google Scholar] [CrossRef] [PubMed]
- González-Ramírez, A.; Pacheco Miranda, A.; Sáez, A.; Arregui Wunderlich, I. Infants from the Tarapacá 40 cemetery (Northern Chile, Formative Period, 1000 BC–AD 600). Int. J. Osteoarchaeol. 2019, 29, 874–880. [Google Scholar] [CrossRef]
- Geber, J. Skeletal manifestations of stress in child victims of the Great Irish Famine (1845–1852): Prevalence of enamel hypoplasia, Harris lines, and growth retardation. Am. J. Phys. Anthropol. 2014, 155, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Primeau, C.; Homøe, P.; Lynnerup, N. Childhood health as reflected in an adult urban and a rural samples from medieval Denmark. Homo 2018, 69, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jerszyńska, B.; Nowak, O. Application of Byers method for reconstruction of age at formation of Harris lines in adults from a cemetery of Cedynia (Poland). Var. Evol. 1996, 5, 75–82. [Google Scholar]
- Ameen, S.; Staub, L.; Ulrich, S.; Vock, P.; Ballmer, F.; Anderson, S.E. Harris lines of the tibia across centuries: A comparison of two populations, medieval and contemporary in Central Europe. Skeletal Radiol. 2005, 34, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Nowak, O.; Piontek, J. The frequency of appearance of transverse (Harris) lines in the tibia in relationship to age at death. Ann. Hum. Biol. 2002, 29, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Heylings, D.J.A.; Power, C. Transverse (Harris) lines in Irish archaeological remains. Am. J. Phys. Anthropol. 1996, 101, 115–131. [Google Scholar] [CrossRef]
- Wojenka, M.; Jaskulska, E.; Popović, D.; Baca, M.; Frog; Fetner, R.; Wertz, K.; Rataj, K.; Gryczewska, N.; Kosiński, T.; et al. The girl with finches: A unique post-medieval burial in Tunel Wielki Cave, southern Poland. Praehist. Zeitschrift 2021, 96, 286–309. [Google Scholar] [CrossRef]
- Welsh, H. Investigating Patterns of Growth and Development in Subadults from the 10th–13th Century Cemetery of St. Étienne de Toulouse, France, McMaster University. 2021. Available online: https://macsphere.mcmaster.ca/bitstream/11375/26917/2/Welsh_Hayley_finalsubmission2021September_MA.pdf (accessed on 11 April 2024).
- Debard, J. Les Conditions Socio-Économiques Pendant L’âge du Fer en Suisse Occidentale: Intégration des Paramètres Archéologiques, Bioanthropologiques, Paléopathologiques et Paléoalimentaires; l’Université de Genève: Geneva, Switzerland, 2020. [Google Scholar]
- Allison, M.J.; Mendoza, D.; Pezzia, A. A radiographic approach to Childhood illness in precolumbian inhabitants of Southern Peru. Am. J. Phys. Anthropol. 1974, 40, 409–415. [Google Scholar] [CrossRef] [PubMed]
- McHenry, H.M.; Schulz, P.D. The association between Harris lines and enamel hypoplasia in prehistoric California Indians. Am. J. Phys. Anthropol. 1976, 44, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.E.; Hatch, J.W. The estimation of age at death and ages of formation of transverse lines from measurements of human long bones. Am. J. Phys. Anthropol. 1981, 54, 461–469. [Google Scholar] [CrossRef]
- Clarke, S.K. The association of early childhood enamel hypoplasias an radiopaque transverse lines in a culturally diverse prehistoric skeletal sample. Hum. Biol. 1982, 54, 77–84. [Google Scholar] [PubMed]
