Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a global health crisis, exacerbating issues like malnutrition due to increased metabolic demands and reduced intake during illness. Malnutrition, a significant risk factor, is linked to worse outcomes in patients with COVID-19, such as increased mortality and extended hospital stays. This retrospective cohort study investigated the relationship between malnutrition and clinical outcomes within 90–180 days using data obtained from the TriNetX database. Patients aged >18 years diagnosed with COVID-19 between 1 January 2022, and 31 March 2024 were enrolled in the study. The propensity score-matching (PSM) method was used to match patients with malnutrition (malnutrition group) and those without malnutrition (control group). The primary composite outcome was the cumulative hazard ratio (HR) for post-COVID-19 condition, all-cause hospitalization, and all-cause mortality between 90 days and 180 days after COVID-19 diagnosis. The secondary outcomes were the individual components of the primary outcomes. Two cohorts, each consisting of 15,004 patients with balanced baseline characteristics, were identified using PSM. During the 90–180-day follow-up period, the malnutrition group exhibited a higher incidence of all-cause hospitalization, mortality, or post-COVID-19 condition (HR = 2.315, 95% confidence interval: 2.170–2.471, p < 0.0001). Compared with patients with COVID-19 without malnutrition, those with malnutrition may be associated with a higher risk of adverse clinical outcomes.
1. Introduction
Coronavirus disease (COVID-19), an acute respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has triggered a global pandemic and public health outbreak [1]. As of 5 May 2024, over 700 million cases and 7 million deaths have been reported worldwide [2]. The spectrum of symptoms in infected individuals varies widely; some patients remain asymptomatic, while others develop mild symptoms such as cough, chills, fever, fatigue, and dyspnea [3,4]. More severe manifestations include sepsis, acute respiratory distress syndrome, heart failure, septic shock, and multi-organ dysfunction due to the acute inflammatory response [5,6]. Beyond these immediate effects, approximately 10% of individuals experience long-term consequences known as the post-acute sequelae of SARS-CoV-2 infection (PASC) or “long COVID.” PASC can involve a range of persistent symptoms, including chronic fatigue, respiratory difficulties, cognitive impairment, and cardiovascular issues, extending for months beyond the initial recovery period [7,8,9].
Building on an understanding of the impact of COVID-19, the factors that can exacerbate the severity of the disease must be investigated. Malnutrition, which can manifest in acute, subacute, or chronic form, is one such factor. It is characterized by progressive weight loss, insufficient energy intake, muscle and fat loss, fluid accumulation, and reduced grip strength [10,11]. It is a significant independent risk factor for increased morbidity and mortality across various diseases, as it leads to heightened susceptibility to infections or superinfections [12].
Malnutrition may influence the severity of COVID-19 through several mechanisms, including impairment of the immune response, increased inflammation, and delayed recovery. Deficiencies in key nutrients, such as vitamins A, C, and D, zinc, and protein–energy malnutrition can reduce lymphocyte proliferation, impair immune function and antibody production, and weaken the mucosal barriers that are crucial for preventing pathogen entry. These deficiencies can compromise pulmonary function, which can weaken the respiratory muscles and reduce the pulmonary immune defenses, thereby facilitating more severe respiratory symptoms and extensive viral damage [10,11,12].
Previous meta-analyses have indicated that nearly 49% of patients with COVID-19 experience malnutrition [13]. Observational studies have suggested that malnutrition in these patients correlates with poorer outcomes, including increased rates of mechanical ventilation, mortality, and prolonged hospital stay [14,15,16,17,18,19].
However, much of the existing evidence is derived from retrospective studies conducted at a single center. Therefore, more comprehensive studies with larger sample sizes are needed. This study aimed to explore the association between malnutrition and clinical outcomes in patients with SARS-CoV-2 infection over a period of 90–180 days using an international database.
2. Materials and Methods
2.1. Data Source
The data used in this study were collected from the TriNetX Research Network. The TriNetX database shares electronic medical record data (diagnoses, procedures, medications, laboratory values, genomic information, and types of visits) of approximately 140 million individuals at 119 healthcare organizations (HCOs) [20]. Numerous observational studies have utilized the TriNetX database, particularly when conducting COVID-19 research [21,22,23,24]. The TriNetX platform provides integrated tools for analyzing patient-level data and presents outcomes to researchers as consolidated reports. Considering that the data utilized from TriNetX were anonymized, written informed consent was not required. This study was approved by the Institutional Review Board of the Chi Mei Medical Center (approval no. 11302-E01).
