The Intersection of the Pathogenic Processes Underlying Psoriasis and the Comorbid Condition of Obesity
Abstract
1. Introduction
2. Materials and Methods
3. Psoriasis
3.1. Epidemiology, Clinical Features, and Quality of Life
3.2. Pathogenesis
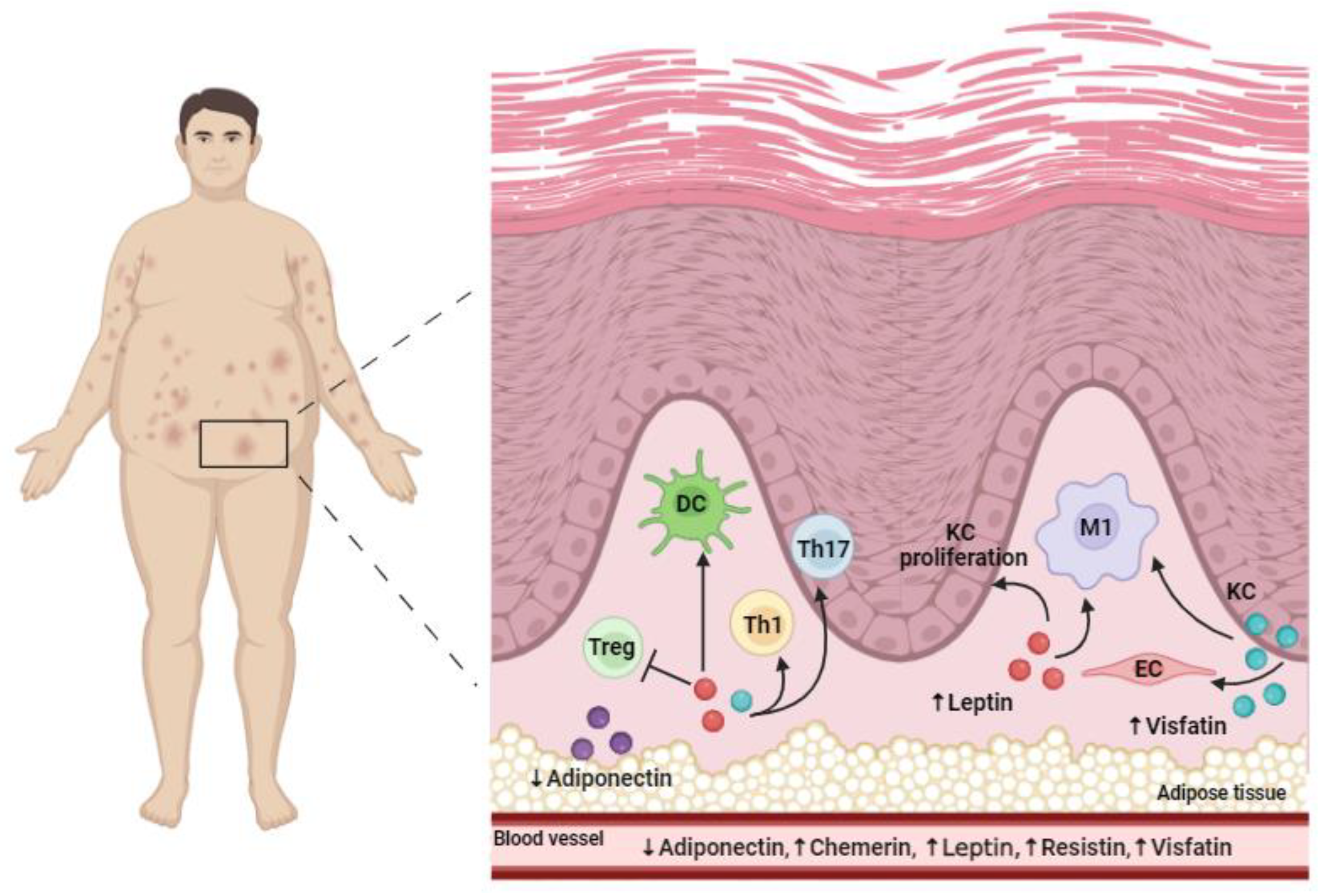
4. Obesity
4.1. The Relationship between Psoriasis and Obesity
4.2. Adipokines
5. Pharmacological Treatment of Psoriasis in Obese Patients
5.1. Conventional Systemic Treatments
5.2. Non-Conventional Systemic Treatments
5.3. Adipokines as Possible Markers of Response to Biologics
5.4. Management of Obesity and the Influence on Psoriasis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Elder, J.T. Psoriasis: Epidemiology. Clin. Dermatol. 2007, 25, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Pavlova, N.T.; Kioskli, K.; Smith, C.; Picariello, F.; Rayner, L.; Moss-Morris, R. Psychosocial aspects of obesity in adults with psoriasis: A systematic review. Ski. Health Dis. 2021, 1, e33. [Google Scholar] [CrossRef]
- Carrascosa, J.M.; Rocamora, V.; Fernandez-Torres, R.M.; Jimenez-Puya, R.; Moreno, J.C.; Coll-Puigserver, N.; Fonseca, E. Obesity and psoriasis: Inflammatory nature of obesity, relationship between psoriasis and obesity, and therapeutic implications. Actas Dermo-Sifiliogr. 2014, 1, 31–44. [Google Scholar] [CrossRef]
- Vata, D.; Tarcau, B.M.; Popescu, I.A.; Halip, I.A.; Patrascu, A.I.; Gheuca Solovastru, D.F.; Mocanu, M.; Chiriac, P.C.; Gheuca Solovastru, L. Update on Obesity in Psoriasis Patients. Life 2023, 13, 1947. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, T.F. HLA-Cw6 and psoriasis. Br. J. Dermatol. 2018, 178, 854–862. [Google Scholar] [CrossRef]
- Smith, R.L.; Warren, R.B.; Eyre, S.; Ho, P.; Ke, X.; Young, H.S.; Griffiths, C.E.; Worthington, J. Polymorphisms in the IL-12beta and IL-23R genes are associated with psoriasis of early onset in a UK cohort. J. Investig. Dermatol. 2008, 128, 1325–1327. [Google Scholar] [CrossRef]
- Israel, L.; Mellett, M. Clinical and Genetic Heterogeneity of CARD14 Mutations in Psoriatic Skin Disease. Front. Immunol. 2018, 9, 2239. [Google Scholar] [CrossRef]
- Gupta, R.; Debbaneh, M.G.; Liao, W. Genetic Epidemiology of Psoriasis. Curr. Dermatol. Rep. 2014, 3, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Zink, A.; Babino, G.; Buononato, D.; Kiani, C.; Eyerich, K.; Ziehfreund, S.; Scala, E. The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life 2022, 12, 2026. [Google Scholar] [CrossRef]
- Jankowiak, B.; Kowalewska, B.; Krajewska-Kułak, E.; Khvorik, D.F. Stigmatization and Quality of Life in Patients with Psoriasis. Dermatol. Ther. 2020, 10, 285–296. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Di Meglio, P.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4, a015354. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Pallotta, S.; Daniele, R.; Bosisio, D.; Madonna, S.; Fortugno, P.; Gonzalvo-Feo, S.; Franssen, J.D.; Parmentier, M.; et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009, 206, 249–258. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1616–1626. [Google Scholar] [CrossRef]
- Schlaak, J.F.; Buslau, M.; Jochum, W.; Hermann, E.; Girndt, M.; Gallati, H.; Meyer zum Büschenfelde, K.H.; Fleischer, B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J. Investig. Dermatol. 1994, 102, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.M.; Ozawa, M.; Kikuchi, T.; Walters, I.B.; Krueger, J.G. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: A type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Investig. Dermatol. 1999, 113, 752–759. [Google Scholar] [PubMed]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Di Meglio, P.; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef]
