The In Vitro Antioxidant and Anti-Inflammatory Activities of Selected Australian Seagrasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
2.1.2. Solvents, Reagents and Consumables
2.2. Extraction and Isolation
2.2.1. General Procedures
HPLC
Structural Elucidation by NMR Spectroscopy
Mass Determination by Mass Spectrometry
2.2.2. Extract Preparation for Initial Screening
2.2.3. Large-Scale Preparation of Z. muelleri Aerial and Root Extracts
2.2.4. Purification of Z. muelleri Aerial Extract
2.3. Biological Screening
2.3.1. Radical Scavenging Antioxidant Assay
2.3.2. Anti-Inflammatory Activity and Quantification
PBMC Collection and Culture Conditions
Sample Treatment
Determination of Cell Viability
Quantification of Proinflammatory Cytokines
3. Results
3.1. DPPH Radical Scavenging Activity of Seagrasses
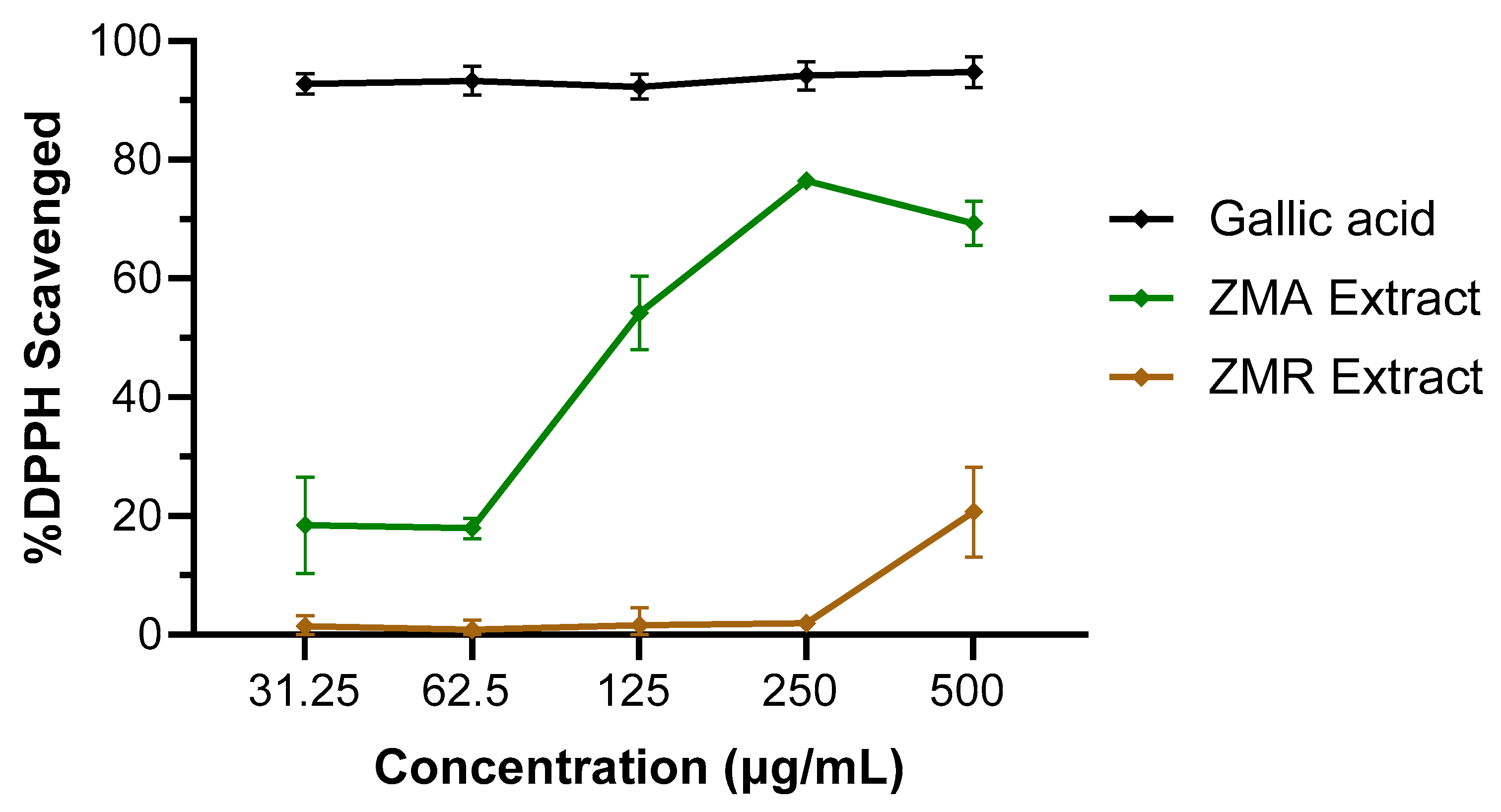
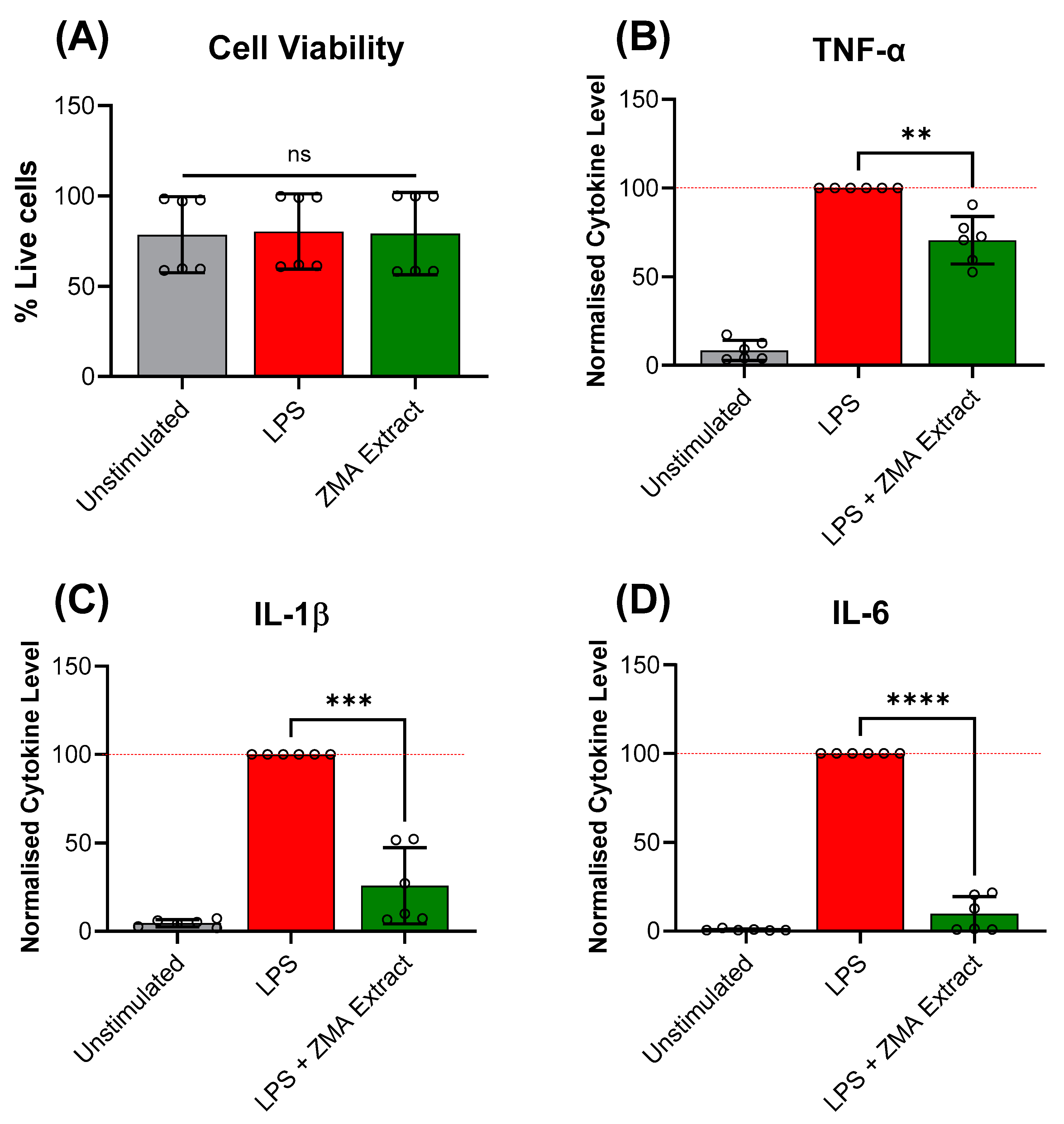
3.2. In-Depth Investigation of Z. muelleri Aerial and Root Parts
3.3. Bioactive Compounds Isolated Using HPLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation; StatPearls, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Gm Naidu, V.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–147. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and inflammation: Possible effects on immunity, chronic diseases, and life span. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I. Functional foods for health: The interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.S.; Mahmoud, A.-B.S.E.-D.; EL-Swaify, Z.A.; Salah El-Din, R.A. A comparative Evaluation of Phytochemical and Antimicrobial Properties of Selected Aquatic and Terrestrial Halophyte Plants Growing in Egypt. Int. J. Theor. Appl. Res. 2023, 2, 169–182. [Google Scholar] [CrossRef]