2.2. Patient Selection and Exposure
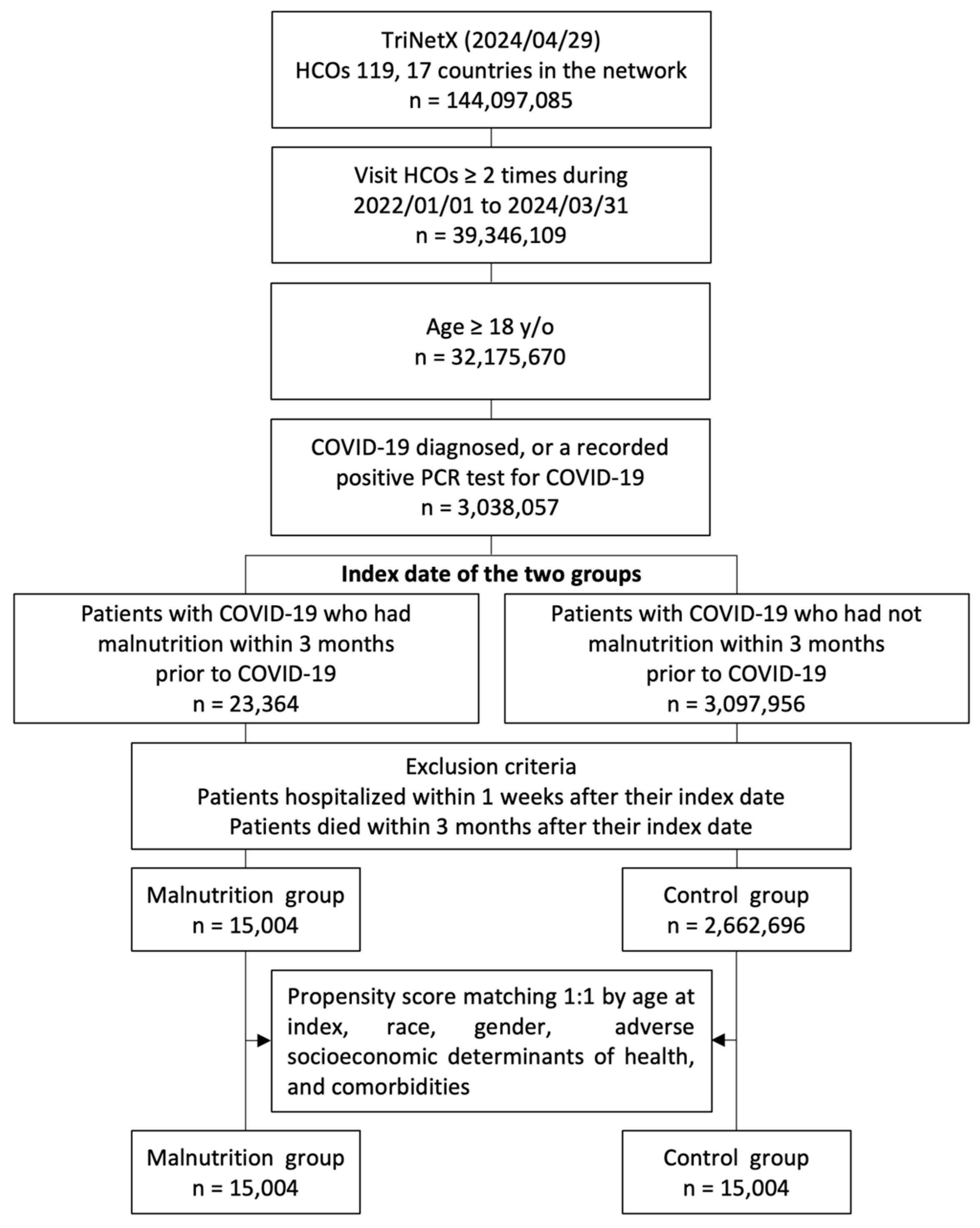
Patients aged >18 years who had more than two visits to HCOs from 1 January 2022 to 31 March 2024 and were diagnosed with COVID-19 (confirmed by a positive polymerase chain reaction test [laboratory test code with TNX: LAB:9088] or an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code U07.1) [25,26,27] were included in the study. To ensure consistency in terms of disease severity, patients who died within 90 days or were hospitalized within 7 days after contracting COVID-19 were excluded. Patients were further divided into the malnutrition and control groups based on whether they exhibited signs of malnutrition (ICD-10 cm codes: E40–E46) within 90 days before the index date. Our initial cohort from 1 January 2022 to 31 March 2024, consisted of 3,038,057 patients with COVID-19, including 23,364 with malnutrition and 3,014,693 without malnutrition (Figure 1).