- Nograles, K.E.; Zaba, L.C.; Guttman-Yassky, E.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Cardinale, I.; Khatcherian, A.; Gonzalez, J.; Pierson, K.C.; White, T.R.; et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008, 159, 1092–1102. [Google Scholar] [CrossRef]
- Albanesi, C.; Madonna, S.; Gisondi, P.; Girolomoni, G. The Interplay Between Keratinocytes and Immune Cells in the Pathogenesis of Psoriasis. Front. Immunol. 2018, 9, 1549. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef]
- Albanesi, C.; Pastore, S. Pathobiology of chronic inflammatory skin diseases: Interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr. Drug Metab. 2010, 11, 210–227. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Nograles, K.E.; Johnson-Huang, L.M.; Fuentes-Duculan, J.; Cardinale, I.; Bonifacio, K.M.; Gulati, N.; Mitsui, H.; Guttman-Yassky, E.; Suárez-Fariñas, M.; et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE 2014, 9, e90284. [Google Scholar] [CrossRef]
- Capon, F. The Genetic Basis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of Psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef]
- Balato, A.; Scala, E.; Balato, N.; Caiazzo, G.; Di Caprio, R.; Monfrecola, G.; Raimondo, A.; Lembo, S.; Ayala, F. Biologics that inhibit the Th17 pathway and related cytokines to treat inflammatory disorders. Expert. Opin. Biol. Ther. 2017, 17, 1363–1374. [Google Scholar] [CrossRef]
- Scala, E.; Kaczmarczyk, R.; Zink, A.; Balato, A.; PSES study group. Sociodemographic, clinical and therapeutic factors as predictors of life quality impairment in psoriasis: A cross-sectional study in Italy. Dermatol. Ther. 2022, 35, e15622. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sinica. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef]
- Aronne, L.J. Classification of obesity and assessment of obesity-related health risks. Obes. Res. 2002, 10 (Suppl. S2), 105S–115S. [Google Scholar] [CrossRef]
- Gisondi, P.; Bellinato, F.; Girolomoni, G.; Albanesi, C. Pathogenesis of Chronic Plaque Psoriasis and Its Intersection With Cardio-Metabolic Comorbidities. Front. Pharmacol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Costache, D.O.; Blejan, H.; Cojocaru, D.L.; Ioniță, G.A.; Poenaru, M.; Constantin, M.M.; Costache, A.C.; Căruntu, C.; Balaban, D.V.; Costache, R.S. Intersecting Pathways: Nonalcoholic Fatty Liver Disease and Psoriasis Duet—A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 2660. [Google Scholar] [CrossRef]
- Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B.; Gelfand, J.M. Prevalence of cardiovascular risk factors in patients with psoriasis. J. Am. Acad. Dermatol. 2006, 55, 829–835. [Google Scholar] [CrossRef]
- Phan, C.; Sigal, M.L.; Lhafa, M.; Barthélémy, H.; Maccari, F.; Estève, E.; Reguiai, Z.; Perrot, J.L.; Chaby, G.; Maillard, H.; et al. Metabolic comorbidities and hypertension in psoriasis patients in France. Comparisons with French national databases. Ann. Dermatol. Venereol. 2016, 143, 264–274. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef]
- Kumar, S.; Han, J.; Li, T.; Qureshi, A.A. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J. Eur. Acad. Dermatol. Venereol. JEADV 2013, 27, 1293–1298. [Google Scholar] [CrossRef]
- Ogawa, K.; Stuart, P.E.; Tsoi, L.C.; Suzuki, K.; Nair, R.P.; Mochizuki, H.; Elder, J.T.; Okada, Y. A Transethnic Mendelian Randomization Study Identifies Causality of Obesity on Risk of Psoriasis. J. Investig. Dermatol. 2019, 139, 1397–1400. [Google Scholar] [CrossRef]
- Czarnecka, A.; Purzycka-Bohdan, D.; Zabłotna, M.; Bohdan, M.; Nowicki, R.J.; Szczerkowska-Dobosz, A. Considerations of the Genetic Background of Obesity among Patients with Psoriasis. Genes 2023, 14, 594. [Google Scholar] [CrossRef]