- Maat, G.J.R. Dating and rating of Harris’s lines. Am. J. Phys. Anthropol. 1984, 63, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hummert, J.R.; Van Gerven, D.P. Observations on the formation and persistence of radiopaque transverse lines. Am. J. Phys. Anthropol. 1985, 66, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Byers, S. Calculation of age at formation of radiopaque transverse lines. Am. J. Phys. Anthropol. 1991, 85, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.J.; Dean, M.C. Variation in modern human enamel formation times. J. Hum. Evol. 2006, 50, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.H.; Song, R.J. Sources of Variation in Estimated Ages at Formation of Linear Enamel Hypoplasias. In Human Growth in the Past: Studies from Bones and Teeth; Hoppa, R., FitzGerald, C., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 210–239. [Google Scholar]
- Swärdstedt, T. Odontological Aspects of a Medieval Population in the Province of Jamtland, Mid-Sweden; University of Lund: Lund, Sweden, 1966. [Google Scholar]
- Belcastro, G.; Rastelli, E.; Mariotti, V.; Consiglio, C.; Facchini, F.; Bonfiglioli, B. Continuity or discontinuity of the life-style in central Italy during the Roman Imperial Age-Early Middle Ages transition: Diet, health, and behavior. Am. J. Phys. Anthropol. 2007, 132, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Ham, A.C.; Temple, D.H.; Klaus, H.D.; Hunt, D.R. Evaluating life history trade-offs through the presence of linear enamel hypoplasia at Pueblo Bonito and Hawikku: A biocultural study of early life stress and survival in the Ancestral Pueblo Southwest. Am. J. Hum. Biol. 2020, 33, e23506. [Google Scholar] [CrossRef] [PubMed]
- Ritzman, T.B.; Baker, B.J.; Schwartz, G.T. A fine line: A comparison of methods for estimating ages of linear enamel hypoplasia formation. Am. J. Phys. Anthropol. 2008, 135, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, A.C.; Oxenham, M.F. New distance-based exponential regression method and equations for estimating the chronology of linear enamel hypoplasia (LEH) defects on the anterior dentition. Am. J. Phys. Anthropol. 2019, 168, 510–520. [Google Scholar] [CrossRef]
- Burak, M.; Okólska, H. Cmentarze Dawnego Wrocławia; Wydawnictwo Muzeum Architektury: Wroclaw, Poland, 2007. [Google Scholar]
- Dąbrowski, P.; Kulus, M.J.; Grzelak, J.; Olchowy, C.; Staniowski, T.; Paulsen, F. Nutritional reconstruction in an early modern population: Searching for a relationship between dental microwear and bone element composition. Ann. Anat.-Anat. Anzeiger 2022, 240, 151884. [Google Scholar] [CrossRef] [PubMed]
- Labuda, A.S. Wrocławski Ołtarz św. Barbary i Jego Twórcy: Studium o Malarstwie Śląskim Połowy XV Wieku; Wydawnictwo Uniwersytetu Adama Mickiewicza: Poznań, Poland, 1984. [Google Scholar]
- Maresh, M.M. Linear Growth of Extremities from Infancy Through Adolescence. Am. J. Dis. Child. 1955, 89, 725–742. [Google Scholar]
- Gindhart, P.S. Growth standards for the tibia and radius in children aged one month through eighteen years. Am. J. Phys. Anthropol. 1973, 39, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Green, W.T. Lengths of the femur and the tibia; norms derived from orthoroentgenograms of children from 5 years of age until epiphysial closure. Am. J. Dis. Child. 1948, 75, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Green, W.T.; Messner, M.B. Growth and predictions of growth in the lower extremities. J. Bone Jt. Surg. Am. 1963, 45-A, 1–14. [Google Scholar] [CrossRef]
- Papageorgopoulou, C.; Suter, S.K.; Rühli, F.J.; Siegmund, F. Harris lines revisited: Prevalence, comorbidities, and possible etiologies. Am. J. Hum. Biol. 2011, 23, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pan, W. Akaike’s Information Criterion in Generalized Estimating Equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Points of Significance: Model selection and overfitting. Nat. Methods 2016, 13, 703–704. [Google Scholar] [CrossRef]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). 2020. Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 11 April 2024).
