The Impact of Lifestyle on the Secondary Sex Ratio: A Review
Abstract
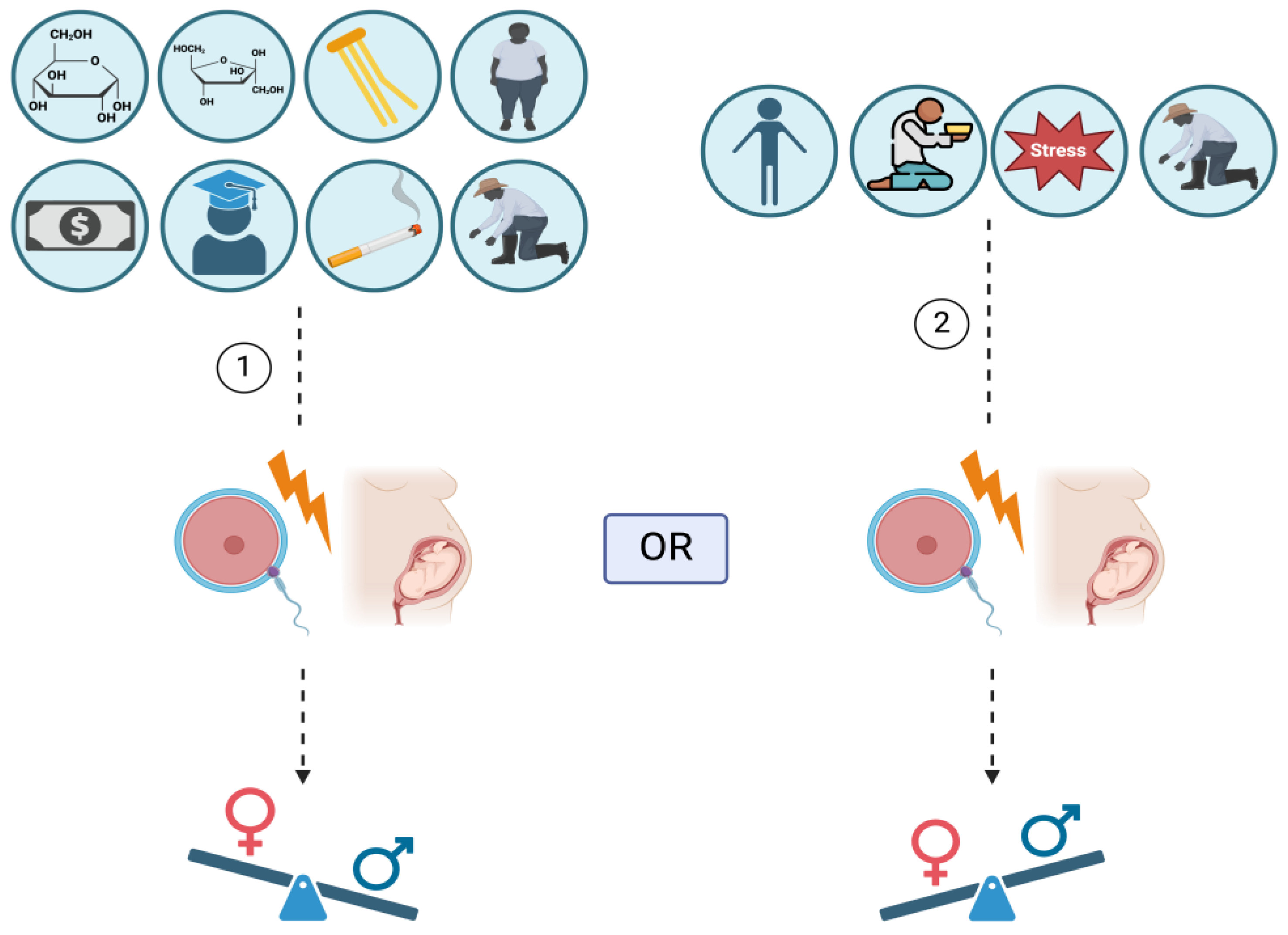
1. Introduction
2. Review Methodology
3. Dietary Habits and Calorie Intake
3.1. Dietary Habits and SSR
3.1.1. Fatty Acids
3.1.2. Sugar Intake
3.2. Calorie Intake and SSR
4. Socioeconomic Conditions
5. Psychological Distress
6. Occupation and Other Factors
6.1. Occupation and SSR
6.2. Other Lifestyle Factors and the SSR
6.2.1. Smoking Habits
6.2.2. Cultural Beliefs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, L.; Grech, V. A Systematic Search of the Factors That Influence the Sex Ratio at Birth. Early Hum. Dev. 2020, 140, 104865. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Gerland, P.; Cook, A.R.; Alkema, L. Systematic Assessment of the Sex Ratio at Birth for All Countries and Estimation of National Imbalances and Regional Reference Levels. Proc. Natl. Acad. Sci. USA 2019, 116, 9303–9311. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Miliaras, D.; Kesidou, E.; Boziki, M.; Petratos, S.; Grigoriadis, N.; Theotokis, P. Developmental Cues and Molecular Drivers in Myelinogenesis: Revisiting Early Life to Re-Evaluate the Integrity of CNS Myelin. Curr. Issues Mol. Biol. 2022, 44, 3208–3237. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Tremblay, M.-È.; Petratos, S.; Zoupi, L.; Boziki, M.; Kesidou, E.; Simeonidou, C.; Theotokis, P. Origin and Emergence of Microglia in the CNS—An Interesting (Hi)Story of an Eccentric Cell. Curr. Issues Mol. Biol. 2023, 45, 2609–2628. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Theotokis, P.; Evangelidis, P.; Delilampou, E.; Evangelidis, N.; Chatzisavvidou, A.; Avramidou, E.; Manthou, M.E. CNS Border-Associated Macrophages: Ontogeny and Potential Implication in Disease. Curr. Issues Mol. Biol. 2023, 45, 4285–4300. [Google Scholar] [CrossRef] [PubMed]
- Fakorede, S.T.; Ojo, S.D.; Shonde, K.M.; Adekoya, K.O.; Ogunkanmi, L.A.; Oboh, B. Trends and Seasonal Variations in Human Secondary Sex Ratio in Southwest Nigeria: A 10-Year Survey. Adv. Hum. Biol. 2022, 12, 271–276. [Google Scholar] [CrossRef]
- Catalano, R.; Bruckner, T.; Marks, A.R.; Eskenazi, B. Exogenous Shocks to the Human Sex Ratio: The Case of September 11, 2001 in New York City. Hum. Reprod. 2006, 21, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Graffelman, J.; Hoekstra, R.F. A Statistical Analysis of the Effect of Warfare on the Human Secondary Sex Ratio. Hum. Biol. 2000, 72, 433–445. [Google Scholar] [PubMed]
- Bae, J.; Kim, S.; Kannan, K.; Buck Louis, G.M. Couples’ Urinary Bisphenol A and Phthalate Metabolite Concentrations and the Secondary Sex Ratio. Environ. Res. 2015, 137, 450–457. [Google Scholar] [CrossRef]
- Masukume, G.; Ryan, M.; Masukume, R.; Zammit, D.; Grech, V.; Mapanga, W. COVID-19 Onset Reduced the Sex Ratio at Birth in South Africa. PeerJ 2022, 10, e13985. [Google Scholar] [CrossRef]
