An Update on Physiopathological Roles of Akt in the ReprodAKTive Mammalian Ovary
Abstract
:1. Introduction
2. PI3K/Akt and the Control of Follicle Activation and Growth
3. PI3K/Akt Role during Oocyte Maturation and Fertilization
4. PI3K/Akt Pathway in Reproductive Pathologies
4.1. Polycystic Ovary Syndrome
4.2. Premature Ovarian Failure
| Modulator | Level | Effect on PI3K/Akt Related Proteins | Reproductive Disease | Research Subjects | References |
|---|---|---|---|---|---|
| HMGB1 | Increased | ↓ GLUT4 ↓ pAkt | PCOS | Human | [89] |
| HOTAIR | Increased | ↓ Bcl2 ↑ BAX ↑ IGF1 ↑ pAkt | PCOS | Rat model | [84] |
| IL-4 | Increased | ↓ Bcl2 ↑ Caspase-3 ↑ Caspase-9 ↑ BAX ↑ pAkt | POF | Human | [98] |
| kisspeptin | Reduced | ↓ Bcl2 ↑ Caspase-3 ↑ BAX ↓ pAkt | PCOS | Human Rat model | [85] |
| miR-18b-5p | Reduced | ↑PTEN | PCOS | Human Rat model | [93] |
| miR-133a-3p | Increased | ↓ GLUT4 ↑ pGSK3β ↑ pFOXO1 ↓ pAkt | PCOS | Human | [88] |
| miR-190a-5p | Increased | ↓ PHLPP1 ↑ pFOXO3a ↑ pAkt | POF | Rat model | [99,100] |
| miR-497-3p | Increased | ↓ KLF4 ↓ Klotho ↓ pAkt | POF | Human | [101] |
| USP25 | Increased | ↓ GLUT4 ↓ IRS-1 | PCOS | Human Mouse model | [91] |
| Zinc | Reduced | ↓ pAkt ↓ pGSK3β | POF | Rat model | [97] |
4.3. Potential Treatments for Reproductive Pathologies Involving the PI3K/Akt Pathway
| Treatment | Effect on PI3K/Akt Related Proteins | Reproductive Disease | Research Subjects | References |
|---|---|---|---|---|
| Berberine | ↑ pAkt ↑ GLUT4 | PCOS | Rat model | [103] |
| ESC-sEVs | ↑ pAkt | POF | Mouse model | [106] |
| GH | ↑ IGF1 ↑ PI3K ↑ Akt ↑ Bcl-2 ↓ Caspase-3 ↓ Caspase-9 ↓ BAX | PCOS | Human | [85] |
| hucMSCEVs | ↓ PIK3R2 ↑ pAkt | POF | Human | [107,108] |
| Melatonin | ↑ SIRT1 ↑ pAkt | PCOS | Human | [105] |
| Resveratrol | ↑ pPI3K ↑ pAkt ↑ Bcl-2 ↓ Caspase-3 ↓ BAX | POF | Rat model | [109] |
| SeNPs and MET | ↑ PI3K ↑ Akt | PCOS | Rat model | [104] |
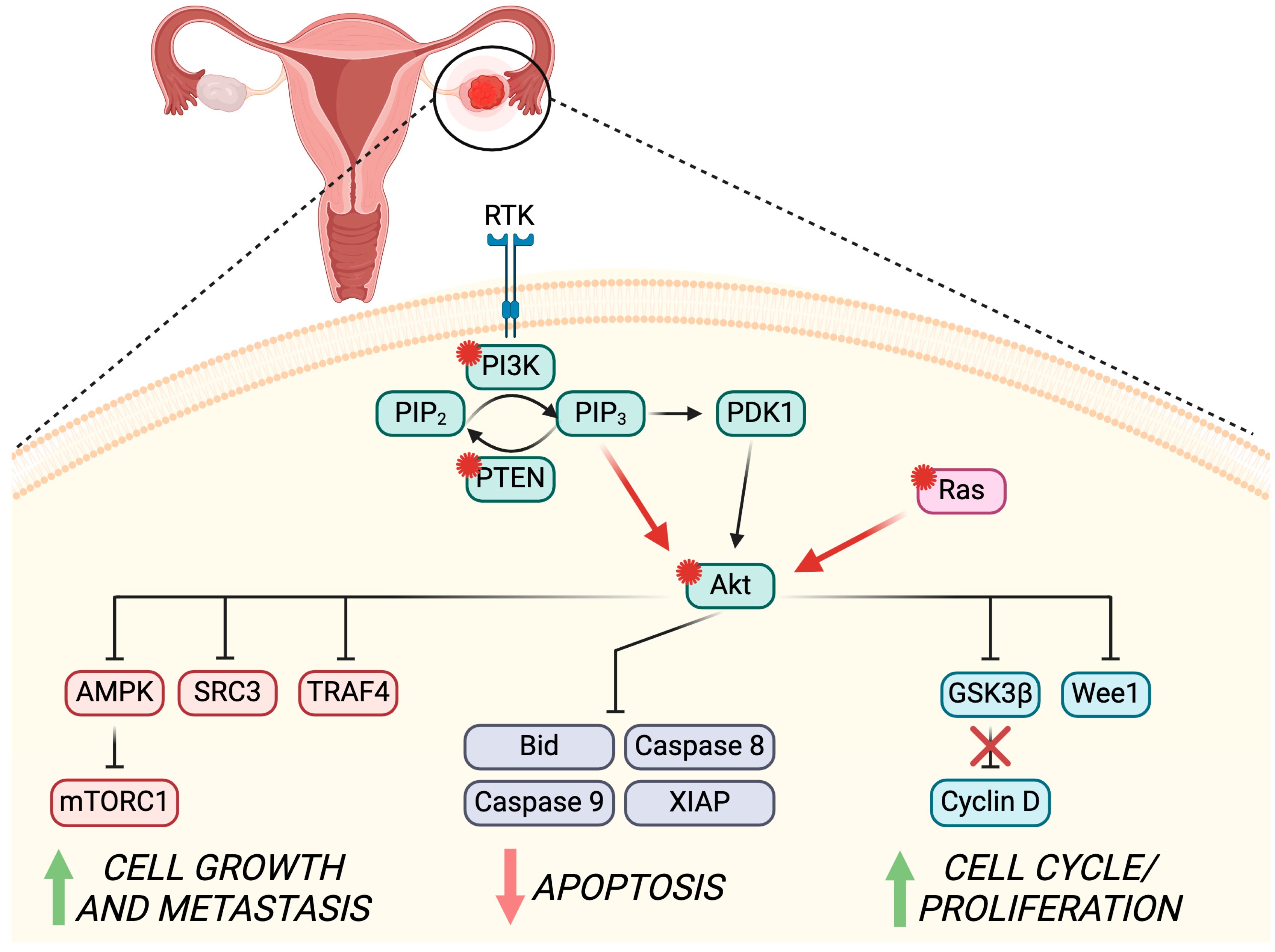
5. PI3K/Akt Pathway in Ovarian Cancer
5.1. PI3K/Akt Role in OC Pathogenesis
5.2. PI3K/Akt Role in OC Development
5.3. Possible Strategies to Counteract PI3K/Akt Role in OC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cecconi, S.; Mauro, A.; Cellini, V.; Patacchiola, F. The Role of Akt Signalling in the Mammalian Ovary. Int. J. Dev. Biol. 2012, 56, 809–817. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt Signalling in Health and Disease. Cell Signal 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Kalous, J.; Aleshkina, D.; Anger, M. A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development. Cells 2023, 12, 1830. [Google Scholar] [CrossRef]
- Günesdogan, U.; Surani, M.A. Developmental Competence for Primordial Germ Cell Fate. Curr. Top. Dev. Biol. 2016, 117, 471–496. [Google Scholar] [CrossRef]
- De Felici, M. Origin, Migration, and Proliferation of Human Primordial Germ Cells. In Oogenesis; Coticchio, G., Albertini, D.F., De Santis, L., Eds.; Springer: London, UK, 2013; pp. 19–37. ISBN 978-0-85729-826-3. [Google Scholar]
- De Felici, M.; Klinger, F.G. PI3K/PTEN/AKT Signaling Pathways in Germ Cell Development and Their Involvement in Germ Cell Tumors and Ovarian Dysfunctions. Int. J. Mol. Sci. 2021, 22, 9838. [Google Scholar] [CrossRef]
- Hackett, J.A.; Huang, Y.; Günesdogan, U.; Gretarsson, K.A.; Kobayashi, T.; Surani, M.A. Tracing the Transitions from Pluripotency to Germ Cell Fate with CRISPR Screening. Nat. Commun. 2018, 9, 4292. [Google Scholar] [CrossRef]
- Sekita, Y.; Nakamura, T.; Kimura, T. Reprogramming of Germ Cells into Pluripotency. World J. Stem Cells 2016, 8, 251–259. [Google Scholar] [CrossRef]
- Albamonte, M.I.; Albamonte, M.S.; Bou-Khair, R.M.; Zuccardi, L.; Vitullo, A.D. The Ovarian Germinal Reserve and Apoptosis-Related Proteins in the Infant and Adolescent Human Ovary. J. Ovarian Res. 2019, 12, 22. [Google Scholar] [CrossRef]
- Ikami, K.; Shoffner-Beck, S.; Weh, M.T.; Schnell, S.; Yoshida, S.; Miranda, E.A.D.; Ko, S.; Lei, L. Branched Germline Cysts and Female-Specific Cyst Fragmentation Facilitate Oocyte Determination in Mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2219683120. [Google Scholar] [CrossRef]
- Depmann, M.; Faddy, M.J.; Van der Schouw, Y.T.; Peeters, P.H.M.; Broer, S.L.; Kelsey, T.W.; Nelson, S.M.; Broekmans, F.J.M. The Relationship between Variation in Size of the Primordial Follicle Pool and Age at Natural Menopause. J. Clin. Endocrinol. Metab. 2015, 100, E845–E851. [Google Scholar] [CrossRef]
- Pelosi, E.; Forabosco, A.; Schlessinger, D. Genetics of the Ovarian Reserve. Front. Genet. 2015, 6, 308. [Google Scholar] [CrossRef]
- Iorio, R.; Castellucci, A.; Ventriglia, G.; Teoli, F.; Cellini, V.; Macchiarelli, G.; Cecconi, S. Ovarian Toxicity: From Environmental Exposure to Chemotherapy. Curr. Pharm. Des. 2014, 20, 5388–5397. [Google Scholar] [CrossRef]
- Aloisi, M.; Rossi, G.; Colafarina, S.; Guido, M.; Cecconi, S.; Poma, A.M.G. The Impact of Metal Nanoparticles on Female Reproductive System: Risks and Opportunities. Int. J. Environ. Res. Public. Health 2022, 19, 13748. [Google Scholar] [CrossRef]