- R Core Team R: A Language And Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Buikstra, J.E.J.; Ubelaker, D.H.D. Standards for Data Collection from Human Skeletal Remains; Arkansas Archeological Survey: Fayetteville, AR, USA, 1994; Volume 44, ISBN 9781563490750. [Google Scholar]
- Miles, A.E.W.; Bulman, J.S. Growth curves of immature bones from a Scottish island population of sixteenth to mid-nineteenth century: Limb-bone diaphyses and some bones of the hand and foot. Int. J. Osteoarchaeol. 1994, 4, 121–136. [Google Scholar] [CrossRef]
- Aldegheri, R.; Agostini, S. A chart of anthropometric values. J. Bone Jt. Surg. Br. 1993, 75, 86–88. [Google Scholar] [CrossRef]
- Taranger, J.; Hägg, U. The timing and duration of adolescent growth. Acta Odontol. Scand. 1980, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mays, S.A. The relationship between harris line formation and bone growth and development. J. Archaeol. Sci. 1985, 12, 207–220. [Google Scholar] [CrossRef]
- Mays, S. The relationship between harris lines and other aspects of skeletal development in adults and juveniles. J. Archaeol. Sci. 1995, 22, 511–520. [Google Scholar] [CrossRef]
- Alfonso, M.P.; Thompson, J.L.; Standen, V.G. Reevaluating Harris lines—A comparison between Harris lines and enamel hypoplasia. Coll. Antropol. 2005, 29, 393–408. [Google Scholar] [PubMed]
- Nelson, S.J. Wheeler’s Dental Anatomy, Physiology, and Occlusion, 3rd ed.; Elsevier: St. Louis, MI, USA, 2015; ISBN 9780323263238. [Google Scholar]
- Reid, D.J.; Dean, M.C. Brief communication: The timing of linear hypoplasia on human anterior teeth. Am. J. Phys. Anthr. 2000, 113, 135–139. [Google Scholar] [CrossRef]
- Krenz-Niedbała, M.; Kozłowski, T. Comparing the chronological distribution of enamel hypoplasia in Rogowo, Poland (2nd century AD) using two methods of defect timing estimation. Int. J. Osteoarchaeol. 2013, 23, 410–420. [Google Scholar] [CrossRef]
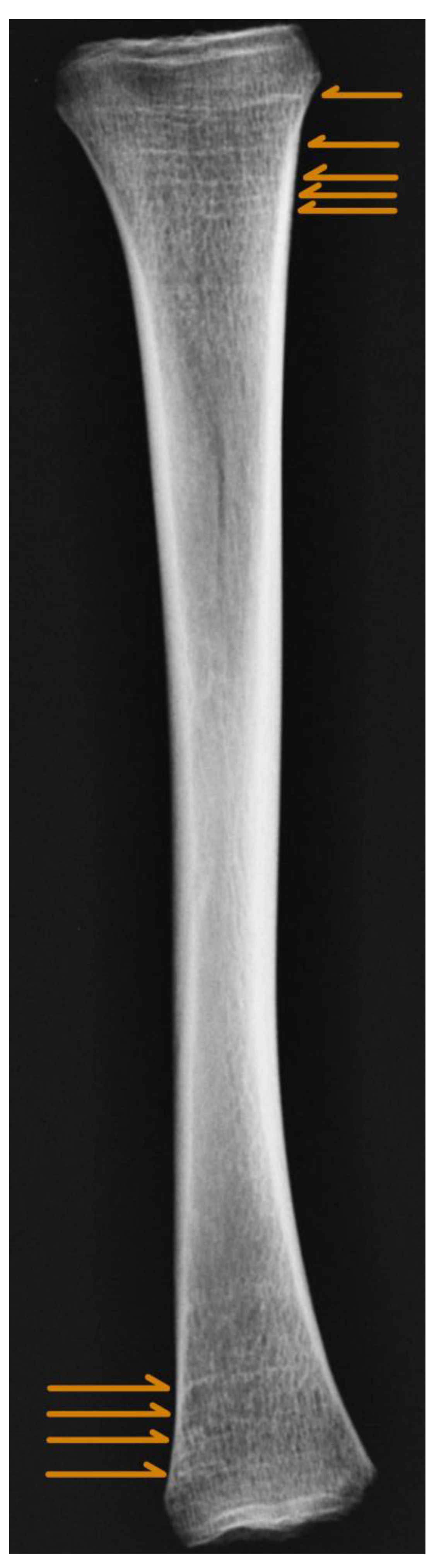


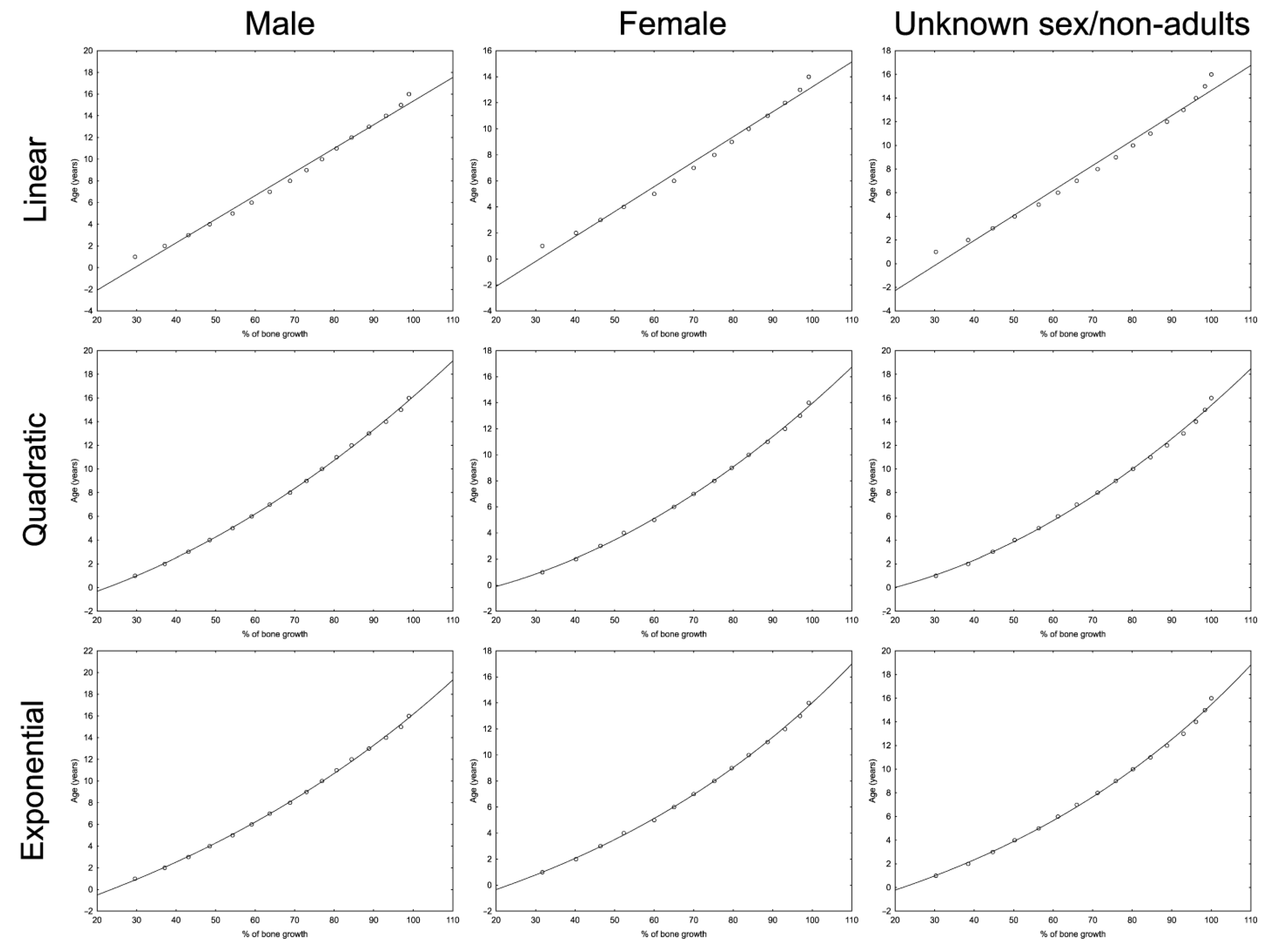
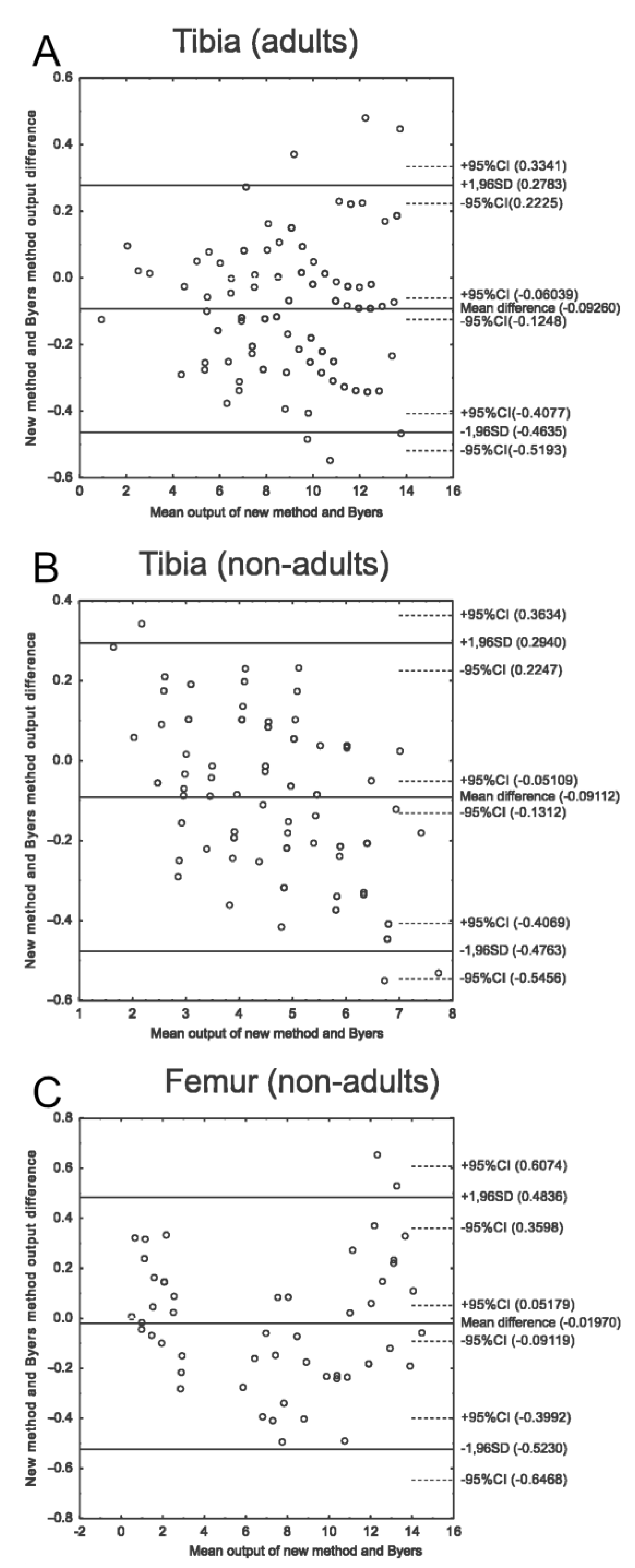
| Bone | Equation Type | Equation | AIC | AICc | Residuals |
|---|---|---|---|---|---|
| Femur (males) | Linear | y = 0.217859 × x − 6.43422 | 25.82 | 27.82 | 3.234 |
| Quadratic | y= 0.00106893 × x2 + 0.077086 × x − 2.28129 | −18.35 | −14.71 | 0.1804 | |
| Exponential | y = 11.5213 × e0.00979342×x – 14.5196 | −15.94 | −12.30 | 0.2098 | |
