Cardiac Computed Tomography Identification of the Septal Vein—A Small Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Protocol
2.3. Image Analysis
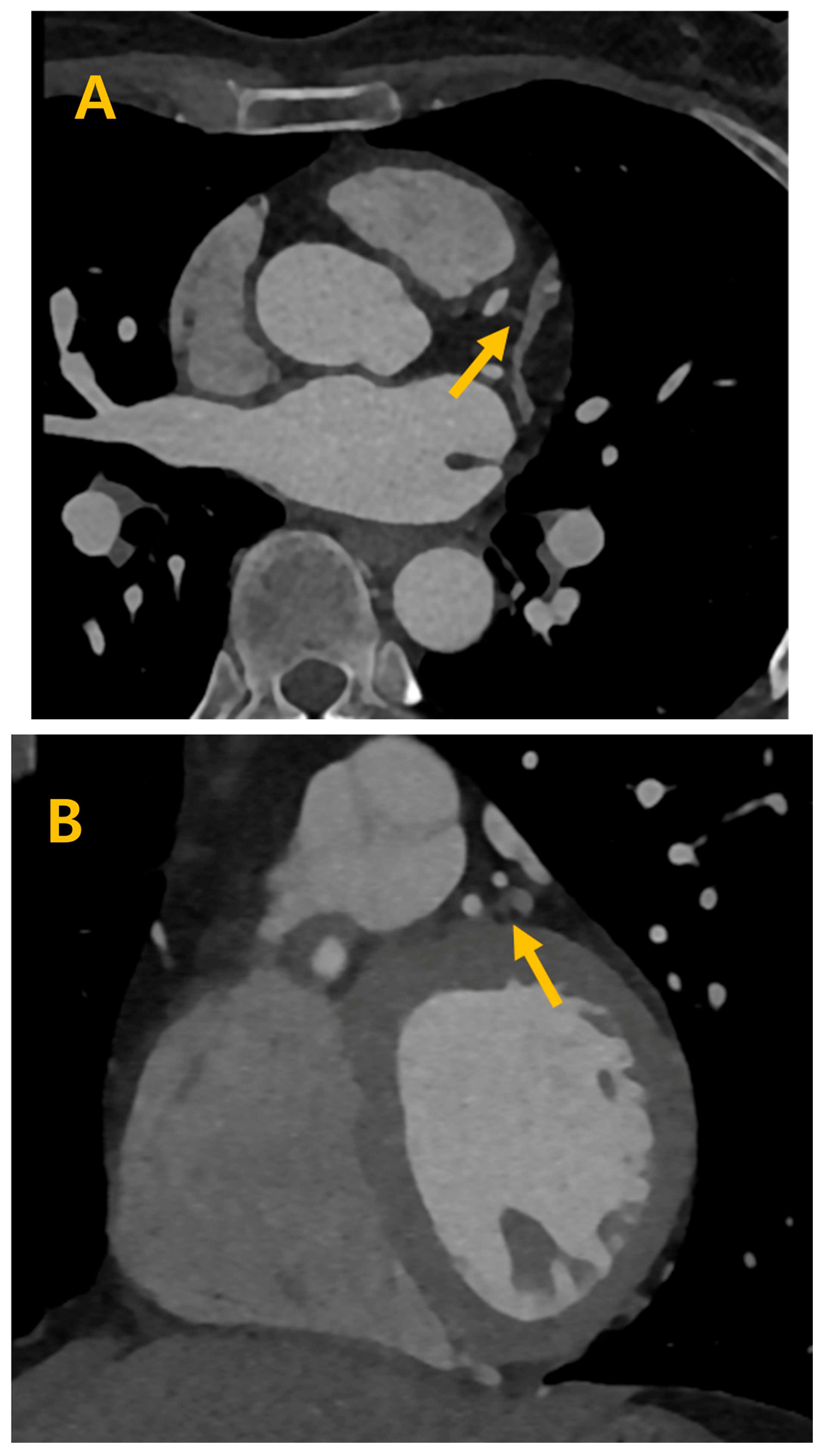
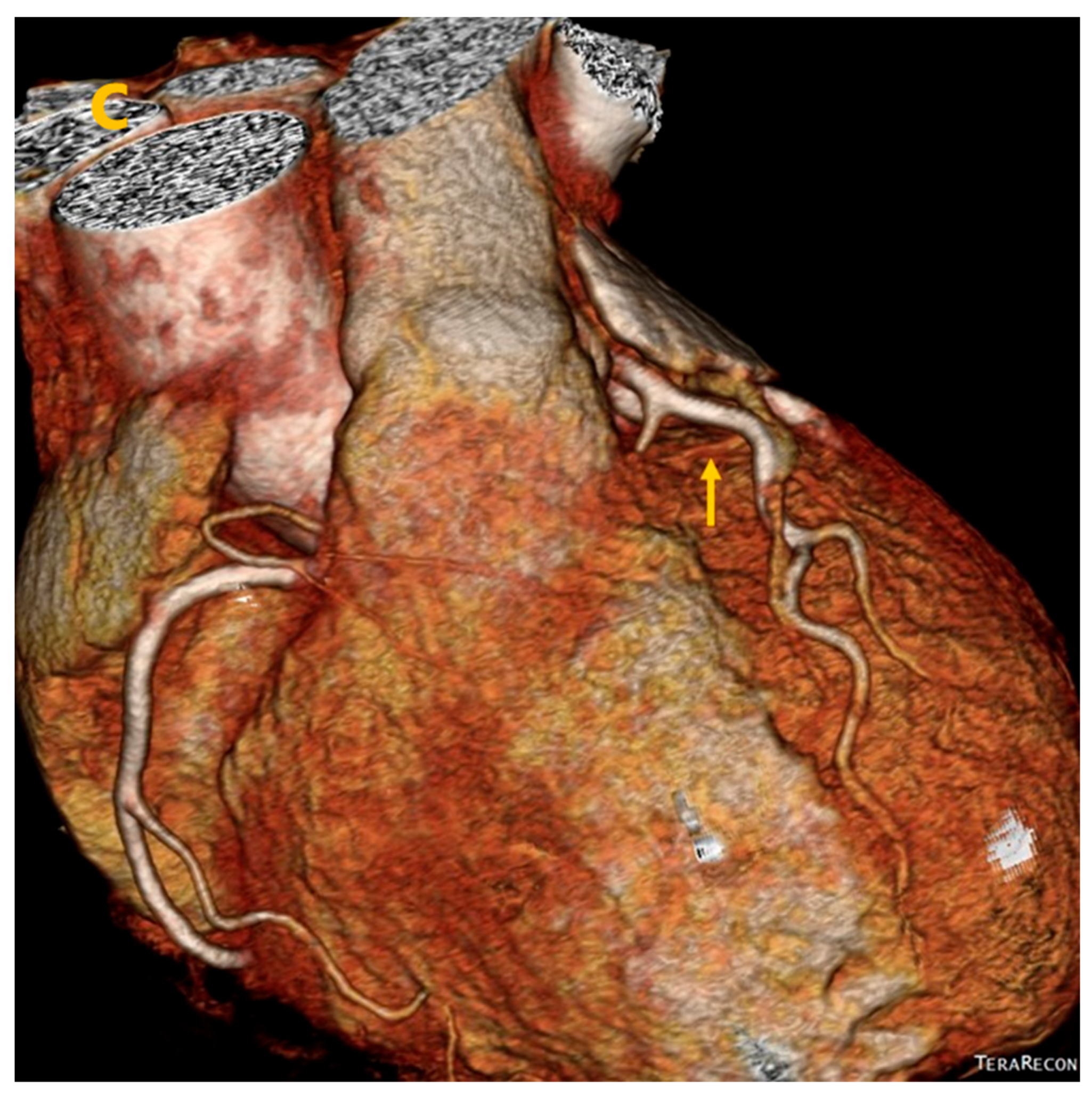
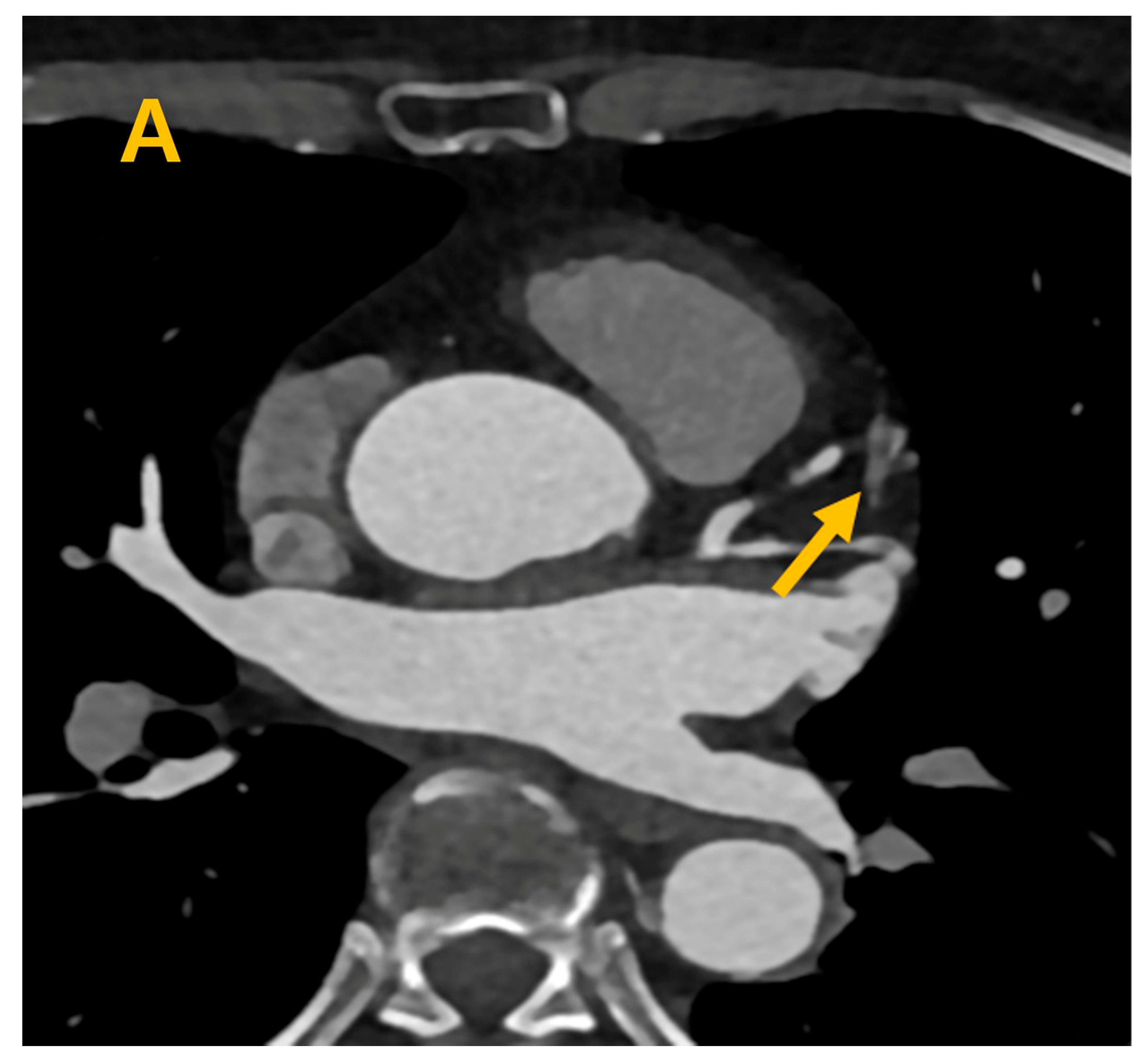
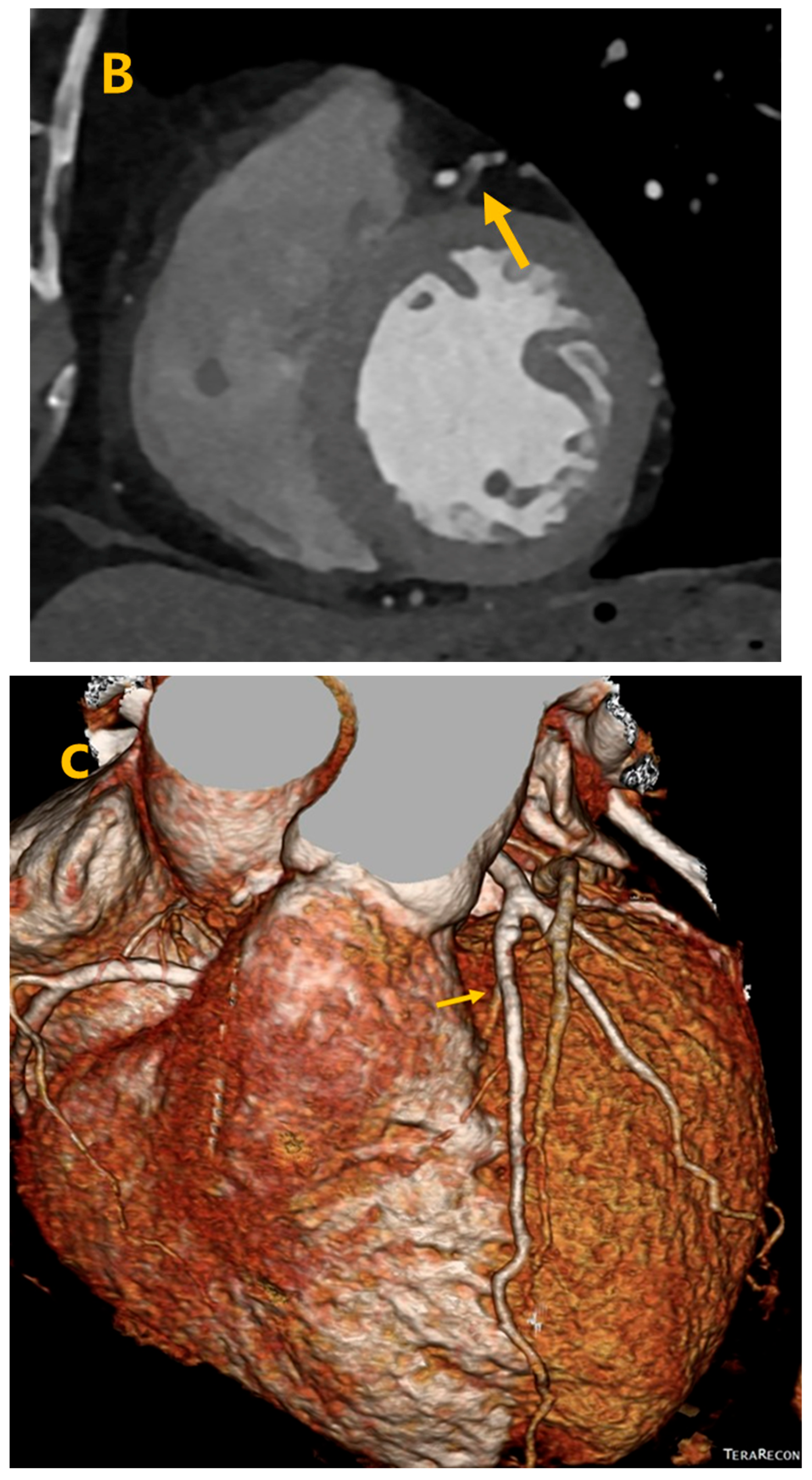
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.; Shreenivas, S.S.; Pratt, D.N.; Lynch, D.R.; Kereiakes, D.J. Percutaneous Interventions for Secondary Mitral Regurgitation. Circ. Cardiovasc. Interv. 2020, 13, e008998. [Google Scholar] [CrossRef] [PubMed]
- Denti, P.; Sala, A.; Belluschi, I.; Alfieri, O. Over 15 years: The advancement of transcatheter mitral valve repair. Ann. Cardiothorac. Surg. 