- Panagiotou, E.M.; Ojasalo, V.; Damdimopoulou, P. Phthalates, Ovarian Function and Fertility in Adulthood. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101552. [Google Scholar] [CrossRef]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; Mclaughlin, E.A.; Sutherland, J.M. Advances in Human Primordial Follicle Activation and Premature Ovarian Insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef]
- Tobler, K.J.; Shoham, G.; Christianson, M.S.; Zhao, Y.; Leong, M.; Shoham, Z. Use of Anti-Mullerian Hormone for Testing Ovarian Reserve: A Survey of 796 Infertility Clinics Worldwide. J. Assist. Reprod. Genet. 2015, 32, 1441–1448. [Google Scholar] [CrossRef]
- Dai, Y.; Bo, Y.; Wang, P.; Xu, X.; Singh, M.; Jia, L.; Zhang, S.; Niu, S.; Cheng, K.; Liang, J.; et al. Asynchronous Embryonic Germ Cell Development Leads to a Heterogeneity of Postnatal Ovarian Follicle Activation and May Influence the Timing of Puberty Onset in Mice. BMC Biol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and Molecular Regulation of the Activation of Mammalian Primordial Follicles: Somatic Cells Initiate Follicle Activation in Adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Saatcioglu, H.D.; Cuevas, I.; Castrillon, D.H. Control of Oocyte Reawakening by Kit. PLoS Genet. 2016, 12, e1006215. [Google Scholar] [CrossRef]
- Ueda, S.; Mizuki, M.; Ikeda, H.; Tsujimura, T.; Matsumura, I.; Nakano, K.; Daino, H.; Honda, Z.-I.; Sonoyama, J.; Shibayama, H.; et al. Critical Roles of C-Kit Tyrosine Residues 567 and 719 in Stem Cell Factor-Induced Chemotaxis: Contribution of Src Family Kinase and PI3-Kinase on Calcium Mobilization and Cell Migration. Blood 2002, 99, 3342–3349. [Google Scholar] [CrossRef]
- Mi, X.; Jiao, W.; Yang, Y.; Qin, Y.; Chen, Z.J.; Zhao, S. HGF Secreted by Mesenchymal Stromal Cells Promotes Primordial Follicle Activation by Increasing the Activity of the PI3K-AKT Signaling Pathway. Stem Cell Rev. Rep. 2022, 18, 1834–1850. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol. Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/MTOR Signaling Pathways, Ovarian Dysfunction, and Infertility: An Update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef]
- De Felici, M.; Klinger, F.G. Programmed Cell Death in Mouse Primordial Germ Cells. Int. J. Dev. Biol. 2015, 59, 41–49. [Google Scholar] [CrossRef]
- Terren, C.; Nisolle, M.; Munaut, C. Pharmacological Inhibition of the PI3K/PTEN/Akt and MTOR Signalling Pathways Limits Follicle Activation Induced by Ovarian Cryopreservation and in Vitro Culture. J. Ovarian Res. 2021, 14, 95. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Q. Role of MTOR Signaling in Female Reproduction. Front. Endocrinol. 2019, 10, 692. [Google Scholar] [CrossRef]
- Gorre, N.; Adhikari, D.; Lindkvist, R.; Brännström, M.; Liu, K.; Shen, Y. MTORC1 Signaling in Oocytes Is Dispensable for the Survival of Primordial Follicles and for Female Fertility. PLoS ONE 2014, 9, e110491. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Miao, L.; Kollipara, R.; Horner, J.W.; DePinho, R.A. Suppression of Ovarian Follicle Activation in Mice by the Transcription Factor Foxo3a. Science 2003, 301, 215–218. [Google Scholar] [CrossRef]
- Albamonte, M.I.; Calabró, L.Y.; Albamonte, M.S.; Zuccardi, L.; Stella, I.; Halperin, J.; Vitullo, A.D. PTEN and FOXO3 Expression in the Prenatal and Postnatal Human Ovary. J. Assist. Reprod. Genet. 2020, 37, 1613–1622. [Google Scholar] [CrossRef]
- Tarnawa, E.D.; Baker, M.D.; Aloisio, G.M.; Carr, B.R.; Castrillon, D.H. Gonadal Expression of Foxo1, but Not Foxo3, Is Conserved in Diverse Mammalian Species. Biol. Reprod. 2013, 88, 103. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Liu, H.; Luo, L.L.; Qian, Y.S.; Fu, Y.C.; Sui, X.X.; Geng, Y.J.; Huang, D.N.; Gao, S.T.; Zhang, R.L. FOXO3a Is Involved in the Apoptosis of Naked Oocytes and Oocytes of Primordial Follicles from Neonatal Rat Ovaries. Biochem. Biophys. Res. Commun. 2009, 381, 722–727. [Google Scholar] [CrossRef]
- Reddy, P.; Shen, L.; Ren, C.; Boman, K.; Lundin, E.; Ottander, U.; Lindgren, P.; Liu, Y.X.; Sun, Q.Y.; Liu, K. Activation of Akt (PKB) and Suppression of FKHRL1 in Mouse and Rat Oocytes by Stem Cell Factor during Follicular Activation and Development. Dev. Biol. 2005, 281, 160–170. [Google Scholar] [CrossRef]
- Froment, P.; Bontoux, M.; Pisselet, C.; Monget, P.; Dupont, J. PTEN Expression in Ovine Granulosa Cells Increases during Terminal Follicular Growth. FEBS Lett. 2005, 579, 2376–2382. [Google Scholar] [CrossRef]
- Ding, W.; Wang, W.; Zhou, B.; Zhang, W.; Huang, P.; Shi, F.; Taya, K. Formation of Primordial Follicles and Immunolocalization of PTEN, PKB and FOXO3A Proteins in the Ovaries of Fetal and Neonatal Pigs. J. Reprod. Dev. 2010, 56, 162–168. [Google Scholar] [CrossRef]
- Makin, E.; Davenport, M. Teratomas. In Handbook of Pediatric Surgery; Springer International Publishing: Berlin/Heidelberg, Germany,, 2022; pp. 391–396. ISBN 9783030844677. [Google Scholar]