- Scherb, H.; Grech, V. The Secondary Sex Ratio in Italy over the Past Eighty Years (1940 to 2019) and Potential Impact of Radiological Contamination after Atmospheric Nuclear Testing and after Chernobyl: Temporal Change-Point Analysis Using Markov Chain Monte Carlo. Reprod. Toxicol. 2021, 100, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Brown, S.I. Effects of Lifestyle Factors on Fertility: Practical Recommendations for Modification. Reprod. Fertil. 2021, 2, R13–R26. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Khalil, W.A.; Pushpakumara, A.P.G.; El-Harairy, M.A.; Abo El-Atta, A.M.A.; Wathes, D.C.; Fouladi-Nashta, A. Polyunsaturated Fatty Acids Influence Offspring Sex Ratio in Cows. Int. J. Vet. Sci. Med. 2018, 6, S36–S40. [Google Scholar] [CrossRef]
- Cagnacci, A. Influences of Maternal Weight on the Secondary Sex Ratio of Human Offspring. Hum. Reprod. 2004, 19, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Behie, A.M.; O’Donnell, M.H. Higher Parental Perceptions of Wealth Associated with the Birth of More Sons in an Australian Population. J. Biosoc. Sci. 2018, 50, 569–572. [Google Scholar] [CrossRef]
- Catalano, R.; Bruckner, T.; Hartig, T.; Ong, M. Population Stress and the Swedish Sex Ratio. Paediatr. Perinat. Epidemiol. 2005, 19, 413–420. [Google Scholar] [CrossRef]
- Alexopoulos, E.C.; Alamanos, Y. Secondary Sex Ratio in Greece: Evidence of an Influence by Father’s Occupational Exposure. Hum. Reprod. 2007, 22, 2999–3001. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Roberts, R.M. Maternal Diet and Other Factors Affecting Offspring Sex Ratio: A Review. Biol. Reprod. 2004, 71, 1063–1070. [Google Scholar] [CrossRef]
- Foster, H.; Polz, P.; Mair, F.; Gill, J.; O’Donnell, C.A. Understanding the Influence of Socioeconomic Status on the Association between Combinations of Lifestyle Factors and Adverse Health Outcomes: A Systematic Review Protocol. BMJ Open 2021, 11, e042212. [Google Scholar] [CrossRef]
- Catalano, R.A. Sex Ratios in the Two Germanies: A Test of the Economic Stress Hypothesis. Hum. Reprod. 2003, 18, 1972–1975. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The Impact of Stress on Body Function: A Review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Sawaki, J.; Tokuda, N.; Tanaka, H.; Sawai, H.; Takeshima, Y.; Shibahara, H.; Shima, M. Paternal Occupational Exposure to Chemicals and Secondary Sex Ratio: Results from the Japan Environment and Children’s Study. Lancet Planet. Health 2019, 3, e529–e538. [Google Scholar] [CrossRef] [PubMed]
- Chahnazarian, A. Determinants of the Sex Ratio at Birth: Review of Recent Literature. Soc. Biol. 1988, 35, 214–235. [Google Scholar] [CrossRef]
- James, W.H. Proximate Causes of the Variation of the Human Sex Ratio at Birth. Early Hum. Dev. 2015, 91, 795–799. [Google Scholar] [CrossRef]
- James, W.H. Further Support for the Hypothesis That Parental Hormone Levels around the Time of Conception Are Associated with Human Sex Ratios at Birth. J. Biosoc. Sci. 2008, 40, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.C.; Nesse, R.M.; Hofferth, S. The Trivers–Willard Hypothesis of Parental Investment: No Effect in the Contemporary United States. Evol. Hum. Behav. 2001, 22, 343–360. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural Selection of Parental Ability to Vary the Sex Ratio of Offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef]