- Girija, K.; Parthiban, C.; Hemalatha, A.; Saranya, C.; Anantharaman, P. Evaluation of antioxidant activities and preliminary phytochemical analysis of seagrasses Halodule pinifolia, Halophila ovalis and Syringodium isoetifolium. J. Phytochem. 2013, 114, 181–187. [Google Scholar]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Iyapparaj, P.; Thangaradjou, T.; Anantharaman, P. In vitro antibacterial, cytotoxicity and haemolytic activities and phytochemical analysis of seagrasses from the Gulf of Mannar, South India. Food Chem. 2013, 136, 1484–1489. [Google Scholar] [CrossRef]
- Jafriati, J.; Jumadi, O.; Hatta, M.; Natzir, R.; Budu, B.; Eddyman, W.; Husain, D.; Ahmad, A.; Sjahril, R.; Dwiyanti, R. Analysis of phythology components and potentials of antioxidant activities of Thalassia hemprichii extract. Int. Med. J. 2019, 24, 145–152. [Google Scholar]
- McMillan, C. The condensed tannins (proanthocyanidins) in seagrasses. Aquat. Bot. 1984, 20, 351–357. [Google Scholar] [CrossRef]
- McMillan, C.; Williams, S.; Escobar, L.; Zapata, O. Isozymes, secondary compounds and experimental cultures of Australian seagrasses in Halophila, Halodule, Zostera, Amphibolis and Posidonia. Aust. J. Bot. 1981, 29, 247–260. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Gono, C.M.P.; Ahmadi, P.; Hertiani, T.; Septiana, E.; Putra, M.Y.; Chianese, G. A Comprehensive Update on the Bioactive Compounds from Seagrasses. Mar. Drugs 2022, 20, 406. [Google Scholar] [CrossRef]
- de la Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses—An example from tropical East Africa. Ocean Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am. Eur. J. Agric. Environ. Sci 2014, 14, 685–697. [Google Scholar]