- McLaughlin, M.; Innell, H.L.; Anderson, R.A.; Telfer, E.E. Inhibition of Phosphatase and Tensin Homologue (PTEN) in Human Ovary in Vitro Results in Increased Activation of Primordial Follicles but Compromises Development of Growing Follicles. Mol. Hum. Reprod. 2014, 20, 736–744. [Google Scholar] [CrossRef]
- Grosbois, J.; Devos, M.; Demeestere, I. Implications of Nonphysiological Ovarian Primordial Follicle Activation for Fertility Preservation. Endocr. Rev. 2020, 41, bnaa020. [Google Scholar] [CrossRef]
- Canipari, R.; Cellini, V.; Cecconi, S. The Ovary Feels Fine When Paracrine and Autocrine Networks Cooperate with Gonadotropins in the Regulation of Folliculogenesis. Curr. Pharm. Des. 2012, 18, 245–255. [Google Scholar] [CrossRef]
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 Play Central Roles in the FSH Signaling Pathway to PI3K and AKT in Ovarian Granulosa Cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Li, J.; Zheng, N.; Xu, X.; Yang, J.; Xia, G.; Zhang, M. MAPK3/1 Participates in the Activation of Primordial Follicles through MTORC1-KITL Signaling. J. Cell Physiol. 2018, 233, 226–237. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective Mechanism of FSH against Oxidative Damage in Mouse Ovarian Granulosa Cells by Repressing Autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Yao, J.; Ma, Y.; Zhou, S.; Bao, T.; Mi, Y.; Zeng, W.; Li, J.; Zhang, C. Metformin Prevents Follicular Atresia in Aging Laying Chickens through Activation of PI3K/Akt and Calcium Signaling Pathways. Oxid. Med. Cell Longev. 2020, 2020, 3648040. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, L.; Tian, Y.; Wang, H.; Su, Y.; Feng, G.; Wang, C.; Lu, Z. Tyrosine Phosphatase SHP2 in Ovarian Granulosa Cells Balances Follicular Development by Inhibiting PI3K/AKT Signaling. J. Mol. Cell Biol. 2022, 14, mjac048. [Google Scholar] [CrossRef]
- Alcaráz, L.P.; Prellwitz, L.; Alves, G.; Souza-Fabjan, J.M.G.; Dias, A.J.B. Role of Phosphoinositide 3-Kinase/ Protein Kinase B/ Phosphatase and Tensin Homologue (PI3K/AKT/PTEN) Pathway Inhibitors during in Vitro Maturation of Mammalian Oocytes on in Vitro Embryo Production: A Systematic Review. Theriogenology 2022, 189, 42–52. [Google Scholar] [CrossRef]
- Shimada, M.; Idris Anas, M.-K.; Terada, T. Effects of Phosphatidylinositol 3-Kinase Inhibitors, Wortmannin and LY294002, on Germinal Vesicle Breakdown (GVBD) in Porcine Oocytes. J. Reprod. Dev. 1998, 44, 281–288. [Google Scholar] [CrossRef]
- Song, B.S.; Jeong, P.S.; Lee, J.H.; Lee, M.H.; Yang, H.J.; Choi, S.A.; Lee, H.Y.; Yoon, S.B.; Park, Y.H.; Jeong, K.J.; et al. The Effects of Kinase Modulation on in Vitro Maturation According to Different Cumulusoocyte Complex Morphologies. PLoS ONE 2018, 13, e0205495. [Google Scholar] [CrossRef]
- Anas, M.K.I.; Shimada, M.; Terada, T. Possible Role for Phosphatidylinositol 3-Kinase in Regulating Meiotic Maturation of Bovine Oocytes in Vitro. Theriogenology 1998, 50, 347–356. [Google Scholar] [CrossRef]
- Kalous, J.; Solc, P.; Baran, V.; Kubelka, M.; Schultz, R.M.; Motlik, J. PKB/AKT Is Involved in Resumption of Meiosis in Mouse Oocytes. Biol. Cell 2006, 98, 111–123. [Google Scholar] [CrossRef]
- Saskova, A.; Solc, P.; Baran, V.; Kubelka, M.; Schultz, R.M.; Motlik, J. Aurora Kinase a Controls Meiosis I Progression in Mouse Oocytes. Cell Cycle 2008, 7, 2368–2376. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Li, H.; Yu, D.; Li, C.; Li, G.; Cao, Y.; Feng, C.; Deng, X. Protein Kinase Cδ (PKCδ) Involved in the Regulation of PAkt1 (Ser473) on the Release of Mouse Oocytes from Diplotene Arrest. Cell Biochem. Funct. 2018, 36, 221–227. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Labbe, B.; Wang, Y.; Mao, S.; Cao, Y.; Zhao, M.; Liu, S.; Yu, H.; Deng, X. Involvement of CaMKII in Regulating the Release of Diplotene-Arrested Mouse Oocytes by PAkt1 (Ser473). Cell Cycle 2019, 18, 2986–2997. [Google Scholar] [CrossRef]
- Kalous, J.; Kubelka, M.; Šolc, P.; Šušor, A.; Motlík, J. AKT (Protein Kinase B) Is Implicated in Meiotic Maturation of Porcine Oocytes. Reproduction 2009, 138, 645–654. [Google Scholar] [CrossRef]
- Pereira, J.L.; Curcio, A.G.; Barroso, L.M.; Mogollón-Waltero, E.M.; Gomes, H.F.; Maia, R.C.; Viana, K.S.; Caldas Bussiere, M.C.; Marin, D.F.D.; Dias, A.J.B. Modulation of Phosphatidylinositol 3-Kinase Activity during in Vitro Oocyte Maturation Increases the Production of Bovine Blastocysts. Zygote 2020, 28, 371–376. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.L.; Kong, I.K. Melatonin Abrogates the Anti-Developmental Effect of the Akt Inhibitor Sh6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Zheng, P.; Baibakov, B.; Wang, X.H.; Dean, J. PtdIns(3,4,5)P3 Is Constitutively Synthesized and Required for Spindle Translocation during Meiosis in Mouse Oocytes. J. Cell Sci. 2013, 126, 715–721. [Google Scholar] [CrossRef]