- Popkin, B.M. Global Nutrition Dynamics: The World Is Shifting Rapidly toward a Diet Linked with Noncommunicable Diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of Saturated and Trans Unsaturated Fatty Acids and Risk of All Cause Mortality, Cardiovascular Disease, and Type 2 Diabetes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Grimm, K.M.; Livingston, K.A.; Brokman, A.M.; Lamberson, W.E.; Roberts, R.M. Striking Variation in the Sex Ratio of Pups Born to Mice According to Whether Maternal Diet Is High in Fat or Carbohydrate. Proc. Natl. Acad. Sci. USA 2003, 100, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Chaudhary, I.; Jain, M.; Pandey, S. Sex Ratio Trajectory in Mouse. Reprod. Biol. 2021, 21, 100514. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Spate, L.D.; Green, M.P.; Roberts, R.M. Effects of D-Glucose Concentration, D-Fructose, and Inhibitors of Enzymes of the Pentose Phosphate Pathway on the Development and Sex Ratio of Bovine Blastocysts. Mol. Reprod. Dev. 2005, 72, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Kimura, K.; Hashimoto, S.; Ohta, M.; Tominaga, K.; Minami, N. Role of G6PD Activity on Sex Ratio and Developmental Competence of Bovine Embryos under Oxidative Stress. J. Reprod. Dev. 2002, 48, 447–453. [Google Scholar] [CrossRef]
- Filosa, S.; Fico, A.; Paglialunga, F.; Balestrieri, M.; Crooke, A.; Verde, P.; Abrescia, P.; Bautista, J.M.; Martini, G. Failure to Increase Glucose Consumption through the Pentose-Phosphate Pathway Results in the Death of Glucose-6-Phosphate Dehydrogenase Gene-Deleted Mouse Embryonic Stem Cells Subjected to Oxidative Stress. Biochem. J. 2003, 370, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Akamatsu, S.; Minami, N.; Yamada, M. Allopurinol, an Inhibitor of Xanthine Oxidase, Improves the Development of IVM/IVF Bovine Embryos (>4 Cell) in Vitro under Certain Culture Conditions. Theriogenology 1999, 51, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Saran, M. To What End Does Nature Produce Superoxide? NADPH Oxidase as an Autocrine Modifier of Membrane Phospholipids Generating Paracrine Lipid Messengers. Free Radic. Res. 2003, 37, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, D.; John, D.; Kiessling, A.A. Hypoxanthine Causes a 2-Cell Block in Random-Bred Mouse Embryos. Biol. Reprod. 1987, 37, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rieger, D. Relationships between Energy Metabolism and Development of Early Mammalian Embryos. Theriogenology 1992, 37, 75–93. [Google Scholar] [CrossRef]
- Salvemini, F.; Franzé, A.; Iervolino, A.; Filosa, S.; Salzano, S.; Ursini, M.V. Enhanced Glutathione Levels and Oxidoresistance Mediated by Increased Glucose-6-Phosphate Dehydrogenase Expression. J. Biol. Chem. 1999, 274, 2750–2757. [Google Scholar] [CrossRef]
- Babior, B.M. Phagocytes and Oxidative Stress. Am. J. Med. 2000, 109, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.Z.; Lemons, P.R.; Bateman, P.W.; Bennett, N.C. Experimental Alteration of Litter Sex Ratios in a Mammal. Proc. R. Soc. B Biol. Sci. 2008, 275, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Burén, J.; Liu, H.-X.; Jensen, J.; Eriksson, J.W. Dexamethasone Impairs Insulin Signalling and Glucose Transport by Depletion of Insulin Receptor Substrate-1, Phosphatidylinositol 3-Kinase and Protein Kinase B in Primary Cultured Rat Adipocytes. Eur. J. Endocrinol. 