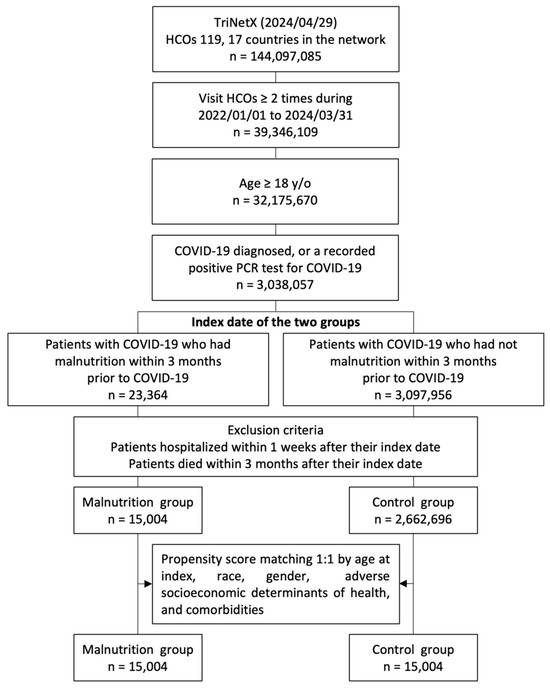
Figure 1.
Flowchart of the participant selection process. HCOs: healthcare organizations; y/o: years old; PCR: polymerase chain reaction.
2.3. Covariates
To balance the distribution between groups at baseline, we selected covariates for matching based on CDC [28]. The baseline variables used to match the two study groups included (a) demographics such as age, sex, and race; (b) potential health risks linked to socioeconomic factors, including housing and financial conditions, educational attainment and literacy levels, employment status, and occupational exposure to hazards; and (c) comorbid conditions such as alcohol-related disorders, nicotine dependence, hypertension, hyperlipidemia, diabetes mellitus, neoplasms, chronic diseases of the lower respiratory tract, liver diseases, chronic kidney disease, end-stage renal disease, cerebrovascular disease, heart failure, and atrial fibrillation.
2.4. Outcomes
The primary outcome of this study was a composite of all-cause hospitalization, all-cause mortality, or post-COVID-19 condition. The secondary outcomes were the individual components of the primary outcome, including all-cause hospitalization, all-cause mortality, and post-COVID-19 condition. The follow-up period was 90–180 days after the index date.
2.5. Statistical Analysis
Statistical analyses were performed using the integrated functions of the TriNetX platform. The baseline characteristics of the malnutrition and control groups were expressed as the means, standard deviations, counts, and percentages. To correct imbalances in baseline covariates, a 1:1 PSM was employed. The PSM technique utilized a nearest-neighbor matching algorithm with a caliper width set at 0.1 of pooled standard deviations. Variables with a standardized difference of less than 0.1 between groups were considered adequately matched. Following PSM, the cumulative incidence of each outcome was estimated using Cox regression models and the results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Additionally, Kaplan–Meier curves were generated to assess the survival distributions between the groups, with significance evaluated using the log-rank test. A p value of <0.05 was considered significant.
2.6. Subgroup Analysis
Subgroup analyses were performed using a stratified approach to explore variations in outcomes based on sex, age, race, vaccination status, and the antiviral agent used.