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The Role of Epigenetic Factors in Psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Labitigan, M.; Bahče-Altuntas, A.; Kremer, J.M.; Reed, G.; Greenberg, J.D.; Jordan, N.; Putterman, C.; Broder, A. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res. 2014, 66, 600–607. [Google Scholar] [CrossRef]
- Li, W.; Han, J.; Qureshi, A.A. Obesity and risk of incident psoriatic arthritis in US women. Ann. Rheum. Dis. 2012, 71, 1267–1272. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Raimondo, A.; Lembo, S.; Fausti, F.; Dini, V.; Costanzo, A.; Monfrecola, G.; Balato, N.; Ayala, F.; Romanelli, M.; et al. Crosstalk between skin inflammation and adipose tissue-derived products: Pathogenic evidence linking psoriasis to increased adiposity. Expert Rev. Clin. Immunol. 2016, 12, 1299–1308. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Gisondi, P.; Girolomoni, G. The pharmacological management of patients with comorbid psoriasis and obesity. Expert Opin. Pharmacother. 2019, 20, 863–872. [Google Scholar] [CrossRef]
- Kanemaru, K.; Matsuyuki, A.; Nakamura, Y.; Fukami, K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp. Dermatol. 2015, 24, 436–442. [Google Scholar] [CrossRef]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef]
- Kovács, D.; Fazekas, F.; Oláh, A.; Törőcsik, D. Adipokines in the Skin and in Dermatological Diseases. Int. J. Mol. Sci. 2020, 21, 9048. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Kawai, K.; Kageyama, A.; Tsumano, T.; Nishimoto, S.; Fukuda, K.; Yokoyama, S.; Oguma, T.; Fujita, K.; Yoshimoto, S.; Yanai, A.; et al. Effects of adiponectin on growth and differentiation of human keratinocytes–implication of impaired wound healing in diabetes. Biochem. Biophys. Res. Commun. 2008, 374, 269–273. [Google Scholar] [CrossRef]
- Takahashi, H.; Honma, M.; Ishida-Yamamoto, A.; Iizuka, H. Adiponectin and leptin modulate cell proliferation and cytokine secretion of normal human keratinocytes and T lymphocytes. J. Dermatol. Sci. 2010, 59, 143–145. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, H.S.; Hong, H.J.; Youn, B.S.; Kim, T.S. Adiponectin induces dendritic cell activation via PLCγ/JNK/NF-κB pathways, leading to Th1 and Th17 polarization. J. Immunol. 2012, 188, 2592–2601. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Surendar, J.; Frohberger, S.J.; Karunakaran, I.; Schmitt, V.; Stamminger, W.; Neumann, A.L.; Wilhelm, C.; Hoerauf, A.; Hübner, M.P. Adiponectin Limits IFN-γ and IL-17 Producing CD4 T Cells in Obesity by Restraining Cell Intrinsic Glycolysis. Front. Immunol. 2019, 10, 2555. [Google Scholar] [CrossRef]
- Kaur, S.; Zilmer, K.; Kairane, C.; Kals, M.; Zilmer, M. Clear differences in adiponectin level and glutathione redox status revealed in obese and normal-weight patients with psoriasis. Br. J. Dermatol. 2008, 159, 1364–1367. [Google Scholar] [CrossRef]
- Shibata, S.; Saeki, H.; Tada, Y.; Karakawa, M.; Komine, M.; Tamaki, K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J. Dermatol. Sci. 2009, 55, 62–63. [Google Scholar] [CrossRef]
- Kulig, P.; Kantyka, T.; Zabel, B.A.; Banas, M.; Chyra, A.; Stefanska, A.; Tu, H.; Allen, S.J.; Handel, T.M.; Kozik, A.; et al. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J. Immunol. 2011, 187, 1403–1410. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Banas, M.; Zegar, A.; Kwitniewski, M.; Zabieglo, K.; Marczynska, J.; Kapinska-Mrowiecka, M.; LaJevic, M.; Zabel, B.A.; Cichy, J. The expression and regulation of chemerin in the epidermis. PLoS ONE 2015, 10, e0117830. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, J.; Zhang, D.; Hu, G.; Zhang, Y. Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-κB signaling. J. Cell. Biochem. 2019, 120, 6459–6470. [Google Scholar] [CrossRef]