2002, 146, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Long, S.; Green, C.; Gardiner, S.M.; Craigon, J.; Gardner, D.S. Maternal Fructose and/or Salt Intake and Reproductive Outcome in the Rat: Effects on Growth, Fertility, Sex Ratio, and Birth Order1. Biol. Reprod. 2013, 89, 51. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Li, D.; Bay, J.L. Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region. Nutrients 2022, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational Weight Gain across Continents and Ethnicity: Systematic Review and Meta-Analysis of Maternal and Infant Outcomes in More than One Million Women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Meikle, D.B.; Thornton, M.W. Premating and Gestational Effects of Maternal Nutrition on Secondary Sex Ratio in House Mice. Reproduction 1995, 105, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.P.W.; Crawford, M.A. Maternal Nutrition and the Sex Ratio at Birth. Nature 1974, 252, 297–298. [Google Scholar] [CrossRef]
- Labov, J.B.; William Huck, U.; Vaswani, P.; Lisk, R.D. Sex Ratio Manipulation and Decreased Growth of Male Offspring of Undernourished Golden Hamsters (Mesocricetus auratus). Behav. Ecol. Sociobiol. 1986, 18, 241–249. [Google Scholar] [CrossRef]
- Wright, S.L.; Crawford, C.B.; Anderson, J.L. Allocation of Reproductive Effort in Mus domesticus: Responses of Offspring Sex Ratio and Quality to Social Density and Food Availability. Behav. Ecol. Sociobiol. 1988, 23, 357–365. [Google Scholar] [CrossRef]
- Bulik, C.M.; Von Holle, A.; Gendall, K.; Lie, K.K.; Hoffman, E.; Mo, X.; Torgersen, L.; Reichborn-Kjennerud, T. Maternal Eating Disorders Influence Sex Ratio at Birth. Acta Obstet. Gynecol. Scand. 2008, 87, 979–981. [Google Scholar] [CrossRef]
- Mathews, F.; Johnson, P.J.; Neil, A. You Are What Your Mother Eats: Evidence for Maternal Preconception Diet Influencing Foetal Sex in Humans. Proc. R. Soc. B Biol. Sci. 2008, 275, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.S.; Lumey, L.H. Maternal Preconception Diet and the Sex Ratio. Hum. Biol. 2010, 82, 103–107. [Google Scholar] [CrossRef]
- Stein, A.D.; Zybert, P.A.; Lumey, L.H. Acute Undernutrition Is Not Associated with Excess of Females at Birth in Humans: The Dutch Hunger Winter. Proc. R. Soc. B Biol. Sci. 2004, 271, S138–S141. [Google Scholar] [CrossRef]
- Song, S. Does Famine Influence Sex Ratio at Birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc. Biol. Sci. 2012, 279, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Mucci, N.; Giorgi, G.; Roncaioli, M.; Fiz Perez, J.; Arcangeli, G. The Correlation between Stress and Economic Crisis: A Systematic Review. Neuropsychiatr. Dis. Treat. 2016, 12, 983–993. [Google Scholar] [CrossRef]
- Riis, I. The Secondary Sex Ratio in Israel: 1980–1995. Soc. Biol. 1999, 46, 33–46. [Google Scholar] [CrossRef]
- Stuckler, D.; Basu, S.; Suhrcke, M.; Coutts, A.; McKee, M. The Public Health Effect of Economic Crises and Alternative Policy Responses in Europe: An Empirical Analysis. Lancet 2009, 374, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Jetten, J.; Mols, F.; Healy, N.; Spears, R. “Fear of Falling”: Economic Instability Enhances Collective Angst among Societies’ Wealthy Class. J. Soc. Issues 2017, 73, 61–79. [Google Scholar] [CrossRef]