2021, 10, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Chon, M.K.; Lederman, R.J.; Sung, S.C.; Je, H.G.; Choo, K.S.; Lee, S.H.; Shin, E.S.; Kim, J.S.; Hwang, K.W.; et al. Mitral Loop Cerclage Annuloplasty for Secondary Mitral Regurgitation: First Human Results. JACC Cardiovasc. Interv. 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Peigh, G.; Wasserlauf, J.; Choudhury, L.; Knight, B.P.; Kim, S. From 100 to 0: Endocardial Radiofrequency Ablation of Septal Hypertrophy for Management of Hypertrophic Cardiomyopathy. JACC Case Rep. 2020, 2, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, J.; Qi, Y.; Wang, F.; Guo, L.; Shi, X.; Wu, W.; Zhou, X.; Li, R. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019, 16, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Alhous, M.H.; Small, G.R.; Hannah AHillis, G.S.; Frenneaux, M.; Broadhurst, P.A. Right ventricular septal pacing as alternative for failed left ventricular lead implantation in cardiac resynchronization therapy candidates. Europace 2015, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Chon, M.K.; Jun, E.J.; Park, Y.H.; Lee, S.H.; Kim, J.S.; Shin, D.H.; Lee, S.Y.; Cho, M.S.; Lee, S.W.; et al. Septal Reduction Using Transvenous Intramyocardial Cerclage Radiofrequency Ablation: Preclinical Feasibility. JACC Basic Transl. Sci. 2020, 30, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Choo, K.S.; Kim, J.H.; Hwang, J.Y.; Park, C.K.; Lee, J.W. Comparison