- Chyl-Surdacka, K.M.; Gerkowicz, A.; Bartosińska, J.; Kowal, M.; Przepiórka-Kosińska, J.; Surdacki, G.; Krasowska, D.; Chodorowska, G. Analysis of serum chemerin concentrations in psoriatic patients in relation to metabolic abnormalities. Adv. Dermatol. Allergol. 2020, 36, 531–537. [Google Scholar] [CrossRef]
- Gisondi, P.; Lora, V.; Bonauguri, C.; Russo, A.; Lippi, G.; Girolomoni, G. Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br. J. Dermatol. 2013, 168, 749–755. [Google Scholar] [CrossRef]
- Muoio, D.M.; Lynis Dohm, G. Peripheral metabolic actions of leptin. Best Pract. Research. Clin. Endocrinol. Metab. 2002, 16, 653–666. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Al-Hussaniy, H.A.; Alburghaif, A.H.; Naji, M.A. Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions. J. Med. Life 2021, 14, 600–605. [Google Scholar] [CrossRef]
- Xue, K.; Liu, H.; Jian, Q.; Liu, B.; Zhu, D.; Zhang, M.; Gao, L.; Li, C. Leptin induces secretion of pro-inflammatory cytokines by human keratinocytes in vitro—A possible reason for increased severity of psoriasis in patients with a high body mass index. Exp. Dermatol. 2013, 22, 406–410. [Google Scholar] [CrossRef]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Investig. 2000, 106, 501–509. [Google Scholar] [CrossRef]
- Murad, A.; Nath, A.K.; Cha, S.T.; Demir, E.; Flores-Riveros, J.; Sierra-Honigmann, M.R. Leptin is an autocrine/paracrine regulator of wound healing. FASEB J. 2003, 17, 1895–1897. [Google Scholar] [CrossRef]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Bassi, E.J.; Larocca, R.A.; Castoldi, A.; Burghos, M.; Lepique, A.P.; Quintana, F.J.; Araujo, R.C.; Basso, A.S.; Strom, T.B.; et al. Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile. Am. J. Transplant. 2013, 13, 36–44. [Google Scholar] [CrossRef]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef]
- Loffreda, S.; Yang, S.Q.; Lin, H.Z.; Karp, C.L.; Brengman, M.L.; Wang, D.J.; Klein, A.S.; Bulkley, G.B.; Bao, C.; Noble, P.W.; et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998, 12, 57–65. [Google Scholar] [CrossRef]
- Zarkesh-Esfahani, H.; Pockley, G.; Metcalfe, R.A.; Bidlingmaier, M.; Wu, Z.; Ajami, A.; Weetman, A.P.; Strasburger, C.J.; Ross, R.J. High-dose leptin activates human leukocytes via receptor expression on monocytes. J. Immunol. 2001, 167, 4593–4599. [Google Scholar] [CrossRef]
- Mattioli, B.; Straface, E.; Quaranta, M.G.; Giordani, L.; Viora, M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 2005, 174, 6820–6828. [Google Scholar] [CrossRef]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Effects of treatment for psoriasis on circulating levels of leptin, adiponectin and resistin: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Rajala, M.W.; Qi, Y.; Patel, H.R.; Takahashi, N.; Banerjee, R.; Pajvani, U.B.; Sinha, M.K.; Gingerich, R.L.; Scherer, P.E.; Ahima, R.S. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 2004, 53, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Słuczanowska-Głabowska, S.; Staniszewska, M.; Marchlewicz, M.; Duchnik, E.; Łuczkowska, K.; Safranow, K.; Machaliński, B.; Pawlik, A. Adiponectin, Leptin and Resistin in Patients with Psoriasis. J. Clin. Med. 2023, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Grolla, A.A.; Travelli, C.; Genazzani, A.A.; Sethi, J.K. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br. J. Pharmacol. 2016, 173, 2182–2194. [Google Scholar] [CrossRef]