- Katayama, K.; Fujita, N.; Tsuruo, T. Akt/Protein Kinase B-Dependent Phosphorylation and Inactivation of WEE1Hu Promote Cell Cycle Progression at G 2/M Transition. Mol. Cell Biol. 2005, 25, 5725–5737. [Google Scholar] [CrossRef]
- Baldin, V.; Theis-Febvre, N.; Benne, C.; Froment, C.; Cazales, M.; Burlet-Schiltz, O.; Ducommun, B. PKB/Akt Phosphorylates the CDC25B Phosphatase and Regulates Its Intracellular Localisation. Biol. Cell 2003, 95, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Vaccari, S.; Nedachi, T.; Andersen, C.B.; Kovacina, K.S.; Roth, R.A.; Conti, M. Protein Kinase B/Akt Phosphorylation of PDE3A and Its Role in Mammalian Oocyte Maturation. EMBO J. 2006, 25, 5716–5725. [Google Scholar] [CrossRef] [PubMed]
- Newhall, K.J.; Criniti, A.R.; Cheah, C.S.; Smith, K.C.; Kafer, K.E.; Burkart, A.D.; McKnight, G.S. Dynamic Anchoring of PKA Is Essential during Oocyte Maturation. Curr. Biol. 2006, 16, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, D.; Aono, R.; Hanada, S.I.; Okumura, E.; Kishimoto, T. Two New Competing Pathways Establish the Threshold for Cyclin-B-Cdk1 Activation at the Meiotic G2/M Transition. J. Cell Sci. 2016, 129, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Okumura, E.; Fukuhara, T.; Yoshida, H.; Hanada, S.I.; Kozutsumi, R.; Mori, M.; Tachibana, K.; Kishimoto, T. Akt Inhibits Myt1 in the Signalling Pathway That Leads to Meiotic G2/M-Phase Transition. Nat. Cell Biol. 2002, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Khan, P.P.; Maitra, S. Participation of PI3-Kinase/Akt Signalling in Insulin Stimulation of P34cdc2 Activation in Zebrafish Oocyte: Phosphodiesterase 3 as a Potential Downstream Target. Mol. Cell Endocrinol. 2013, 374, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. MPF-Based Meiotic Cell Cycle Control: Half a Century of Lessons from Starfish Oocytes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 180–203. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, W.B.; Morris, P.L. SUMOylation Regulates Germinal Vesicle Breakdown and the Akt/PKB Pathway during Mouse Oocyte Maturation. Am. J. Physiol. Cell Physiol. 2018, 315, 115–121. [Google Scholar] [CrossRef]
- Pozuelo Rubio, M.; Campbell, D.G.; Morrice, N.A.; MacKintosh, C. Phosphodiesterase 3A Binds to 14-3-3 Proteins in Response to PMA-Induced Phosphorylation of Ser428. Biochem. J. 2005, 392, 163–172. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sato, E. Protein Kinase B (PKB/Akt) Is Required for the Completion of Meiosis in Mouse Oocytes. Dev. Biol. 2008, 314, 215–223. [Google Scholar] [CrossRef]
- Cecconi, S.; Rossi, G.; Santilli, A.; Di Stefano, L.; Hoshino, Y.; Sato, E.; Palmerini, M.G.; Macchiarelli, G. Akt Expression in Mouse Oocytes Matured in Vivo and in Vitro. Reprod. Biomed. Online 2010, 20, 35–41. [Google Scholar] [CrossRef]
- Jansova, D.; Koncicka, M.; Tetkova, A.; Cerna, R.; Malik, R.; Del Llano, E.; Kubelka, M.; Susor, A. Regulation of 4E-BP1 Activity in the Mammalian Oocyte. Cell Cycle 2017, 16, 927–939. [Google Scholar] [CrossRef]
- Conti, M.; Franciosi, F. Acquisition of Oocyte Competence to Develop as an Embryo: Integrated Nuclear and Cytoplasmic Events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef]
- Franciosi, F.; Manandhar, S.; Conti, M. FSH Regulates MRNA Translation in Mouse Oocytes and Promotes Developmental Competence. Endocrinology 2016, 157, 872–882. [Google Scholar] [CrossRef]
- Procházka, R.; Bartková, A.; Němcová, L.; Murín, M.; Gad, A.; Marcollová, K.; Kinterová, V.; Lucas-Hahn, A.; Laurinčík, J. The Role of Mapk3/1 and Akt in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured in Vitro in Fli Medium. Int. J. Mol. Sci. 2021, 22, 11148. [Google Scholar] [CrossRef] [PubMed]
- Němcová, L.; Nagyová, E.; Petlach, M.; Tománek, M.; Procházka, R. Molecular Mechanisms of Insulin-like Growth Factor 1 Promoted Synthesis and Retention of Hyaluronic Acid in Porcine Oocyte-Cumulus Complexes. Biol. Reprod. 2007, 76, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Procházka, R.; Petlach, M.; Nagyová, E.; Němcová, L. Effect of Epidermal Growth Factor-like Peptides on Pig Cumulus Cell Expansion, Oocyte Maturation, and Acquisition of Developmental Competence in Vitro: Comparison with Gonadotropins. Reproduction 2011, 141, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.; Prochazka, R.; Adamkova, K.; Nevoral, J.; Nemcova, L. Prostaglandin E2 Stimulates the Expression of Cumulus Expansion-Related Genes in Pigs: The Role of Protein Kinase B. Prostaglandins Other Lipid Mediat. 2017, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Baran, V.; Fabian, D.; Rehak, P. Akt/PKB Plays Role of Apoptosis Relay on Entry into First Mitosis of Mouse Embryo. Zygote 2013, 21, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, M.T.; Torcia, S.; Canterini, S.; Bevilacqua, A.; Narducci, M.G.; Ragone, G.; Croce, C.M.; Russo, G.; Mangia, F. TCL1 Promotes Blastomere Proliferation through Nuclear Transfer, but Not Direct Phosphorylation, of AKT/PKB in Early Mouse Embryos. Cell Death Differ. 2008, 15, 420–422. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Cui, W. Proliferation, Survival and Metabolism: The Role of PI3K/AKT/ MTOR Signalling in Pluripotency and Cell Fate Determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lian, X.; Du, J.; Xu, S.; Wei, J.; Pang, L.; Song, C.; He, L.; Wang, S. Inhibition of Phosphorylated Ser473-Akt from Translocating into the Nucleus Contributes to 2-Cell Arrest and Defective Zygotic Genome Activation in Mouse Preimplantation Embryogenesis. Dev. Growth Differ. 2016, 58, 280–292. [Google Scholar] [CrossRef]