| Femur (females) | Linear | y = 0.191588 × x − 5.93988 | 24.19 | 26.59 | 3.007 |
| Quadratic | y = 0.00114036 × x2 + 0.0387797 × x − 1.33028 | −12.01 | −7.56 | 0.1963 | |
| Exponential | y= 6.84604 × e0.0121551×x − 9.07047 | −14.22 | −9.77 | 0.1676 | |
| Tibia (males) | Linear | y = 0.216145 × x − 6.14786 | 20.56 | 22.56 | 2.328 |
| Quadratic | y= 0.000860092 × x2 + 0.103426 × x − 2.84765 | −13.35 | −9.71 | 0.2467 | |
| Exponential | y = 16.227 × e0.00787971×x − 19.5643 | −11.19 | −7.55 | 0.2823 | |
| Tibia (females) | Linear | y = 0.19219 × x − 5.90254 | 22.61 | 25.01 | 2.685 |
| Quadratic | y = 0.00105703 × x2+ 0.0508895 × x − 1.64875 | −4.59 | −0.15 | 0.3333 | |
| Exponential | y= 7.78153 × e0.011363×x −10.1631 | 6.38 | −1.94 | 0.2933 | |
| Tibia (unisex) | Liner | y = (0.211442) × x + (−6.50326) | 33.78 | 35.78 | 5.318 |
| Quadratic | y = 0.00128889 × x2 + (0.0374516) × x + (−1.24991) | 4.29 | 7.93 | 0.7431 | |
| Exponential | y= (6.79694) × exp0.0128072×x + (−9.00042) | 1.11 | 4.75 | 0.6093 | |
| Femur (unisex) | Linear | y = (0.211759) × x + (−6.65994) | 36.57 | 38.57 | 6.331 |
