Association of HLA Haplotypes with Autoimmune Pathogenesis in Newly Diagnosed Type 1 Romanian Diabetic Children: A Pilot, Single-Center Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Subjects and Study Design
2.3. Laboratory Procedures
2.4. HLA Genotyping
2.5. Statistical Analysis
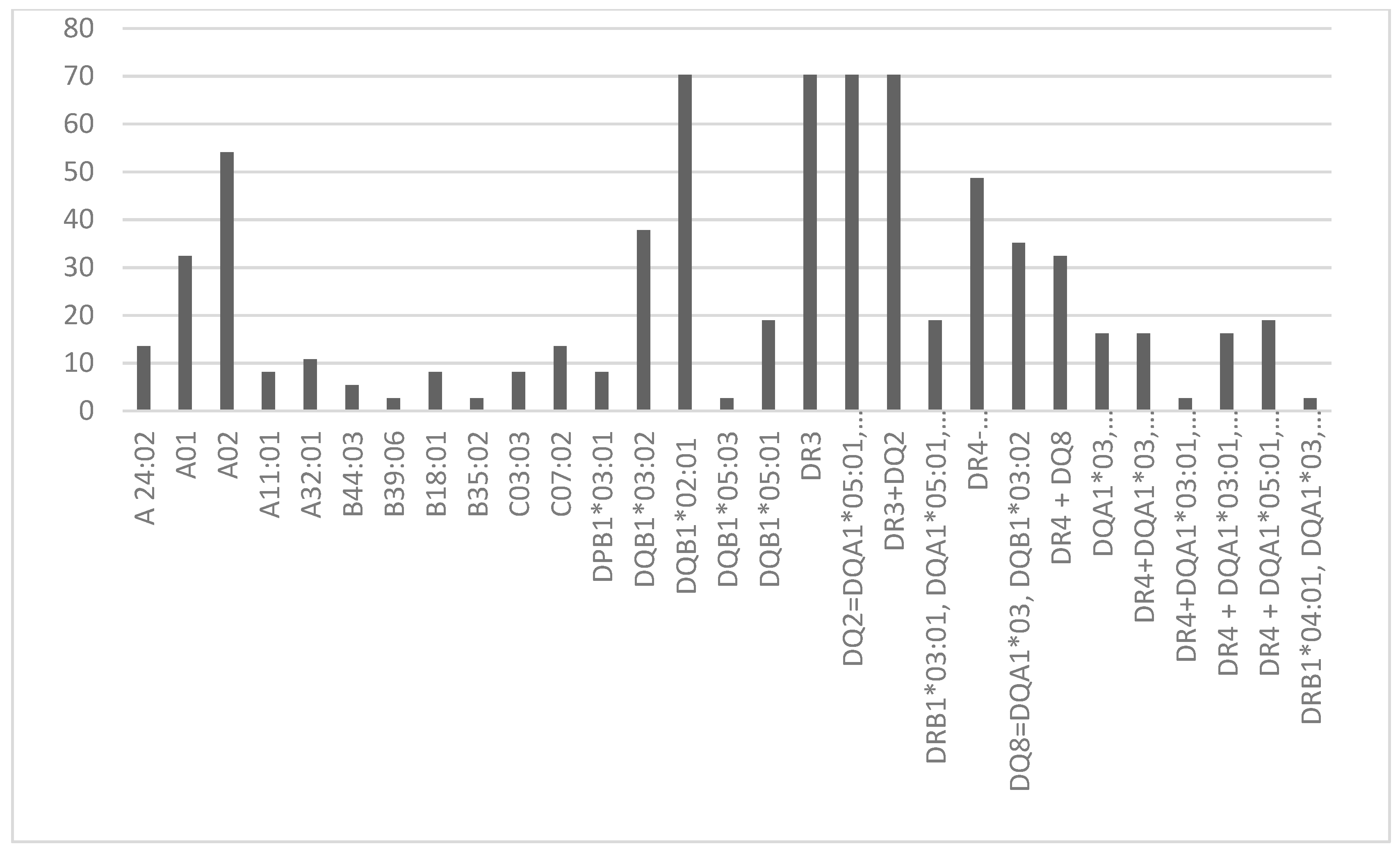
3. Results
3.1. Clinical and Biological Characteristics of the Patients
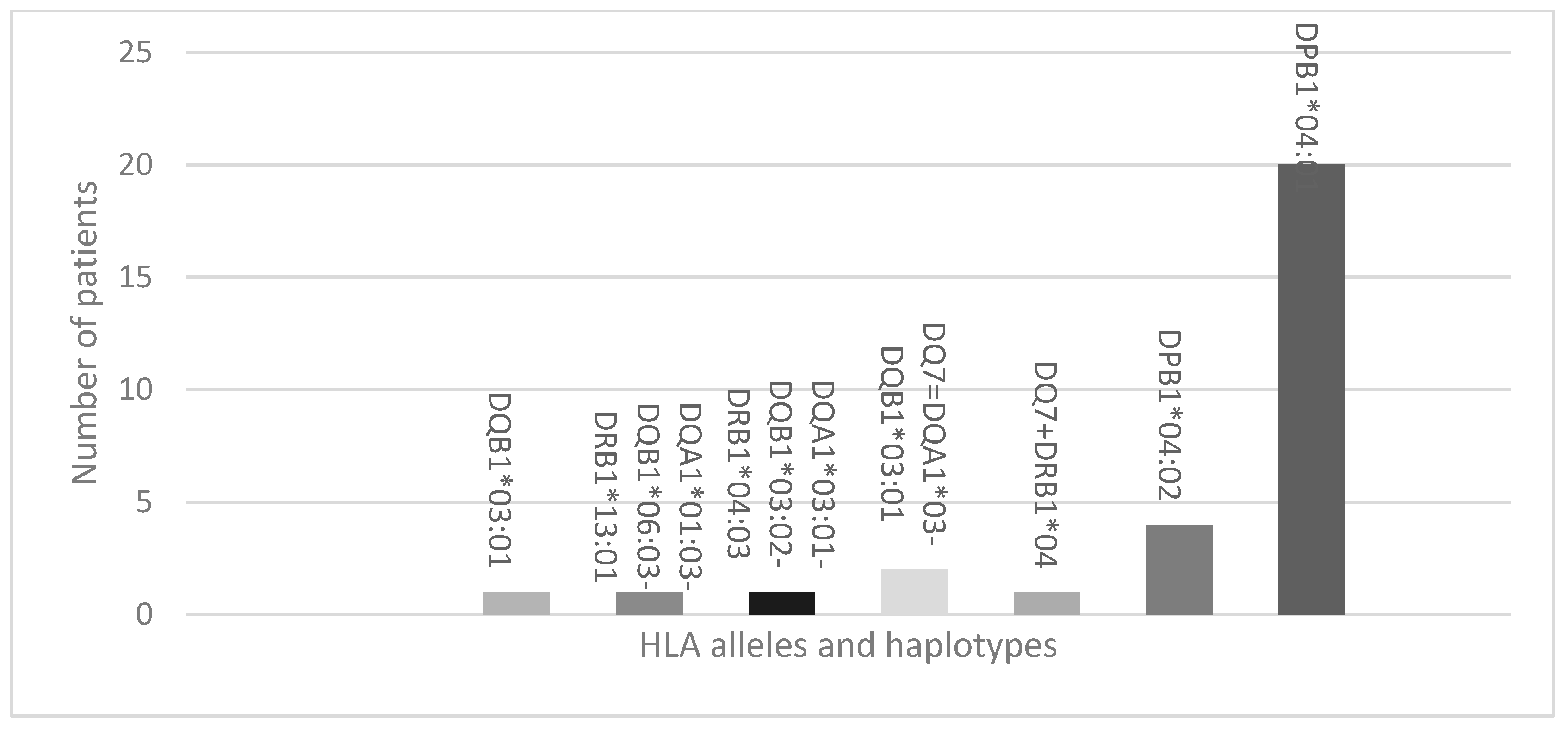
3.2. Protecting HLA Allele and Haplotypes
3.3. HLA Alleles and Haplotypes Linked to Elevated ATPO and ATG Levels
3.4. HLA Alleles and Haplotypes Correlated with Positive Transglutaminase Antibodies
3.5. HLA Alleles and Haplotypes Associated with Vitamin D Deficiency
4. Discussions
4.1. Protecting and Predisposing Alleles and Haplotypes in T1DM Patients
4.2. Thyroid Autoimmunity and T1DM
4.3. Vitamin D Deficiency and T1DM
4.4. Celiac Disease and T1DM
4.5. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Undlien, D.E.; Thorsby, E. HLA associations in type 1 diabetes: Merging genetics and immunology. Trends Immunol. 2001, 22, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.; Serban, V.; Green, A.; Möller, S.; Vlad, M.; Timar, B.; Sima, A.; ONROCAD Study Group. Time Trends, Regional Variability and Seasonality Regarding the Incidence of Type 1 Diabetes Mellitus in Romanian Children Aged 0–14 Years, Between 1996 and 2015. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Steenkiste, A.; Valdes, A.M.; Feolo, M.; Hoffman, D.; Concannon, P.; Noble, J.; Schoch, G.; Hansen, J.; Helmberg, W.; Dorman, J.S.; et al. 13th IHWS 1 diabetes component participating investigators. 14th international HLA and immunogenetics workshop: Report on the HLA component of type 1 diabetes. Tissue Antigens 2007, 69 (Suppl. S1), 214–225. [Google Scholar] [CrossRef]