- Zulkifli, L.; Muksin, Y.; Hartanto, P.; Desimarlina, Y.; Idrus, A.; Syukur, A. Phytochemical profiles and ethnomedicine preliminary studies on seagrass species in the Southern Coast of Lombok Island Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012102. [Google Scholar] [CrossRef]
- Noor, N.; Febriani, D.; Ali, M. Seagrass of Enhalus Acoroides as a Traditional Body Scrubs in Preventing Malarial Bites by Pahawang Island Community in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1012, 012037. [Google Scholar] [CrossRef]
- Newmaster, A.; Berg, K.; Ragupathy, S.; Palanisamy, M.; Sambandan, K.; Newmaster, S. Local knowledge and conservation of seagrasses in the Tamil Nadu State of India. J. Ethnobiol. Ethnomedicine 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Waycott, M.; Longstaff, B.J.; Mellors, J. Seagrass population dynamics and water quality in the Great Barrier Reef region: A review and future research directions. Mar. Pollut. Bull. 2005, 51, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Bio. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Ritmejerytė, E.; Ryan, R.Y.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem.-Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Suga, K.; Sato, K.; Sato, Y. A Strained Disilane-Promoted Carboxylation of Organic Halides with CO2 under Transition-Metal-Free Conditions. Org. Lett. 2015, 17, 5276–5279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Zhao, B.T.; Le, D.D.; Kim, K.Y.; Kim, Y.H.; Yoon, Y.H.; Ko, J.Y.; Woo, K.S.; Woo, M.H. Phenolic constituents and their anti-inflammatory activity from Echinochloa utilis grains. Nat. Prod. Sci. 2016, 22, 140–145. [Google Scholar] [CrossRef]