| Quadratic | y = (0.00142038) × x2 + (0.0191624) × x + (−0.812149) | 6.1 | 9.74 | 0.8323 | |
| Exponential | y= (5.61797) × exp0.0140969×x + (−7.60929) | 2.58 | 6.21 | 0.6677 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulus, M.J.; Cebulski, K.; Kmiecik, P.; Sputa-Grzegrzółka, P.; Grzelak, J.; Dąbrowski, P. New Equations for the Estimation of the Age of the Formation of the Harris Lines. Life 2024, 14, 501. https://doi.org/10.3390/life14040501
Kulus MJ, Cebulski K, Kmiecik P, Sputa-Grzegrzółka P, Grzelak J, Dąbrowski P. New Equations for the Estimation of the Age of the Formation of the Harris Lines. Life. 2024; 14(4):501. https://doi.org/10.3390/life14040501
Chicago/Turabian StyleKulus, Michał J., Kamil Cebulski, Piotr Kmiecik, Patrycja Sputa-Grzegrzółka, Joanna Grzelak, and Paweł Dąbrowski. 2024. "New Equations for the Estimation of the Age of the Formation of the Harris Lines" Life 14, no. 4: 501. https://doi.org/10.3390/life14040501
APA StyleKulus, M. J., Cebulski, K., Kmiecik, P., Sputa-Grzegrzółka, P., Grzelak, J., & Dąbrowski, P. (2024). New Equations for the Estimation of the Age of the Formation of the Harris Lines. Life, 14(4), 501. https://doi.org/10.3390/life14040501