- Pociot, F.; McDermott, M.F. Genetics of type 1 diabetes mellitus. Genes Immun. 2002, 3, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Regnell, S.E.; Lernmark, A. Early prediction of autoimmune (type 1) diabetes. Diabetologia 2017, 60, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diab Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef]
- Thomson, G.; Valdes, A.M.; Noble, J.A.; Kockum, I.; Grote, M.N.; Najman, J.; Erlich, H.A.; Cucca, F.; Pugliese, A.; Steenkiste, A.; et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: A meta-analysis. Tissue Antigens 2007, 70, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Giannini, C.; Primavera, M. Prediction and prevention of type 1 diabetes in children. Clin. Pediatr. Endocrinol. 2019, 28, 43–57. [Google Scholar] [CrossRef]
- Ziegler, A.-G.; Hoffmann, G.F.; Hasford, J.; Larsson, H.E.; Danne, T.; Berner, R.; Penno, M.; Koralova, A.; Dunne, J.; Bonifacio, E. Screening for asymptomatic β-cell autoimmunity in young children. Lancet Child. Adolesc. Health 2019, 3, 288–290. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef]
- Frommer, L.; Kahaly, G.J. Type 1 diabetes and autoimmune thyroid disease-the genetic link. Front. Endocrinol. 2021, 12, 618213. [Google Scholar] [CrossRef]
- Gomes, M.B.; Braga, F.O.; Drummond, K.G.; Pinheiro, A.; Leal, F.; Porto, L.C.; Ferreira, L.L.; Pinheiro, G.D.R.C.; Negrato, C.A. Prevalence of autoimmune diseases in an admixed population of patients with type 1 diabetes: A multicenter study in Brazil. Diabetol. Metab. Syndr. 2024, 16, 31. [Google Scholar] [CrossRef]