- Fiorenza, M.T.; Russo, G.; Narducci, M.G.; Bresin, A.; Mangia, F.; Bevilacqua, A. Protein Kinase Akt2/PKBβ Is Involved in Blastomere Proliferation of Preimplantation Mouse Embryos. J. Cell Physiol. 2020, 235, 3393–3401. [Google Scholar] [CrossRef]
- Ashry, M.; Rajput, S.K.; Folger, J.K.; Knott, J.G.; Hemeida, N.A.; Kandil, O.M.; Ragab, R.S.; Smith, G.W. Functional Role of AKT Signaling in Bovine Early Embryonic Development: Potential Link to Embryotrophic Actions of Follistatin. Reprod. Biol. Endocrinol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic Ovary Syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth Hormone Activates PI3K/Akt Signaling and Inhibits ROS Accumulation and Apoptosis in Granulosa Cells of Patients with Polycystic Ovary Syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Argyrakopoulou, G.; Economou, F.; Kandaraki, E.; Koutsilieris, M. Defects in Insulin Signaling Pathways in Ovarian Steroidogenesis and Other Tissues in Polycystic Ovary Syndrome (PCOS). J. Steroid Biochem. Mol. Biol. 2008, 109, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, H.; Cui, Y.; Wang, W.; Qin, Y.; You, L.; Chan, W.Y.; Sun, Y.; Chen, Z.J. Metabolic Actions of Insulin in Ovarian Granulosa Cells Were Unaffected by Hyperandrogenism. Endocrine 2016, 53, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.; Wang, W.; Sun, Y.; Sun, K. HMGB1-Induced Aberrant Autophagy Contributes to Insulin Resistance in Granulosa Cells in PCOS. FASEB J. 2020, 34, 9563–9574. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Goto, M.; Harata, T.; Takigawa, S.; Nakahara, T.; Suzuki, K.; Manabe, S.; Kikkawa, F. Insulin Attenuates the Insulin-like Growth Factor-I (IGF-I)-Akt Pathway, Not IGF-I-Extracellularly Regulated Kinase Pathway, in Luteinized Granulosa Cells with an Increase in PTEN. J. Clin. Endocrinol. Metab. 2009, 94, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, J.; Ji, R.; Ding, J.; Zhang, Y.; Yang, J. USP25 Regulates the Proliferation and Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome by Modulating the PI3K/AKT Pathway via Deubiquitinating PTEN. Front. Cell Dev. Biol. 2021, 9, 779718. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Lang, J.; Guo, D.; Gao, H.; Qiu, Y.; Jin, X.; Zhang, M.; Shi, J.; Ma, Q.Q.; et al. Up-Regulation of MiR-133a-3p Promotes Ovary Insulin Resistance on Granulosa Cells of Obese PCOS Patients via Inhibiting PI3K/AKT Signaling. BMC Womens Health 2022, 22, 412. [Google Scholar] [CrossRef]
- Machtinger, R.; Rodosthenous, R.S.; Adir, M.; Mansour, A.; Racowsky, C.; Baccarelli, A.A.; Hauser, R. Extracellular MicroRNAs in Follicular Fluid and Their Potential Association with Oocyte Fertilization and Embryo Quality: An Exploratory Study. J. Assist. Reprod. Genet. 2017, 34, 525–533. [Google Scholar] [CrossRef]
- Zhou, Z.; Tu, Z.; Zhang, J.; Tan, C.; Shen, X.; Wan, B.; Li, Y.; Wang, A.; Zhao, L.; Hu, J.; et al. Follicular Fluid-Derived Exosomal MicroRNA-18b-5p Regulates PTEN-Mediated PI3K/Akt/MTOR Signaling Pathway to Inhibit Polycystic Ovary Syndrome Development. Mol. Neurobiol. 2022, 59, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, Y.; Sun, L.; Sun, N.; Wang, J.; Ma, H. Kisspeptin Regulates the Proliferation and Apoptosis of Ovary Granulosa Cells in Polycystic Ovary Syndrome by Modulating the PI3K/AKT/ERK Signalling Pathway. BMC Womens Health 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, X.; Ding, C.; Sun, X.; Wan, L.; Wang, C. Downregulated LncRNA HOTAIR Ameliorates Polycystic Ovaries Syndrome via IGF-1 Mediated PI3K/Akt Pathway. Gynecol. Endocrinol. 2023, 39, 2227280. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, S. Signaling Pathway Intervention in Premature Ovarian Failure. Front. Med. 2022, 9, 999440. [Google Scholar]
- Han, Y.; Yao, R.; Yang, Z.; Li, S.; Meng, W.; Zhang, Y.; Zhang, Y.; Luo, H. Interleukin-4 Activates the PI3K/AKT Signaling to Promote Apoptosis and Inhibit the Proliferation of Granulosa Cells. Exp. Cell Res. 2022, 412, 113002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.F.; Hu, Y.X.; Liu, X.; Cheng, Z.; Lei, Y.; Liu, Y.M.; Zhao, X.; Mu, M.; Yu, L.; Cheng, M. liang The Role of AKT and FOXO3 in Preventing Ovarian Toxicity Induced by Cyclophosphamide. PLoS ONE 2018, 13, e0201136. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, L.; Wang, W.; Jiang, F.; Ai, H. ZnSO4 Protects against Premature Ovarian Failure through PI3K/AKT/GSK3β Signaling Pathway. Theriogenology 2023, 207, 61–71. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, F.; Jia, C.; Chen, F.; Li, F.; Wang, L. MiR-497-3p Induces Premature Ovarian Failure by Targeting KLF4 to Inactivate Klotho/PI3K/AKT/MTOR Signaling Pathway. Cytokine 2023, 170, 156294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, D.; Yu, X.; Shao, X.; Zong, C.; Zhang, M.; Wang, J.; Liang, J.; Ge, P. MiRNA-190a-5p Promotes Primordial Follicle Hyperactivation by Targeting PHLPP1 in Premature Ovarian Failure. Front. Genet. 2022, 13, 1034832. [Google Scholar] [CrossRef]