of noise-optimized linearly blended images and noise-optimized virtual monoenergetic images evaluated by dual-source, dual-energy CT in cardiac vein assessment. Acta Radiol. 2021, 62, 594–602. [Google Scholar] [CrossRef] [PubMed]
| Order of Prevalence for Ps | Order of Prevalence for Ds |
|---|---|
| 70% (20/25) | 60% (22/29) |
| 60% (17/25) | 70% (20/29) |
| 40% (12/25) | 40% (17/29) |
| 80% (9/25) | 80% (7/29) |
| 30% (6/25) | 30% (6/29) |
| 20% (3/25) | 90% (3/29) |
| 10% (2/25) | 20% (1/29) |
| 10% (1/29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chon, M.K.; Choo, K.S.; Kim, J.H. Cardiac Computed Tomography Identification of the Septal Vein—A Small Retrospective Study. Life 2024, 14, 748. https://doi.org/10.3390/life14060748
Chon MK, Choo KS, Kim JH. Cardiac Computed Tomography Identification of the Septal Vein—A Small Retrospective Study. Life. 2024; 14(6):748. https://doi.org/10.3390/life14060748
Chicago/Turabian StyleChon, Min Ku, Ki Seok Choo, and June Hong Kim. 2024. "Cardiac Computed Tomography Identification of the Septal Vein—A Small Retrospective Study" Life 14, no. 6: 748. https://doi.org/10.3390/life14060748
APA StyleChon, M. K., Choo, K. S., & Kim, J. H. (2024). Cardiac Computed Tomography Identification of the Septal Vein—A Small Retrospective Study. Life, 14(6), 748. https://doi.org/10.3390/life14060748