- Carbone, F.; Liberale, L.; Bonaventura, A.; Vecchiè, A.; Casula, M.; Cea, M.; Monacelli, F.; Caffa, I.; Bruzzone, S.; Montecucco, F.; et al. Regulation and Function of Extracellular Nicotinamide Phosphoribosyltransferase/Visfatin. Compr. Physiol. 2017, 7, 603–621. [Google Scholar]
- Farshchian, F.; Ramezani Tehrani, F.; Amirrasouli, H.; Rahimi Pour, H.; Hedayati, M.; Kazerouni, F.; Soltani, A. Visfatin and resistin serum levels in normal-weight and obese women with polycystic ovary syndrome. Int. J. Endocrinol. Metab. 2014, 12, e15503. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.O.; Cander, S.; Gul, B.; Açıkgoz, E.; Sarandol, E.; Ersoy, C. Evaluation of insulin resistance and plasma levels for visfatin and resistin in obese and non-obese patients with polycystic ovary syndrome. Eur. Cytokine Netw. 2015, 26, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, L.; Morelli, M.; Scarponi, C.; Scaglione, G.L.; Pallotta, S.; Avitabile, D.; Albanesi, C.; Madonna, S. Enhanced NAMPT-Mediated NAD Salvage Pathway Contributes to Psoriasis Pathogenesis by Amplifying Epithelial Auto-Inflammatory Circuits. Int. J. Mol. Sci. 2021, 22, 6860. [Google Scholar] [CrossRef] [PubMed]
- Travelli, C.; Colombo, G.; Mola, S.; Genazzani, A.A.; Porta, C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharmacol. Res. 2018, 135, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Travelli, C.; Porta, C.; Genazzani, A.A. Extracellular nicotinamide phosphoribosyltransferase boosts IFNγ-induced macrophage polarization independently of TLR4. iScience 2022, 25, 104147. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hau, C.S.; Tada, Y.; Tatsuta, A.; Sato, S.; Watanabe, S. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology 2011, 152, 3155–3164. [Google Scholar] [CrossRef]
- Hau, C.S.; Kanda, N.; Noda, S.; Tatsuta, A.; Kamata, M.; Shibata, S.; Asano, Y.; Sato, S.; Watanabe, S.; Tada, Y. Visfatin enhances the production of cathelicidin antimicrobial peptide, human β-defensin-2, human β-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am. J. Pathol. 2013, 182, 1705–1717. [Google Scholar] [CrossRef]
- Ismail, S.A.; Mohamed, S.A. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 2012, 167, 436–439. [Google Scholar] [CrossRef]
- Okan, G.; Baki, A.M.; Yorulmaz, E.; Doğru-Abbasoğlu, S.; Vural, P. Serum Visfatin, Fetuin-A, and Pentraxin 3 Levels in Patients With Psoriasis and Their Relation to Disease Severity. J. Clin. Lab. Anal. 2016, 30, 284–289. [Google Scholar] [CrossRef]
- Torretta, S.; Colombo, G.; Travelli, C.; Boumya, S.; Lim, D.; Genazzani, A.A.; Grolla, A.A. The Cytokine Nicotinamide Phosphoribosyltransferase (eNAMPT; PBEF; Visfatin) Acts as a Natural Antagonist of C-C Chemokine Receptor Type 5 (CCR5). Cells 2020, 9, 496. [Google Scholar] [CrossRef]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFκB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Puig, L. Obesity and psoriasis: Body weight and body mass index influence the response to biological treatment. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 1007–1011. [Google Scholar] [CrossRef]
- Elgaard, C.D.B.; Iversen, L.; Hjuler, K.F. Guselkumab, tildrakizumab, and risankizumab in a real-world setting: Drug survival and effectiveness in the treatment of psoriasis and psoriatic arthritis. J. Dermatol. Treat. 2023, 34, 2133531. [Google Scholar] [CrossRef]
- Iannone, F.; Lopalco, G.; Rigante, D.; Orlando, I.; Cantarini, L.; Lapadula, G. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun. Rev. 2016, 15, 447–450. [Google Scholar] [CrossRef]
- Onsun, N.; Akaslan, T.Ç.; Sallahoglu, K.; Gülcan, A.S.; Bulut, H.; Yabacı, A. Effects of TNF inhibitors and an IL12/23 inhibitor on changes in body weight and adipokine levels in psoriasis patients: A 48-week comparative study. J. Dermatol. Treat. 2022, 33, 1727–1732. [Google Scholar] [CrossRef]