- Grzechnik, A.T.; Newton, A.C. PHLPPing through History: A Decade in the Life of PHLPP Phosphatases. Biochem. Soc. Trans. 2016, 44, 1675–1682. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Zhuang, L.; Liu, X.; Zhao, H.; Shan, Y.; Liu, Z.; Li, F.; Wang, Y.; Fang, J. Berberine Decreases Insulin Resistance in a PCOS Rats by Improving GLUT4: Dual Regulation of the PI3K/AKT and MAPK Pathways. Regul. Toxicol. Pharmacol. 2020, 110, 104544. [Google Scholar] [CrossRef]
- Rabah, H.M.; Mohamed, D.A.; Mariah, R.A.; Abd El-Khalik, S.R.; Khattab, H.A.; AbuoHashish, N.A.; Abdelsattar, A.M.; Raslan, M.A.; Farghal, E.E.; Eltokhy, A.K. Novel Insights into the Synergistic Effects of Selenium Nanoparticles and Metformin Treatment of Letrozole—Induced Polycystic Ovarian Syndrome: Targeting PI3K/Akt Signalling Pathway, Redox Status and Mitochondrial Dysfunction in Ovarian Tissue. Redox Report. 2023, 28, 2160569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Meng, J.; Zhu, Y.; Ding, M.; Zhang, Y.; Zhou, J. Melatonin Enhances SIRT1 to Ameliorate Mitochondrial Membrane Damage by Activating PDK1/Akt in Granulosa Cells of PCOS. J. Ovarian Res. 2021, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qiu, Y.; Xue, Z.; Wu, R.; Li, J.; Niu, X.; Yuan, J.; Wang, Y.; Wu, Q. Small Extracellular Vesicles Derived from Embryonic Stem Cells Restore Ovarian Function of Premature Ovarian Failure through PI3K/AKT Signaling Pathway. Stem Cell Res. Ther. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fan, X.; Liu, L.; Liu, Y. Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles on Ovarian Functions through the PI3K/Akt Cascade in Mice with Premature Ovarian Failure. Eur. J. Histochem. 2023, 67, 3506. [Google Scholar] [CrossRef]
- Qu, Q.; Liu, L.; Cui, Y.; Liu, H.; Yi, J.; Bing, W.; Liu, C.; Jiang, D.; Bi, Y. MiR-126-3p Containing Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Promote Angiogenesis and Attenuate Ovarian Granulosa Cell Apoptosis in a Preclinical Rat Model of Premature Ovarian Failure. Stem Cell Res. Ther. 2022, 13, 352. [Google Scholar] [CrossRef]
- Li, N.; Liu, L. Mechanism of Resveratrol in Improving Ovarian Function in a Rat Model of Premature Ovarian Insufficiency. J. Obstet. Gynaecol. Res. 2018, 44, 1431–1438. [Google Scholar] [CrossRef]
- Guo, T.; Dong, X.; Xie, S.; Zhang, L.; Zeng, P.; Zhang, L. Cellular Mechanism of Gene Mutations and Potential Therapeutic Targets in Ovarian Cancer. Cancer Manag. Res. 2021, 13, 3081–3100. [Google Scholar] [CrossRef] [PubMed]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/MTOR Pathway in Epithelial Ovarian Cancer, Therapeutic Treatment Options for Platinum-Resistant Ovarian Cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef]
- Ghoneum, A.; Abdulfattah, A.Y.; Said, N. Targeting the PI3K/AKT/MTOR/NFκB Axis in Ovarian Cancer. J. Cell Immunol. 2020, 2, 68–73. [Google Scholar]
- Cristiano, B.E.; Chan, J.C.; Hannan, K.M.; Lundie, N.A.; Marmy-Conus, N.J.; Campbell, I.G.; Phillips, W.A.; Robbie, M.; Hannan, R.D.; Pearson, R.B. A Specific Role for AKT3 in the Genesis of Ovarian Cancer through Modulation of G2-M Phase Transition. Cancer Res. 2006, 66, 11718–11725. [Google Scholar] [CrossRef]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A Transforming Mutation in the Pleckstrin Homology Domain of AKT1 in Cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Bessière, L.; Todeschini, A.L.; Auguste, A.; Sarnacki, S.; Flatters, D.; Legois, B.; Sultan, C.; Kalfa, N.; Galmiche, L.; Veitia, R.A. A Hot-Spot of In-Frame Duplications Activates the Oncoprotein AKT1 in Juvenile Granulosa Cell Tumors. EBioMedicine 2015, 2, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, N.; Kurman, R.J.; Cope, L.; Pohl, G.; Samuels, Y.; Velculescu, V.E.; Wang, T.L.; Shih, I.M. Sequence Mutations and Amplification of PIK3CA and AKT2 Genes in Purified Ovarian Serous Neoplasms. Cancer Biol. Ther. 2006, 5, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Greshock, J.; Colligon, T.A.; Wang, Y.; Ward, R.; Katsaros, D.; Lassus, H.; Butzow, R.; Godwin, A.K.; et al. Frequent Genetic Abnormalities of the PI3K/AKT Pathway in Primary Ovarian Cancer Predict Patient Outcome. Genes. Chromosomes Cancer 2011, 50, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT Signaling Pathway and Cancer: An Updated Review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, A.J.; Schultz, N.; Westfal, M.L.; Sakr, R.A.; Giri, D.D.; Scarperi, S.; Janikariman, M.; Olvera, N.; Stevens, E.V.; She, Q.B.; et al. Genomic Complexity and AKT Dependence in Serous Ovarian Cancer. Cancer Discov. 2012, 2, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; Disaia, P.; Gabra, H.; Glenn, P.; et al. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Therachiyil, L.; Anand, A.; Azmi, A.; Bhat, A.; Korashy, H.M. Role of RAS Signaling in Ovarian Cancer. F1000Research 2022, 11, 1253. [Google Scholar] [CrossRef]