- Ersoz, T.; Harput, Ü.Ş.; Saracoglu, İ.; Calis, İ.; Ogihara, Y. Phenolic compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 581–588. [Google Scholar]
- Yeshi, K.; Yangdon, P.; Kashyap, S.; Wangchuk, P. Antioxidant activity and the polyphenolic and flavonoid contents of five high altitude medicinal plants used in Bhutanese Sowa rigpa medicine. J. Biol. Act. Prod. Nat. 2017, 7, 18–26. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Miles, K.; Crayn, D.; Liddell, M.; Wangchuk, P. Antioxidant and Anti-Inflammatory Activities of Endemic Plants of the Australian Wet Tropics. Plants 2022, 11, 2519. [Google Scholar] [CrossRef]
- Gempo, N.; Yeshi, K.; Crayn, D.; Wangchuk, P. Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities. Plants 2024, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, P.; Dilipan, E.; Thangaradjou, T.; Papenbrock, J. Bioactive natural products from marine angiosperms: Abundance and functions. Nat. Prod. Bioprospect. 2013, 3, 129–136. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Muñoz, K.; Ortega, M.J.; Brun, F.G.; Zubía, E. Rosmarinic acid and flavonoids of the seagrass Zostera noltei: New aspects on their quantification and their correlation with sunlight exposure. Plants 2023, 12, 4078. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mahomoodally, M.F.; Sadeer, N.B.; Seok, P.G.; Zengin, G.; Palaniveloo, K.; Khalil, A.A.; Rauf, A.; Rengasamy, K.R. Nutritional and bioactive potential of seagrasses: A review. S. Afr. J. Bot. 2021, 137, 216–227. [Google Scholar] [CrossRef]
- Santoso, J.; Anwariyah, S.; Rumiantin, R.O.; Putri, A.P.; Ukhty, N.; Yoshie-Stark, Y. Phenol content, antioxidant activity and fibers profile of four tropical seagrasses from Indonesia. J. Coast. Dev. 2012, 15, 189–196. [Google Scholar]