3. Results
3.1. Demographic Characteristics
The baseline characteristics of the two groups are presented in Table 1. Prior to PSM, notable differences were noted in the demographic and health profiles of the two groups. Specifically, individuals in the malnutrition group were generally older than those in the control group (mean age: 66.0 ± 17.5 vs. 50.6 ± 19.2). The malnutrition group also had a higher proportion of women (58.7% vs. 46.4%). In terms of comorbidities, the malnutrition group exhibited a higher prevalence of alcohol-related disorders (8.9% vs. 2.6%), nicotine dependence (17.7% vs. 7.9%), hypertension (52.4% vs. 30%), hyperlipidemia (41.4% vs. 28.4%), diabetes mellitus (29.4% vs. 14.4%), neoplasms (34.3% vs. 20.5%), chronic lower respiratory diseases (25.5% vs. 15.3%), liver diseases (15.9% vs. 6.3%), chronic kidney disease (22.4% vs. 6.6%), end-stage renal disease (4.8% vs. 1.1%), cerebrovascular diseases (20.1% vs. 4.9%), atrial fibrillation and flutter (16.5% vs. 4.9%), and ischemic heart diseases (27.3% vs. 9.5%). Following PSM, both groups comprised 15,004 patients, each with well-balanced demographic characteristics, as summarized in Table 1.

Table 1.
Baseline characteristics of the study participants before and after the implementation of propensity-score matching.
3.2. Primary Outcomes
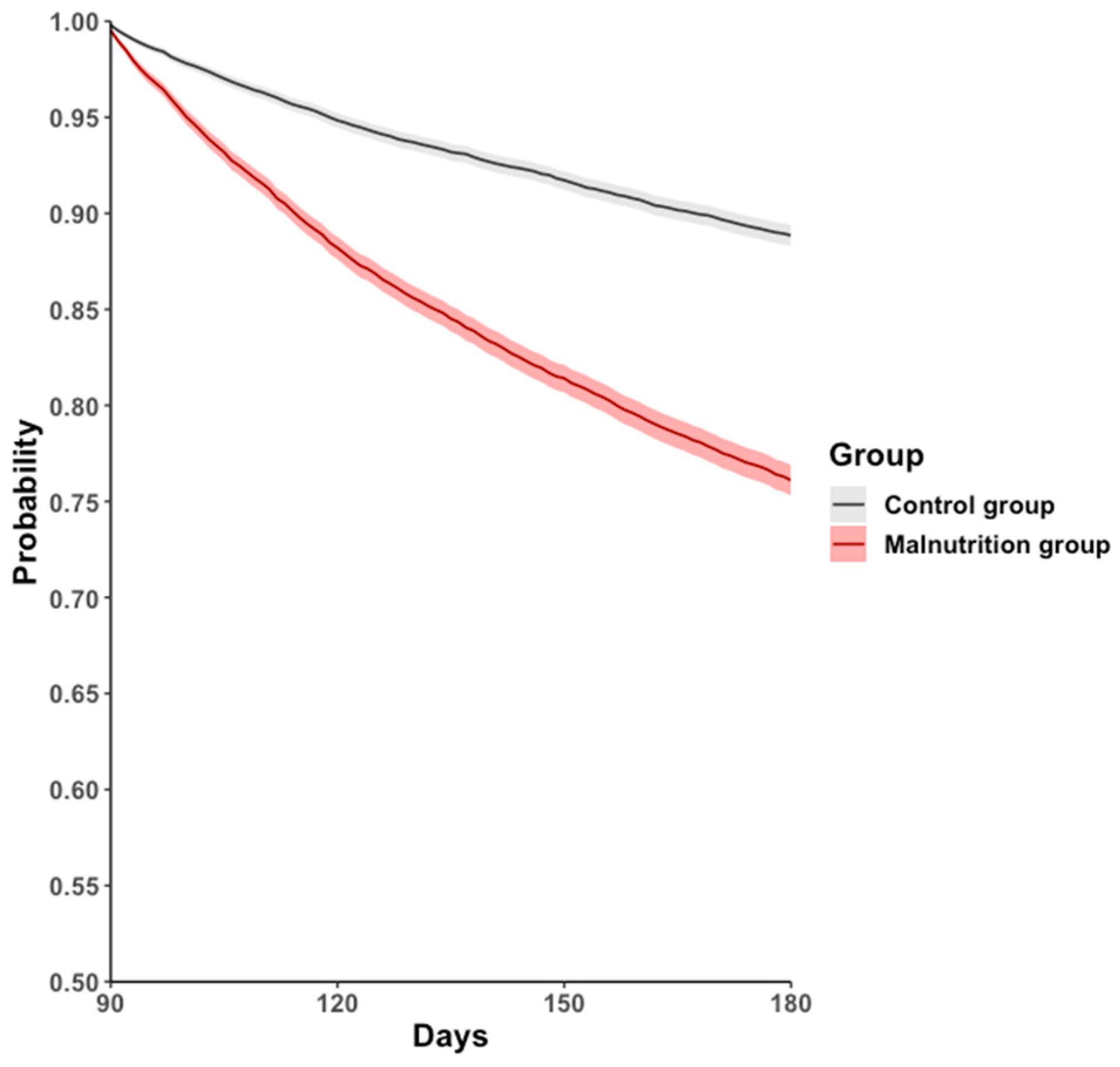
During the 90–180-day follow-up period, the all-cause hospitalization, mortality, or long COVID-19 rate was higher in the malnutrition group (17.9%) than in the control group (9.2%), with an HR of 2.315 (95% CI: 2.170–2.471, p < 0.001; Figure 2, Table 2).