- Cummings, S.; Alfonso, A.; Hughes, E.; Kucera, M.; Mabey, B.; Singh, N.; Eng, C. Cancer Risk Associated With PTEN Pathogenic Variants Identified Using Multigene Hereditary Cancer Panel Testing. JCO Precis. Oncol. 2023, 7, e2200415. [Google Scholar] [CrossRef]
- Zara, L.; Hussain, R.; Masoud, M.S.; Naseem, N.; Ahmad, H.U.; Ashfaq, U.A.; Khaliq, S. Dysregulation of Gene Expression of PTEN and AKT Signaling Pathway in Patients of Ovarian Cancer: A Pilot Study. J. King Saud. Univ. Sci. 2023, 35, 102378. [Google Scholar] [CrossRef]
- Altomare, D.A.; Hui, Q.W.; Skele, K.L.; De Rienzo, A.; Klein-Szanto, A.J.; Godwin, A.K.; Testa, J.R. AKT and MTOR Phosphorylation Is Frequently Detected in Ovarian Cancer and Can Be Targeted to Disrupt Ovarian Tumor Cell Growth. Oncogene 2004, 23, 5853–5857. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, S.; Kawase, C.; Altomare, D.A.; Morishige, K.; Sawada, K.; Hayashi, M.; Tsujimoto, M.; Yamoto, M.; Klein-Szanto, A.J.; Schilder, R.J.; et al. MTOR Is a Promising Therapeutic Target Both in Cisplatin-Sensitive and Cisplatin-Resistant Clear Cell Carcinoma of the Ovary. Clin. Cancer Res. 2009, 15, 5404–5413. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko-Khaider, N.; Lane, D.; Matte, I.; Rancourt, C.; Piché, A. The Inhibition of Bid Expression by Akt Leads to Resistance to TRAIL-Induced Apoptosis in Ovarian Cancer Cells. Oncogene 2010, 29, 5523–5536. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jin, S.; Li, X.; Wang, D. The Involvement of Bcl-2 Family Proteins in AKT-Regulated Cell Survival in Cisplatin Resistant Epithelial Ovarian Cancer. Oncotarget 2017, 8, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Papadopoli, D.; Pollak, M.; Topisirovic, I. The Role of GSK3 in Metabolic Pathway Perturbations in Cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2021, 1868, 119059. [Google Scholar] [CrossRef]
- Cao, Q.; Lu, X.; Feng, Y.J. Glycogen Synthase Kinase-3β Positively Regulates the Proliferation of Human Ovarian Cancer Cells. Cell Res. 2006, 16, 671–677. [Google Scholar] [CrossRef]
- Glibo, M.; Serman, A.; Karin-Kujundzic, V.; Vlatkovic, I.B.; Miskovic, B.; Vranic, S.; Serman, L. The Role of Glycogen Synthase Kinase 3 (GSK3) in Cancer with Emphasis on Ovarian Cancer Development and Progression: A Comprehensive Review. Bosn. J. Basic. Med. Sci. 2021, 21, 5–18. [Google Scholar] [CrossRef]
- Vakana, E.; Altman, J.K.; Platanias, L.C. Targeting AMPK in the Treatment of Malignancies. J. Cell Biochem. 2012, 113, 404–409. [Google Scholar] [CrossRef]
- Li, C.; Liu, V.W.S.; Chiu, P.M.; Yao, K.M.; Ngan, H.Y.S.; Chan, D.W. Reduced Expression of AMPK-Β1 during Tumor Progression Enhances the Oncogenic Capacity of Advanced Ovarian Cancer. Mol. Cancer 2014, 13, 49. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin-Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.R.; Kim, Y.; An, H.; Dhanasekaran, D.N.; Song, Y.S. Metformin Induces Degradation of Cyclin D1 via AMPK/GSK3β Axis in Ovarian Cancer. Mol. Carcinog. 2017, 56, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, X.; Wu, N.; Liao, Q.; Wang, J. SRC-3/TRAF4 Facilitates Ovarian Cancer Development by Activating the PI3K/AKT Signaling Pathway. Med. Oncol. 2023, 40, 76. [Google Scholar] [CrossRef]
- Linnerth-Petrik, N.M.; Santry, L.A.; Moorehead, R.; Jücker, M.; Wootton, S.K.; Petrik, J. Akt Isoform Specific Effects in Ovarian Cancer Progression. Oncotarget 2016, 7, 74820–74833. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, L.; Chen, B.; Zhu, M.; Ma, C.; Mu, C.; Tao, A.; Li, S.; Luo, L.; et al. Cinnamaldehyde Suppressed EGF-Induced EMT Process and Inhibits Ovarian Cancer Progression Through PI3K/AKT Pathway. Front. Pharmacol. 2022, 13, 779608. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Gu, Y.E. Tanshinone IIA Induces Apoptosis of Ovarian Cancer Cells in Vitro and in Vivo through Attenuation of PI3K/AKT/JNK Signaling Pathways. Oncol. Lett. 2019, 17, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/MTOR Signaling Pathway Alleviates Ovarian Cancer Chemoresistance through Reversing Epithelial-Mesenchymal Transition and Decreasing Cancer Stem Cell Marker Expression. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef]
- Parashar, D.; Geethadevi, A.; Mittal, S.; McAlarnen, L.A.; George, J.; Kadamberi, I.P.; Gupta, P.; Uyar, D.S.; Hopp, E.E.; Drendel, H.; et al. Patient-Derived Ovarian Cancer Spheroids Rely on PI3K-AKT Signaling Addiction for Cancer Stemness and Chemoresistance. Cancers 2022, 14, 958. [Google Scholar] [CrossRef]