- Ghandourah, M.; Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M. Fatty acids and other Chemical compositions of some Seagrasses Collected from the Saudi Red Sea with potential of antioxidant and Anticancer agents. Thalassas 2021, 37, 13–22. [Google Scholar] [CrossRef]
- Ramah, S.; Etwarysing, L.; Auckloo, N.; Gopeechund, A.; Bhagooli, R.; Bahorun, T. Prophylactic antioxidants and phenolics of seagrass and seaweed species: A seasonal variation study in a Southern Indian Ocean Island, Mauritius. Internet J. Med. Update 2014, 9, 27–37. [Google Scholar]
- Bragt, P.C.; Bonta, I.L. Oxidant stress during inflammation: Anti-inflammatory effects of antioxidants. Agents Actions 1980, 10, 536–539. [Google Scholar] [CrossRef]
- ElAttar, Y.; Mourad, B.; Alngomy, H.A.; Shams El Deen, A.; Ismail, M. Study of interleukin-1 beta expression in acne vulgaris and acne scars. J. Cosmet. Dermatol. 2022, 21, 4864–4870. [Google Scholar] [CrossRef]
- Triatmakusuma, Y.; Praharsini, I.G.A.A.; Darmaputra, I.G.N.; Winaya, K.K.; Karna, N.L.P.R.V.; Puspawati, N.M.D. Serum Interleukin-6 Levels are Positively Correlated with the Severity of Acne Vulgaris. J. Medihealtico 2024, 5, 158–166. [Google Scholar]
- Younis, S.; Javed, Q. The interleukin-6 and interleukin-1A gene promoter polymorphism is associated with the pathogenesis of acne vulgaris. Arch. Dermatol. Res. 2015, 307, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.; Melvin, S.; Connolly, R.; Pearson, R.; Brown, C. Metabolomic indicators for low-light stress in seagrass. Ecol. Indic. 2020, 114, 106316. [Google Scholar] [CrossRef]