- Renzo, L.D.; Saraceno, R.; Schipani, C.; Rizzo, M.; Bianchi, A.; Noce, A.; Esposito, M.; Tiberti, S.; Chimenti, S.; DE Lorenzo, A. Prospective assessment of body weight and body composition changes in patients with psoriasis receiving anti-TNF-α treatment. Dermatol. Ther. 2011, 24, 446–451. [Google Scholar] [CrossRef]
- Gisondi, P.; Cotena, C.; Tessari, G.; Girolomoni, G. Anti-tumour necrosis factor-α therapy increases body weight in patients with chronic plaque psoriasis: A retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. JEADV 2008, 22, 341–344. [Google Scholar] [CrossRef]
- Esposito, M.; Mazzotta, A.; Saraceno, R.; Schipani, C.; Chimenti, S. Influence and variation of the body mass index in patients treated with etanercept for plaque-type psoriasis. Int. J. Immunopathol. Pharmacol. 2009, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Sun, J.; Huang, J.; He, Y.; Yu, A.; Yu, X.; Yang, Z. TNF-α reduces g0s2 expression and stimulates lipolysis through PPAR-γ inhibition in 3T3-L1 adipocytes. Cytokine 2014, 69, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, M.; Sabin, M.; Holly, J.; Shield, J.; Crowne, E.; Stewart, C. Characterization of differentiated subcutaneous and visceral adipose tissue from children: The influences of TNF-alpha and IGF-I. J. Lipid Res. 2005, 46, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pina, T.; Genre, F.; Lopez-Mejias, R.; Armesto, S.; Ubilla, B.; Mijares, V.; Dierssen-Sotos, T.; Gonzalez-Lopez, M.A.; Gonzalez-Vela, M.C.; Blanco, R.; et al. Relationship of leptin with adiposity and inflammation and resistin with disease severity in psoriatic patients undergoing anti-TNF-alpha therapy. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, T.G.; Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J. Clin. Investig. 1997, 100, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Papadavid, E. Can we better strategize our choice of pharmacotherapy for patients with co-morbid psoriasis and obesity? Expert Opin. Pharmacother. 2019, 20, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Conti, A.; Galdo, G.; Piaserico, S.; De Simone, C.; Girolomoni, G. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: A prospective cohort study. Br. J. Dermatol. 2013, 168, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Puig, L.; Mallbris, L.; Zhang, L.; Osuntokun, O.; Leonardi, C. The effect of bodyweight on the efficacy and safety of ixekizumab: Results from an integrated database of three randomised, controlled Phase 3 studies of patients with moderate-to-severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Langley, R.G.; Sigurgeirsson, B.; Abe, M.; Baker, D.R.; Konno, P.; Haemmerle, S.; Thurston, H.J.; Papavassilis, C.; Richards, H.B. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. Br. J. Dermatol. 2013, 168, 412–421. [Google Scholar] [CrossRef]
- Rompoti, N.; Politou, M.; Stefanaki, I.; Vavouli, C.; Papoutsaki, M.; Neofotistou, A.; Rigopoulos, D.; Stratigos, A.; Nicolaidou, E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37, 689–697. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, Q.; Gu, J.; Liu, X.; Lu, L.; Qian, X.; Shen, J.; Zhang, F.; Li, G. Anti-IL-17 antibody improves hepatic steatosis by suppressing interleukin-17-related fatty acid synthesis and metabolism. Clin. Dev. Immunol. 2013, 2013, 253046. [Google Scholar] [CrossRef]
- Gerdes, S.; Pinter, A.; Papavassilis, C.; Reinhardt, M. Effects of secukinumab on metabolic and liver parameters in plaque psoriasis patients. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 533–541. [Google Scholar] [CrossRef]