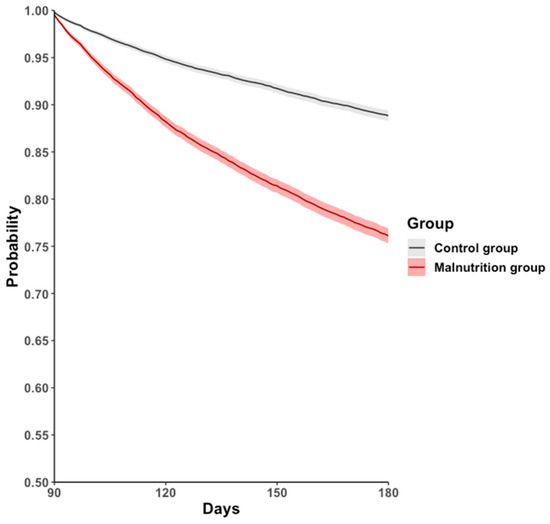
Figure 2.
Kaplan–Meier event-free curves for the primary outcome: a composite of all-cause hospitalization, all-cause mortality, or post-COVID-19 condition.

Table 2.
Hazard ratios of the primary and secondary outcomes between the malnutrition group and control group.
3.3. Secondary Outcomes
For the individual component outcomes during the 90–180-day follow-up period, the malnutrition group showed a higher risk of all-cause hospitalization (HR = 2.151, 95% CI: 2.008–2.304), all-cause mortality (HR = 4.459, 95% CI: 3.699–5.376), and post-COVID-19 condition (HR = 2.707, 95% CI: 1.913–3.832) than the control group (Table 2).
3.4. Subgroup Analysis
The risk of the primary outcome was examined based on age, sex, race, vaccination status, and the antiviral agent used (Table 3). Subgroup analyses showed consistent results for each subgroup. In terms of the other secondary outcomes, similar trends were observed in the subgroup analyses (Table 4).

Table 3.
Subgroup analyses of the primary outcomes between the malnutrition and control groups.

Table 4.
Subgroup analysis of the secondary outcomes between malnutrition and control groups.
4. Discussion
This study used a large sample size to investigate the association between malnutrition and clinical outcomes in patients with COVID-19 over a 90–180-day follow-up period. Our findings highlight that patients with COVID-19 experiencing malnutrition have a significantly higher risk of severe outcomes, including all-cause hospitalization, all-cause mortality, and post-COVID-19 condition. Notably, the effect size of all-cause mortality in the malnutrition group was fourfold higher than that in the control group, indicating a substantial impact of nutritional status on COVID-19 severity.
The underlying mechanisms by which malnutrition exacerbates COVID-19 severity involve both direct and indirect effects on patient health. Malnutrition weakens immune defense mechanisms, as evidenced by a reduced lymphocyte count and impaired phagocytic function, which are critical for combating viral infections [29,30]. Deficiencies in essential proteins, vitamins, and minerals further weaken physical barriers and cellular immunity. This predisposition not only increases susceptibility to severe infections but also increases the risk of prolonged illness and complications [31,32,33,34].
Furthermore, malnutrition exacerbates the inflammatory cascade associated with COVID-19. It induces an imbalanced cytokine profile, promoting inflammatory pathways that can lead to the occurrence of cytokine storm syndrome, a known predictor of severe outcomes in COVID-19. Uncontrolled inflammation can result in tissue damage, multi-organ failure, and heightened mortality risk [32,35,36,37,38,39].