- Victor, G. The Japanese Decline in Secondary Sex Ratio Correlates with Percentage Change in GDP/Annum. Int. J. Trop. Dis. Health 2015, 5, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Grech, V. The Male to Female Ratio at Birth in the Republic of Ireland and Northern Ireland: Influence of Societal Stress. Ulster Med. J. 2015, 84, 157–160. [Google Scholar] [PubMed]
- Daly, M.; Boyce, C.; Wood, A. A Social Rank Explanation of How Money Influences Health. Health Psychol. 2015, 34, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Kim, I.-H.; Khang, Y.-H. [Trends in sex ratio at birth according to parental social positions: Results from vital statistics birth, 1981–2004 in Korea]. J. Prev. Med. Public Health Yebang Uihakhoe Chi 2009, 42, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Fieder, M.; Seidler, H. Ownership of Dwelling Affects the Sex Ratio at Birth in Uganda. PLoS ONE 2012, 7, e51463. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Matthews, S.G. Glucocorticoids and Sex-Dependent Development of Brain Glucocorticoid and Mineralocorticoid Receptors. Endocrinology 2003, 144, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- James, W.H. Hypothesis: High Levels of Maternal Adrenal Androgens Are a Major Cause of Miscarriage and Other Forms of Reproductive Suboptimality. J. Theor. Biol. 2015, 364, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Grant, V.J.; Yang, S. Achieving Women and Declining Sex Ratios. Hum. Biol. 2003, 75, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Grant, V.J. Maternal Dominance and the Conception of Sons. Br. J. Med. Psychol. 1994, 67, 343–351. [Google Scholar] [CrossRef]
- Grant, V.J. The Maternal Dominance Hypothesis: Questioning Trivers and Willard. Evol. Psychol. 2003, 1, 147470490300100100. [Google Scholar] [CrossRef]
- Almond, D.; Edlund, L. Trivers–Willard at Birth and One Year: Evidence from US Natality Data 1983–2001. Proc. R. Soc. B Biol. Sci. 2007, 274, 2491–2496. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative Stress and Male Infertility: Current Knowledge of Pathophysiology and Role of Antioxidant Therapy in Disease Management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, M.S.; Goldman-Mellor, S.J.; Anderson, E.S.; LeWinn, K.Z.; Saxton, K.B.; Shumway, M.; Catalano, R. An Exploration of Secondary Sex Ratios among Women Diagnosed with Anxiety Disorders. Hum. Reprod. 2010, 25, 2084–2091. [Google Scholar] [CrossRef]
- Chason, R.J.; McLain, A.C.; Sundaram, R.; Chen, Z.; Segars, J.H.; Pyper, C.; Louis, G.M.B. Preconception Stress and the Secondary Sex Ratio: A Prospective Cohort Study. Fertil. Steril. 2012, 98, 937–941. [Google Scholar] [CrossRef]
- Bae, J.; Lynch, C.D.; Kim, S.; Sundaram, R.; Sapra, K.J.; Buck Louis, G.M. Preconception Stress and the Secondary Sex Ratio in a Population-Based Preconception Cohort. Fertil. Steril. 2017, 107, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; McCormack, C.A.; Webster, R.; Pinto, A.; Lee, S.; Feng, T.; Krakovsky, H.S.; O’Grady, S.M.; Tycko, B.; Champagne, F.A.; et al. Maternal Prenatal Stress Phenotypes Associate with Fetal Neurodevelopment and Birth Outcomes. Proc. Natl. Acad. Sci. USA 2019, 116, 23996–24005. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, S.; Chen, Z.; Eisenberg, M.; Buck Louis, G. Human Semen Quality and the Secondary Sex Ratio. Asian J. Androl. 2017, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Murthy, L.; Hwang, K.; Lamb, D.J.; Lipshultz, L.I. Sperm Counts and Sperm Sex Ratio in Male Infertility Patients. Asian J. Androl. 2012, 14, 683–686. [Google Scholar] [CrossRef]