- Kuzhiumparambil, U.; Kumar, M.; Ralph, P. Gas and liquid chromatography-mass spectrometry-based metabolic profiling of marine angiosperm Zostera muelleri (Alismatales, Zosteraceae). In Systems Biology of Marine Ecosystems; Kumar, M., Ralph, P., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 189–203. [Google Scholar]
- Volkman, J.; Gllan, F.; Johns, R.; Eglinton, G. Sources of neutral lipids in a temperate intertidal sediment. Geochim. Cosmochim. Acta 1981, 45, 1817–1828. [Google Scholar] [CrossRef]
- Volkman, J.; Johns, R.; Gillan, F.; Perry, G.; Bavor Jr, H. Microbial lipids of an intertidal sediment—I. Fatty acids and hydrocarbons. Geochim. Cosmochim. Acta 1980, 44, 1133–1143. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.Y.; Sim, G.Y.; Ahn, J.-H. Synthesis of 4-hydroxybenzoic acid derivatives in Escherichia coli. J. Agric. Food Chem. 2020, 68, 9743–9749. [Google Scholar] [CrossRef] [PubMed]
- Danaraj, J.; Mariasingarayan, Y.; Ayyappan, S.; Karuppiah, V. Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb. Pathog. 2020, 147, 104392. [Google Scholar] [CrossRef]
- Dumay, O.; Costa, J.; Desjobert, J.-M.; Pergent, G. Variations in the concentration of phenolic compounds in the seagrass Posidonia oceanica under conditions of competition. Phytochemistry 2004, 65, 3211–3220. [Google Scholar]
- Susilo, B.; Oktavianty, O.; Rahayu, F.; Handayani, M.L.W.; Rohim, A. Potential transformation of seagrass (Syringodium isoetifolium) into a bioactive food ingredient using different extraction techniques. F1000Res 2023, 12, 1078. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T. Thalassiolin D: A new flavone O-glucoside Sulphate from the seagrass Thalassia hemprichii. Nat. Prod. Res. 2017, 31, 2369–2374. [Google Scholar] [CrossRef]