Consistent with previous studies, malnutrition amplifies the severity of infectious diseases, including COVID-19 [3,12,40,41,42,43,44]. A meta-analysis of 12 studies demonstrated a similar trend, showing that malnourished patients had significantly higher odds of in-hospital mortality (odds ratio = 3.43, 95% CI: 2.55–4.60) during a 90-day follow-up [45]. Our study builds on these findings by including non-hospitalized patients and extending the follow-up duration, thus providing a more comprehensive view of the impact of malnutrition on COVID-19 outcomes in various settings. Additionally, our study addressed some of the limitations observed in previous studies, such as small sample sizes and short follow-up periods, by utilizing a larger and more diverse cohort with a longer observation period. This approach enhances the generalizability and applicability of our findings, suggesting robust associations across various healthcare settings and populations.
The strong association between malnutrition and adverse COVID-19 outcomes underscores the necessity of early nutritional screening and intervention in patients with COVID-19. The implementation of nutritional support strategies can potentially reduce disease severity and improve clinical outcomes. Physicians and dietitians should consider integrated care pathways that incorporate nutritional assessments and tailored interventions as part of standard care for patients with COVID-19.
Although our findings are significant, they highlight the need for further research to explore the causal relationships and effectiveness of specific nutritional interventions in improving COVID-19 prognosis. Prospective and randomized controlled trials are essential to determine the specific nutrients and dietary interventions that are most effective in mitigating the impact of malnutrition on COVID-19 severity.
This study has several strengths. First, although the existing research often relies on data from a single hospital with limited sample sizes and generalizability, our study draws from a vast and diverse population. This diversity enhances the relevance and generalizability of our findings to broader real-world settings. Second, we employed PSM to ensure comparability between the groups, effectively controlling for potential confounding factors related to the variables of interest and observed outcomes. Finally, the consistency observed across various subgroup analyses adds further credibility to our findings.
This study has some limitations. First, potential information bias and coding errors in the electronic health records database may have occurred; however, these errors were likely consistent between the groups, which minimizes their impact on our findings [46]. Second, relying on ICD-10 cm codes to determine the history of malnutrition may underestimate its true prevalence. Third, although our data suggest significant associations, they do not establish causality. Fourth, due to database limitations, we could not determine the countries from which these patients originated. Finally, to control for disease severity and reduce heterogeneity, we excluded patients who were initially hospitalized and deceased within 90 days from the index date, limiting the generalizability of our findings.
5. Conclusions
This study demonstrated that malnutrition is associated with a significantly higher risk of all-cause hospitalization, mortality, or post-COVID-19 condition compared with the control group. Therefore, malnutrition is a potential risk factor for poor clinical outcomes in patients with COVID-19. These findings highlight the critical role of malnutrition in exacerbating the severity of COVID-19. The robustness of the data, enhanced by the large sample size and diverse patient population, provides compelling evidence supporting the integration of nutritional evaluations and interventions in the management of COVID-19.
Author Contributions
Conceptualization, C.-Y.L., T.-C.W. and J.-Y.W.; methodology, T.-H.L.; software, M.-H.C.; validation, W.-H.H. and Y.-W.T.; writing—original draft preparation, Y.-C.L.; writing—review and editing, K.-C.H., M.-C.L., T.Y., C.-C.L., T.-C.W. and J.-Y.W.; visualization, W.-H.H. and P.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Chi Mei Medical Center (approval no. 11302-E01).
Informed Consent Statement
Informed consent was not obtained, since all data were taken anonymously from the database of the hospital.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 May 2024).
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 19 May 2024).