- Sadeu, J.C.; Hughes, C.L.; Agarwal, S.; Foster, W.G. Alcohol, Drugs, Caffeine, Tobacco, and Environmental Contaminant Exposure: Reproductive Health Consequences and Clinical Implications. Crit. Rev. Toxicol. 2010, 40, 633–652. [Google Scholar] [CrossRef]
- Beratis, N.G.; Asimacopoulou, A.; Varvarigou, A. Association of Secondary Sex Ratio with Smoking and Parity. Fertil. Steril. 2008, 89, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Oniya, O.; Neves, K.; Ahmed, B.; Konje, J.C. A Review of the Reproductive Consequences of Consanguinity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 232, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hamamy, H. Consanguineous Marriages. J. Community Genet. 2012, 3, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bittles, A.H.; Black, M.L. Evolution in Health and Medicine Sackler Colloquium: Consanguinity, Human Evolution, and Complex Diseases. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S1), 1779–1786. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, H. Consanguineous Marriages in India: Prevalence and Determinants. J. Health Manag. 2021, 23, 631–648. [Google Scholar] [CrossRef]
- Bittles, A.H.; Devi, A.R.; Rao, N.A. Consanguinity, Twinning and Secondary Sex Ratio in the Population of Karnataka, South India. Ann. Hum. Biol. 1988, 15, 455–460. [Google Scholar] [CrossRef]
- George, S.M. Millions of Missing Girls: From Fetal Sexing to High Technology Sex Selection in India. Prenat. Diagn. 2006, 26, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, T.; Lu, L.; Xing, Z.W. The Consequences of Son Preference and Sex-Selective Abortion in China and Other Asian Countries. CMAJ Can. Med. Assoc. J. 2011, 183, 1374–1377. [Google Scholar] [CrossRef]
- Auger, N.; Daniel, M.; Moore, S. Sex Ratio Patterns According to Asian Ethnicity in Québec, 1981–2004. Eur. J. Epidemiol. 2009, 24, 17–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermitzakis, I.; Theotokis, P.; Axarloglou, E.; Delilampou, E.; Miliaras, D.; Meditskou, S.; Manthou, M.E. The Impact of Lifestyle on the Secondary Sex Ratio: A Review. Life 2024, 14, 662. https://doi.org/10.3390/life14060662
Dermitzakis I, Theotokis P, Axarloglou E, Delilampou E, Miliaras D, Meditskou S, Manthou ME. The Impact of Lifestyle on the Secondary Sex Ratio: A Review. Life. 2024; 14(6):662. https://doi.org/10.3390/life14060662
Chicago/Turabian StyleDermitzakis, Iasonas, Paschalis Theotokis, Evangelos Axarloglou, Efthymia Delilampou, Dimosthenis Miliaras, Soultana Meditskou, and Maria Eleni Manthou. 2024. "The Impact of Lifestyle on the Secondary Sex Ratio: A Review" Life 14, no. 6: 662. https://doi.org/10.3390/life14060662
APA StyleDermitzakis, I., Theotokis, P., Axarloglou, E., Delilampou, E., Miliaras, D., Meditskou, S., & Manthou, M. E. (2024). The Impact of Lifestyle on the Secondary Sex Ratio: A Review. Life, 14(6), 662. https://doi.org/10.3390/life14060662