- Regalado, E.L.; Menendez, R.; Valdés, O.; Morales, R.A.; Laguna, A.; Thomas, O.P.; Hernandez, Y.; Nogueiras, C.; Kijjoa, A. Phytochemical analysis and antioxidant capacity of BM-21, a bioactive extract rich in polyphenolic metabolites from the sea grass Thalassia testudinum. Nat. Prod. Commun. 2012, 7, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sinyeue, C.; Matsui, M.; Oelgemöller, M.; Bregier, F.; Chaleix, V.; Sol, V.; Lebouvier, N. Synthesis and investigation of flavanone derivatives as potential new anti-inflammatory agents. Molecules 2022, 27, 1781. [Google Scholar] [CrossRef]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P.; Falcone Ferreyra, M.L. Apigenin produced by maize flavone synthase I and II protects plants against UV-B-induced damage. Plant Cell Environ. 2019, 42, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Sen, P.; Sahu, P.K.; Haldar, R.; Sahu, K.; Prasad, P.; Roy, A. Apigenin naturally occurring flavonoids: Occurrence and bioactivity. Pharm. Biosci. J. 2016, 4, 56–68. [Google Scholar]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A therapeutic agent for treatment of skin inflammatory diseases and cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef] [PubMed]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A role for NF-κB–dependent gene transactivation in sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Sundarapandian, M. Antidiabetic Activity of Methanolic Extract of Halodule uninervis in Streptozotocin-Induced Diabetic Mice. J. Pharm. Sci. Res. 2017, 9, 1864–1868. [Google Scholar]
- Styshova, O.N.; Popov, A.M.; Artyukov, A.A.; Klimovich, A.A. Main constituents of polyphenol complex from seagrasses of the genus Zostera, their antidiabetic properties and mechanisms of action. Exp. Ther. Med. 2017, 13, 1651–1659. [Google Scholar] [CrossRef]