- Barazzoni, R.; Bischoff, S.C.; Busetto, L.; Cederholm, T.; Chourdakis, M.; Cuerda, C.; Delzenne, N.; Genton, L.; Schneider, S.; Singer, P.; et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin. Nutr. 2022, 41, 2869–2886. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Hsueh, P.R. Coronavirus disease 2019 rebounds following nirmatrelvir/ritonavir treatment. J. Med. Virol. 2023, 95, e28430. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Ko, W.C.; Lee, P.I.; Jean, S.S.; Hsueh, P.R. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106024. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 2023, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J. Parenter. Enteral Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.M.; Jambou, P.; Filippi, J.; van Obberghen, E.; Hébuterne, X. Malnutrition is an independent factor associated with nosocomial infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef]
- Abate, S.M.; Chekole, Y.A.; Estifanos, M.B.; Abate, K.H.; Kabthymer, R.H. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 43, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A.; Grant, K.; Marano, R.; Arrieta, A.; Grant, K., Jr.; Feaster, W.; Steele, C.; Ehwerhemuepha, L. Long-term effects of malnutrition on severity of COVID-19. Sci. Rep. 2021, 11, 14974. [Google Scholar] [CrossRef] [PubMed]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deng, H.; Wang, Y.; Chen, L.; Gu, X.; Wang, X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition 2021, 84, 111123. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, C.L.; Ba, Y.M.; Wang, Y.M.; Song, B.; Cheng, X.B.; Dong, Q.F.; Wang, L.L.; You, S.S. Nutritional risk and therapy for severe and critical COVID-19 patients: A multicenter retrospective observational study. Clin. Nutr. 2021, 40, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, Z.; Yu, G.; Peng, D.; Feng, Y.; Ling, J.; Wang, Y.; Li, S.; Bian, Y. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin. Nutr. 2021, 40, 534–541. [Google Scholar] [CrossRef] [PubMed]
- TriNetX. TriNetX Research Network. Available online: https://trinetx.com/ (accessed on 9 May 2024).
- Huang, S.W.; Yeh, W.B.; Chen, H.Y.; Yong, S.B. Long-term risk of herpes zoster following COVID-19: A retrospective cohort study of 2,442,686 patients. J. Med. Virol. 2023, 95, e29101. [Google Scholar] [CrossRef]
- Chuang, M.H.; Wu, J.Y.; Liu, T.H.; Hsu, W.H.; Tsai, Y.W.; Huang, P.Y.; Lai, C.C. Efficacy of nirmatrelvir and ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Tsai, Y.W.; Wu, J.Y.; Liu, T.H.; Lai, C.C. Post-acute hospitalization and mortality of nirmatrelvir plus ritonavir for COVID-19 survivors. J. Infect. 2023, 86, e107–e110. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, M.Y.; Liu, T.H.; Chuang, M.H.; Hsu, W.H.; Huang, P.Y.; Tsai, Y.W.; Kuo, C.Y.; Yeh, C.T.; Lai, C.C. Clinical efficacy of nirmatrelvir and ritonavir combination for treating diabetic patients with COVID-19. J. Med. Virol. 2023, 95, e28866. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.W.; Wu, J.Y.; Liu, T.H.; Chuang, M.H.; Hsu, W.H.; Huang, P.Y.; Lai, C.C.; Tsai, K.T.; Shiue, Y.L. Clinical effectiveness of oral antiviral agents in older patients with COVID-19 based on real-world data. J. Med. Virol. 2023, 95, e28869. [Google Scholar] [CrossRef]
- Liu, T.H.; Huang, P.Y.; Wu, J.Y.; Chuang, M.H.; Hsu, W.H.; Tsai, Y.W.; Chang, C.C.; Lai, C.C. Clinical effectiveness of nirmatrelvir plus ritonavir in patients with COVID-19 and substance use disorders based on real-world data. J. Med. Virol. 2023, 95, e28801. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Wu, J.Y.; Huang, P.Y.; Tsai, Y.W.; Lai, C.C. The effect of nirmatrelvir plus ritonavir on the long-term risk of epilepsy and seizure following COVID-19: A retrospective cohort study including 91,528 patients. J. Infect. 2023, 86, 256–308. [Google Scholar] [CrossRef]
- CDC. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 6 April 2024).