- Wang, H.N.; Huang, Y.H. Changes in metabolic parameters in psoriatic patients treated with secukinumab. Ther. Adv. Chronic Dis. 2020, 11, 2040622320944777. [Google Scholar] [CrossRef]
- Egeberg, A.; Wu, J.J.; Korman, N.; Solomon, J.A.; Goldblum, O.; Zhao, F.; Mallbris, L. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: Results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J. Am. Acad. Dermatol. 2018, 79, 104–109.e8. [Google Scholar] [CrossRef]
- Ricceri, F.; Chiricozzi, A.; Peris, K.; Prignano, F. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: A multicentric retrospective study. Dermatol. Ther. 2022, 35, e15793. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Foley, P.; Song, M.; Wasfi, Y.; Randazzo, B.; Shen, Y.K.; You, Y.; Griffiths, C.E.M. Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: A pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br. J. Dermatol. 2018, 178, 132–139. [Google Scholar] [CrossRef]
- Blauvelt, A.; Thaçi, D.; Papp, K.A.; Ho, V.; Ghoreschi, K.; Kim, B.S.; Miller, M.; Shen, Y.K.; You, Y.; Chan, D.; et al. Safety of guselkumab in patients with psoriasis with a history of malignancy: 5-year results from the VOYAGE 1 and VOYAGE 2 trials. Br. J. Dermatol. 2023, 189, 132–134. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Mavropoulos, A.; Bogdanos, D.P. Phosphodiesterase 4 Inhibitors in Immune-mediated Diseases: Mode of Action, Clinical Applications, Current and Future Perspectives. Curr. Med. Chem. 2017, 24, 3054–3067. [Google Scholar] [CrossRef]
- Wouters, E.F.; Bredenbröker, D.; Teichmann, P.; Brose, M.; Rabe, K.F.; Fabbri, L.M.; Göke, B. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2012, 97, E1720–E1725. [Google Scholar] [CrossRef]
- Möllmann, J.; Kahles, F.; Lebherz, C.; Kappel, B.; Baeck, C.; Tacke, F.; Werner, C.; Federici, M.; Marx, N.; Lehrke, M. The PDE4 inhibitor roflumilast reduces weight gain by increasing energy expenditure and leads to improved glucose metabolism. Diabetes Obes. Metab. 2017, 19, 496–508. [Google Scholar] [CrossRef]
- Zhang, R.; Maratos-Flier, E.; Flier, J.S. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology 2009, 150, 3076–3082. [Google Scholar] [CrossRef]
- Jensterle, M.; Kocjan, T.; Janez, A. Phosphodiesterase 4 inhibition as a potential new therapeutic target in obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E1476–E1481. [Google Scholar] [CrossRef]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.; Hu, C.; Stevens, R.M.; Day, R.M.; Gordon, K.B.; et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef]
- Paul, C.; Cather, J.; Gooderham, M.; Poulin, Y.; Mrowietz, U.; Ferrandiz, C.; Crowley, J.; Hu, C.; Stevens, R.M.; Shah, K.; et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: A phase III, randomized controlled trial (ESTEEM 2). Br. J. Dermatol. 2015, 173, 1387–1399. [Google Scholar] [CrossRef]
- Huang, H.; Shen, E.; Tang, S.; Tan, X.; Guo, X.; Wang, Q.; Ding, H. Increased serum resistin levels correlate with psoriasis: A meta-analysis. Lipids Health Dis. 2015, 14, 44. [Google Scholar] [CrossRef]
- Zhu, K.J.; Shi, G.; Zhang, C.; Li, M.; Zhu, C.Y.; Fan, Y.M. Adiponectin levels in patients with psoriasis: A meta-analysis. J. Dermatol. 2013, 40, 438–442. [Google Scholar] [CrossRef]
- Zhu, K.J.; Zhang, C.; Li, M.; Zhu, C.Y.; Shi, G.; Fan, Y.M. Leptin levels in patients with psoriasis: A meta-analysis. Clin. Exp. Dermatol. 2013, 38, 478–483. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–920. [Google Scholar] [CrossRef]
- Baran, A.; Flisiak, I.; Jaroszewicz, J.; Świderska, M. Serum adiponectin and leptin levels in psoriatic patients according to topical treatment. J. Dermatol. Treat. 2015, 26, 134–138. [Google Scholar] [CrossRef]