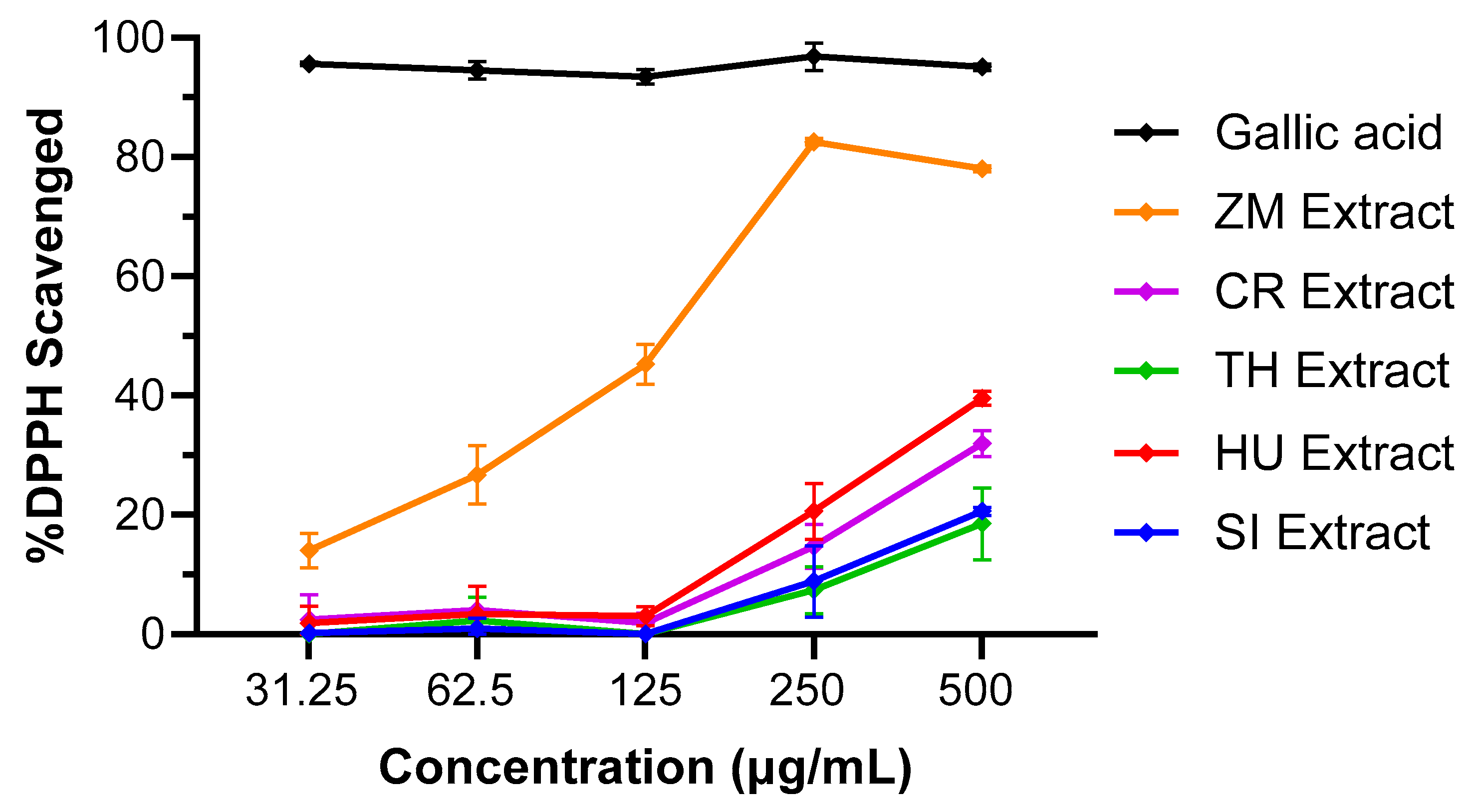
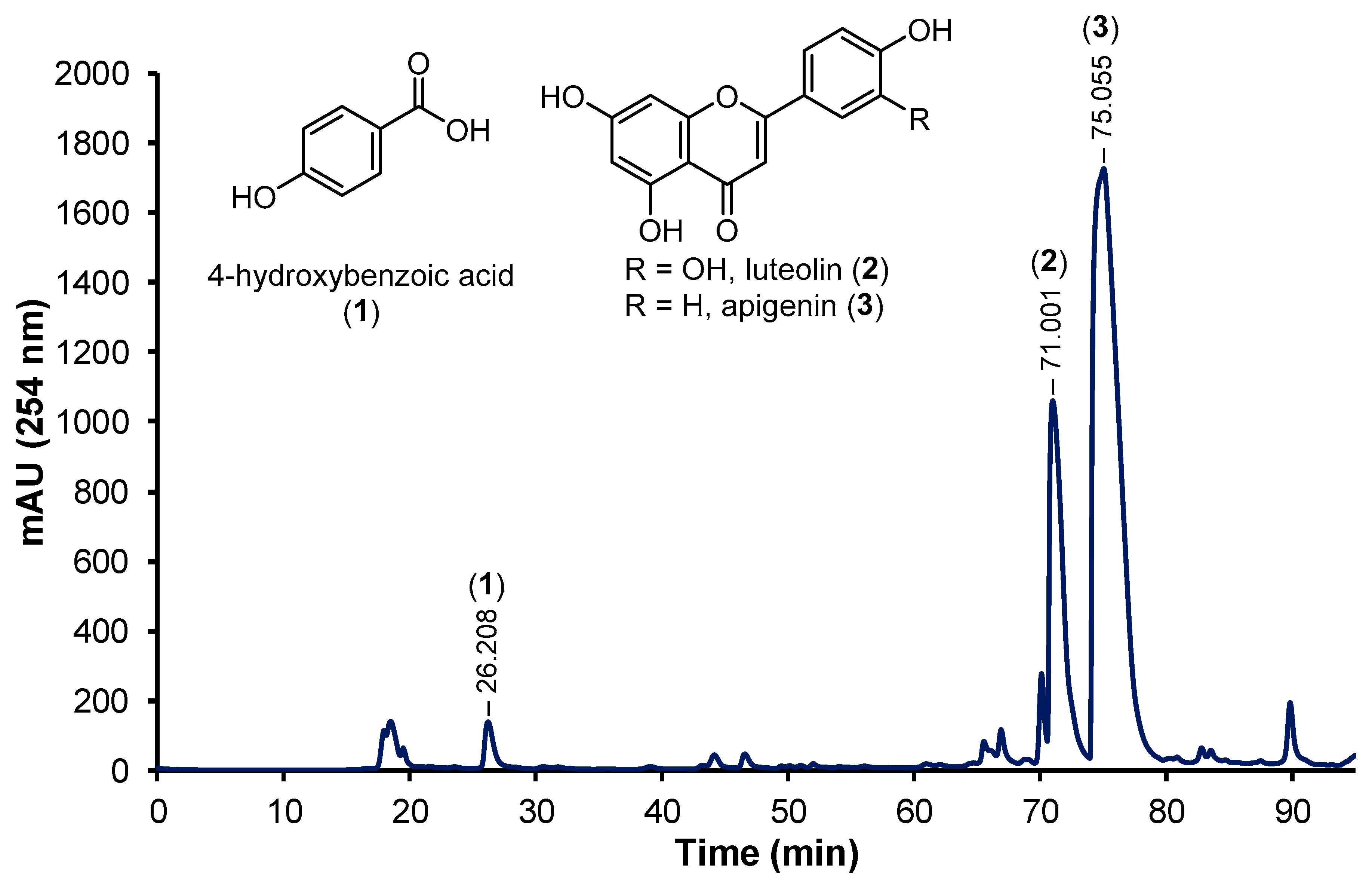
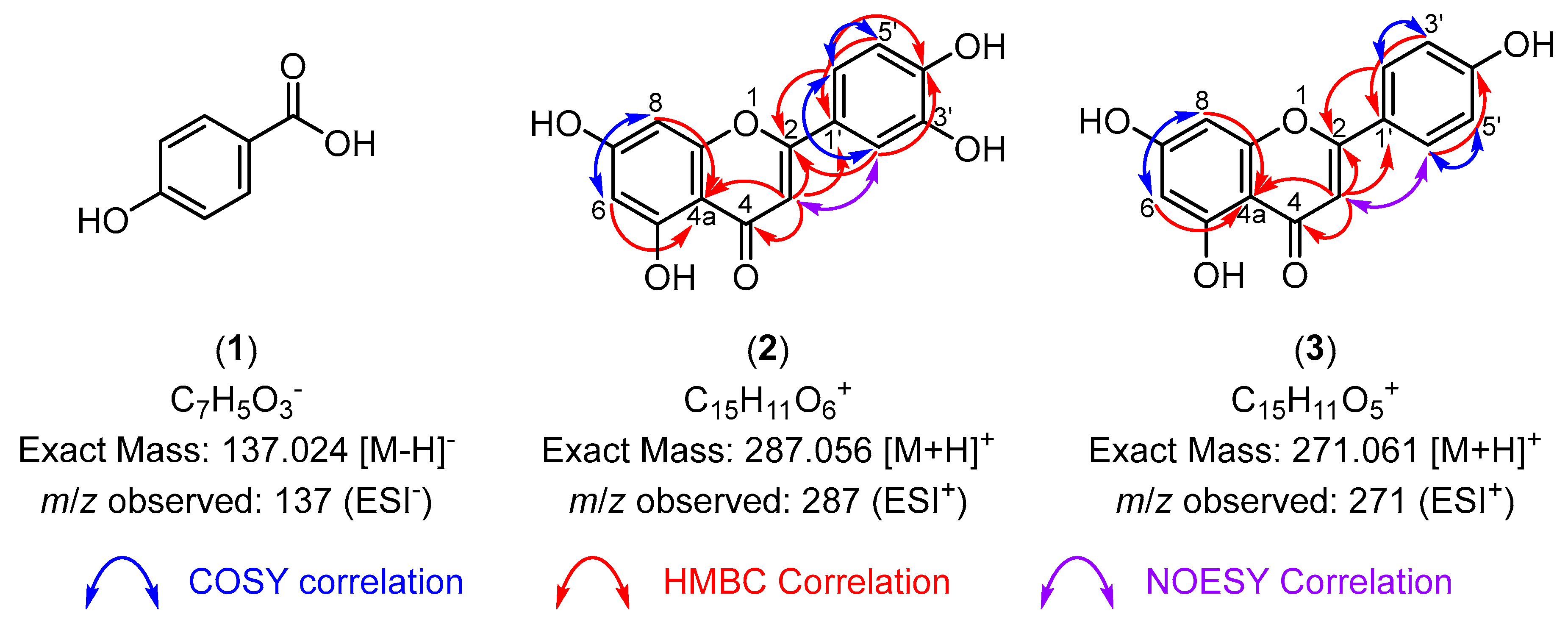
| Sample | %DPPH Radical Scavenging Activity *† | IC50 (µg/mL) |
|---|---|---|
| Gallic acid | 95.1 ± 0.5% | <31.25 |
| ZM Extract | 78.0 ± 0.5% | 138 |
| CR Extract | 32.0 ± 2.2% | >500 |
| TH Extract | 18.5 ± 6.0% | >500 |
| HU Extract | 39.6 ± 1.2% | >500 |
| SI Extract | 20.6 ± 0.7% | >500 |
| Sample | %DPPH Radical Scavenging Activity *† | IC50 (µg/mL) | Normalised Cytokine Levels † | ||
|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-6 | |||
| Gallic acid | 94.8 ± 2.6% | <31.25 | – | – | – |
| ZMA Extract | 69.3 ± 3.7% | 119 | 70.5 ± 13.4 | 25.8 ± 21.6 | 9.6 ± 9.9 |
| ZMR Extract | 20.7 ± 7.5% | >500 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perry, M.J.; Curic, M.; Scott, A.L.; Ritmejerytė, E.; Rahayu, D.U.C.; Keller, P.A.; Oelgemöller, M.; Yeshi, K.; Wangchuk, P. The In Vitro Antioxidant and Anti-Inflammatory Activities of Selected Australian Seagrasses. Life 2024, 14, 710. https://doi.org/10.3390/life14060710
Perry MJ, Curic M, Scott AL, Ritmejerytė E, Rahayu DUC, Keller PA, Oelgemöller M, Yeshi K, Wangchuk P. The In Vitro Antioxidant and Anti-Inflammatory Activities of Selected Australian Seagrasses. Life. 2024; 14(6):710. https://doi.org/10.3390/life14060710
Chicago/Turabian StylePerry, Matthew J., Mara Curic, Abigail L. Scott, Edita Ritmejerytė, Dyah U. C. Rahayu, Paul A. Keller, Michael Oelgemöller, Karma Yeshi, and Phurpa Wangchuk. 2024. "The In Vitro Antioxidant and Anti-Inflammatory Activities of Selected Australian Seagrasses" Life 14, no. 6: 710. https://doi.org/10.3390/life14060710