- Foolchand, A.; Ghazi, T.; Chuturgoon, A.A. Malnutrition and Dietary Habits Alter the Immune System Which May Consequently Influence SARS-CoV-2 Virulence: A Review. Int. J. Mol. Sci. 2022, 23, 2654. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Behjati, M.; Karami, M.; Abari, A.H.; Sobhani-Nasab, A.; Rourani, H.A.; Hazrati, E.; Mirghazanfari, S.M.; Hadi, V.; Hadi, S.; et al. An overview on role of nutrition on COVID-19 immunity: Accumulative review from available studies. Clin. Nutr. Open Sci. 2023, 47, 6–43. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Wei, X.; He, Y.; Zhong, L.; Lu, H.; Lan, J.; Wei, Y.; Liu, Z.; Liu, H. The nutritional roles of zinc for immune system and COVID-19 patients. Front. Nutr. 2024, 11, 1385591. [Google Scholar] [CrossRef]
- Jamilian, A.; Ghalichi, F.; Hamedi Kalajahi, F.; Radkhah, N.; Jourabchi, N.; Musazadeh, V.; Amini-Salehi, E.; Zarezadeh, M.; Ostadrahimi, A. The role of vitamin D in outcomes of critical care in COVID-19 patients: Evidence from an umbrella meta-analysis of interventional and observational studies. Public Health Nutr. 2024, 27, e127. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Shahidi, R.; Mojdeganlou, H.; Akhtaran, F.K.; Maroufi, S.F.; Maroufi, S.P.; Mirzapour, P.; Karimi, A.; Khodaei, S.; Pour, M.M.; et al. The effect of macronutrient and micronutrient supplements on COVID-19: An umbrella review. J. Health Popul. Nutr. 2024, 43, 16. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Vinciguerra, M.; Lamanna, B.; Malvasi, A.; Scacco, S.; Acquaviva, S.; Pinto, V.; Di Vagno, G.; Cormio, G.; et al. Nutrition as Personalized Medicine against SARS-CoV-2 Infections: Clinical and Oncological Options with a Specific Female Groups Overview. Int. J. Mol. Sci. 2022, 23, 9136. [Google Scholar] [CrossRef]
- Grund, S.; Bauer, J.M. Malnutrition and Sarcopenia in COVID-19 Survivors. Clin. Geriatr. Med. 2022, 38, 559–564. [Google Scholar] [CrossRef]
- Chiang, K.C.; Kalantar-Zadeh, K.; Gupta, A. Thymic Dysfunction and Atrophy in COVID-19 Disease Complicated by Inflammation, Malnutrition and Cachexia. Nutr. Health 2022, 28, 199–206. [Google Scholar] [CrossRef]
- Burgos, R.; García-Almeida, J.M.; Matía-Martín, P.; Palma, S.; Sanz-Paris, A.; Zugasti, A.; Alfaro, J.J.; Fullana, A.A.; Continente, A.C.; Chicetru, M.J.; et al. Malnutrition management of hospitalized patients with diabetes/hyperglycemia and COVID-19 infection. Rev. Endocr. Metab. Disord. 2022, 23, 205–213. [Google Scholar] [CrossRef]
- Fedele, D.; De Francesco, A.; Riso, S.; Collo, A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition 2021, 81, 111016. [Google Scholar] [CrossRef]
- Martín-Martínez, A.; Viñas, P.; Carrillo, I.; Martos, J.; Clavé, P.; Ortega, O. The Impact of Frailty, Oropharyngeal Dysphagia and Malnutrition on Mortality in Older Patients Hospitalized for COVID-19. Aging Dis. 2024, 15, 927–938. [Google Scholar] [CrossRef]
- Jima, L.M.; Atomsa, G.E.; Allard, J.P.; Nigatu, Y.D. The effect of malnutrition on adult Covid-19 patient’s ICU admission and mortality in Covid-19 isolation and treatment centers in Ethiopia: A prospective cohort study. PLoS ONE 2024, 19, e0298215. [Google Scholar] [CrossRef]
- Barazzoni, R.; Breda, J.; Cuerda, C.; Schneider, S.; Deutz, N.E.; Wickramasinghe, K. COVID-19: Lessons on malnutrition, nutritional care and public health from the ESPEN-WHO Europe call for papers. Clin. Nutr. 2022, 41, 2858–2868. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, M.Y.; Liu, T.H.; Chuang, M.H.; Hsu, W.H.; Huang, P.Y.; Tsai, Y.W.; Lai, C.C. Association between nirmatrelvir plus ritonavir and the outcomes of non-hospitalized obese patients with COVID-19. Int. J. Antimicrob. Agents 2023, 62, 106984. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, M.Y.; Lee, M.C.; Hung, K.C.; Hsu, W.H.; Tsai, Y.W.; Liu, T.H.; Huang, P.Y.; Chuang, M.H.; Tseng, S.C.; et al. The clinical outcomes and effectiveness of antiviral agents among underweight patients with COVID-19. Expert. Rev. Anti Infect. Ther. 2024, 22, 343–352. [Google Scholar] [CrossRef]
- Boaz, M.; Kaufman-Shriqui, V. Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19. Nutrients 2023, 15, 1298. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.T.; Checkoway, H.; McMichael, A.J.; Holbrook, R.H. Bias due to misclassification in the estimation of relative risk. Am. J. Epidemiol. 1977, 105, 488–495. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).