- Campanati, A.; Ganzetti, G.; Giuliodori, K.; Marra, M.; Bonfigli, A.; Testa, R.; Offidani, A. Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-α inhibitors: Results of a retrospective analysis. Int. J. Dermatol. 2015, 54, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Oliveira, H.; Reis, F.; Belo, L.; Rocha, S.; Quintanilha, A.; Figueiredo, A.; Teixeira, F.; Castro, E.; Rocha-Pereira, P.; et al. Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J. Eur. Acad. Dermatol. Venereol. JEADV 2010, 24, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Ertugrul, D.T.; Kalkan, G.; Bilgili, S.G.; Celik, H.T.; Takci, Z.; Balahoroglu, R.; Calka, O. The effect of acitretin treatment on insulin resistance, retinol-binding protein-4, leptin, and adiponectin in psoriasis vulgaris: A noncontrolled study. Dermatology 2013, 227, 103–108. [Google Scholar] [CrossRef]
- Lora, V.; Bonaguri, C.; Gisondi, P.; Sandei, F.; Battistelli, L.; Russo, A.; Melegari, A.; Trenti, T.; Lippi, G.; Girolomoni, G. Autoantibody induction and adipokine levels in patients with psoriasis treated with infliximab. Immunol. Res. 2013, 56, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Strohal, R.; Fuiman, J.; Pedersen, R.; Szumski, A.; Koenig, A.S.; Robertson, D.; Drexel, H. Cardiometabolic biomarkers in chronic plaque psoriasis before and after etanercept treatment. J. Dermatol. Treat. 2014, 25, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.; Tatsuta, A.; Yamamoto, M.; Kamata, M.; Karakawa, M.; Asano, Y.; Mitsui, H.; Sugaya, M.; et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: Induction of elevated serum adiponectin levels following therapy. Br. J. Dermatol. 2011, 164, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Florea, M.; Diaconu, C.C. Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. J. Pers. Med. 2021, 11, 251. [Google Scholar] [CrossRef]
- Faurschou, A.; Knop, F.K.; Thyssen, J.P.; Zachariae, C.; Skov, L.; Vilsbøll, T. Improvement in psoriasis after treatment with the glucagon-like peptide-1 receptor agonist liraglutide. Acta Diabetol. 2014, 51, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, E.; Nicolau, J.; de la Cueva, P.; Goday, A.; Gallardo, F.; Martorell, A.; Carrascosa, J.M. Agonistas del receptor de GLP-1 para el tratamiento de la obesidad en pacientes con dermatosis inmunomediadas. Actas Dermo-Sifiliogr. 2024, 115, 56–65. [Google Scholar] [CrossRef]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef]
| Biologics | Efficacy in Obese Patients | Effect on Body Weight |
|---|---|---|
| Anti-TNF-α | Potential decrease in efficacy | Could lead to weight gain |
| Anti-IL-12/23 | Effective | Neutral influence |
| Anti-IL-17 | Effective | No significant effects |
| Anti-IL-23 | Effective | Data availability is limited |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, E.; Mercurio, L.; Albanesi, C.; Madonna, S. The Intersection of the Pathogenic Processes Underlying Psoriasis and the Comorbid Condition of Obesity. Life 2024, 14, 733. https://doi.org/10.3390/life14060733
Scala E, Mercurio L, Albanesi C, Madonna S. The Intersection of the Pathogenic Processes Underlying Psoriasis and the Comorbid Condition of Obesity. Life. 2024; 14(6):733. https://doi.org/10.3390/life14060733
Chicago/Turabian StyleScala, Emanuele, Laura Mercurio, Cristina Albanesi, and Stefania Madonna. 2024. "The Intersection of the Pathogenic Processes Underlying Psoriasis and the Comorbid Condition of Obesity" Life 14, no. 6: 733. https://doi.org/10.3390/life14060733
APA StyleScala, E., Mercurio, L., Albanesi, C., & Madonna, S. (2024). The Intersection of the Pathogenic Processes Underlying Psoriasis and the Comorbid Condition of Obesity. Life, 14(6), 733. https://doi.org/10.3390/life14060733