- Hankittichai, P.; Thaklaewphan, P.; Wikan, N.; Ruttanapattanakul, J.; Potikanond, S.; Smith, D.R.; Nimlamool, W. Resveratrol Enhances Cytotoxic Effects of Cisplatin by Inducing Cell Cycle Arrest and Apoptosis in Ovarian Adenocarcinoma SKOV-3 Cells through Activating the P38 MAPK and Suppressing AKT. Pharmaceuticals 2023, 16, 755. [Google Scholar] [CrossRef]
- Shan, K.S.; Bonano-Rios, A.; Theik, N.W.Y.; Hussein, A.; Blaya, M. Molecular Targeting of the Phosphoinositide-3-Protein Kinase (PI3K) Pathway across Various Cancers. Int. J. Mol. Sci. 2024, 25, 1973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Huang, Y.F.; Chen, C.C.; Huang, C.Y.; Chou, C.Y. Comparing PI3K/Akt Inhibitors Used in Ovarian Cancer Treatment. Front. Pharmacol. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Said, N. PI3K-AKT-MTOR and NFkB Pathways in Ovarian Cancer: Implications for Targeted Therapeutics. Cancers 2019, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/MTOR Signaling Pathway in Ovarian Cancer: Biological and Therapeutic Significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Abe, T.; Nagase, H.; Saito, H.; Fujita, R.; Okada, M.; Yonekura, K.; Shimomura, T.; Utsugi, T. Abstract C177: TAS-117, a Highly Selective Non-ATP Competitive Inhibitor of AKT Demonstrated Antitumor Activity in Combination with Chemotherapeutic Agents and Molecular Targeted Drugs. Mol. Cancer Ther. 2013, 12, C177. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, B.Y.H.; Lai, W.T.; Wu, S.F.; Guh, J.H.; Cheng, A.L.; Hsu, L.C. The Akt Inhibitor MK-2206 Enhances the Cytotoxicity of Paclitaxel (Taxol) and Cisplatin in Ovarian Cancer Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 388, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Sootome, H.; Nakatsuru, Y.; Miyama, K.; Taguchi, S.; Tsujioka, K.; Ueno, Y.; Hatch, H.; Majumder, P.K.; Pan, B.S.; et al. MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs in Vitro and in Vivo. Mol. Cancer Ther. 2010, 9, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.B.; Schönhals, T.; Häusler, S.; Krockenberger, M.; Schmidt, M.; Horn, E.; Köster, F.; Dietl, J.; Wischhusen, J.; Honig, A. Induction of Programmed Cell Death by Inhibition of AKT with the Alkylphosphocholine Perifosine in in Vitro Models of Platinum Sensitive and Resistant Ovarian Cancers. Arch. Gynecol. Obstet. 2011, 283, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, T.; Li, J. Co-Administration of Perifosine with Paclitaxel Synergistically Induces Apoptosis in Ovarian Cancer Cells: More than Just AKT Inhibition. Cancer Lett. 2011, 310, 118–128. [Google Scholar] [CrossRef]
- Coleman, N.; Moyers, J.T.; Harbery, A.; Vivanco, I.; Yap, T.A. Clinical Development of AKT Inhibitors and Associated Predictive Biomarkers to Guide Patient Treatment in Cancer Medicine. Pharmgenomics Pers. Med. 2021, 14, 1517–1535. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K Pathway in Cancer: Are We Making Headway? Nat Rev Clin Oncol 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Janku, F. Phosphoinositide 3-Kinase (PI3K) Pathway Inhibitors in Solid Tumors: From Laboratory to Patients. Cancer Treat. Rev. 2017, 59, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Tan-Wasielewski, Z.; Aghajanian, C.; Coleman, R.L.; Curtis, J.; Hirsch, M.S.; Matulonis, U.A.; Cantley, L.C.; Mills, G.B.; Doyle, L.A.; et al. Results of an Abbreviated Phase II Study of AKT Inhibitor MK-2206 in the Treatment of Recurrent Platinum-Resistant High Grade Serous Ovarian, Fallopian Tube, or Primary Peritoneal Carcinoma (NCT 01283035). Gynecol Oncol Rep 2020, 32, 100546. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Hennessy, B.T.; Ng, C.S.; Ju, Z.; Coombes, K.R.; Wolf, J.K.; Sood, A.K.; Levenback, C.F.; Coleman, R.L.; Kavanagh, J.J.; et al. Perifosine plus Docetaxel in Patients with Platinum and Taxane Resistant or Refractory High-Grade Epithelial Ovarian Cancer. Gynecol. Oncol. 2012, 126, 47–53. [Google Scholar] [CrossRef]
- Blagden, S.P.; Hamilton, A.L.; Mileshkin, L.; Wong, S.; Michael, A.; Hall, M.; Goh, J.C.; Lisyanskaya, A.S.; DeSilvio, M.; Frangou, E.; et al. Phase IB Dose Escalation and Expansion Study of Akt Inhibitor Afuresertib with Carboplatin and Paclitaxel in Recurrent Platinum-Resistant Ovarian Cancer. Clin. Cancer Res. 2019, 25, 1472–1478. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaccari, C.; Antonouli, S.; Anifandis, G.; Cecconi, S.; Di Nisio, V. An Update on Physiopathological Roles of Akt in the ReprodAKTive Mammalian Ovary. Life 2024, 14, 722. https://doi.org/10.3390/life14060722
Giaccari C, Antonouli S, Anifandis G, Cecconi S, Di Nisio V. An Update on Physiopathological Roles of Akt in the ReprodAKTive Mammalian Ovary. Life. 2024; 14(6):722. https://doi.org/10.3390/life14060722
Chicago/Turabian StyleGiaccari, Carlo, Sevastiani Antonouli, George Anifandis, Sandra Cecconi, and Valentina Di Nisio. 2024. "An Update on Physiopathological Roles of Akt in the ReprodAKTive Mammalian Ovary" Life 14, no. 6: 722. https://doi.org/10.3390/life14060722