- Skov, J.; Kuja-Halkola, R.; Magnusson, P.K.E.; Gudbjornsdottir, S.; Kampe, O.; Bensing, S. Shared etiology of type 1 diabetes and Hashimoto’s thyroiditis: A population-based twin study. Eur. J. Endocrinol. 2022, 186, 677–685. [Google Scholar] [CrossRef]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 2014, 40, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Wyk, N.V.; Maayer, T.; Gededzha, M.P.; Mayne, E. Coeliac Disease High-Risk Human Leukocyte Antigen Alleles in a South African Type One Diabetic Population. Clin. Lab. 2023, 69, 1775–1778. [Google Scholar] [CrossRef]
- Franzese, A.; Lombardi, F.; Valerio, G.; Spagnuolo, M.I. Update on coeliac disease and type 1 diabetes mellitus in childhood. J. Pediatr. Endocrinol. Metab. 2007, 20, 1257–1264. [Google Scholar] [CrossRef]
- Kim, S.-S.; Hudgins, A.D.; Yang, J.; Zhu, Y.; Tu, Z.; Rosenfeld, M.G.; DiLorenzo, T.P.; Suh, Y. A comprehensive integrated post-GWAS analysis of Type 1 diabetes reveals enhancer-based immune dysregulation. PLoS ONE 2021, 16, e0257265. [Google Scholar] [CrossRef]
- Hyppönen, E. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes Obes. Metab. 2010, 12, 737–743. [Google Scholar] [CrossRef]
- Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, M.; Simell, V.; Mykkänen, J.; Ilonen, J.; Veijola, R.; Hyöty, H.; Knip, M.; Simell, O.; Toppari, J.; Hermann, R. An increase in serum 25-hydroxyvitamin D concentrations preceded a plateau in type 1 diabetes incidence in Finnish children. J. Clin. Endocrinol. Metab. 2014, 99, E2353–E2356. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Pérez-López, F.R.; López-Baena, M.T.; Pérez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G.; Clark, R.M. Methods of lipid analysis. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carbayo, M.; Mauri, M.; Alfayate, R.; Miralles, C.; Soria, F. Analytical and clinical evaluation of TSH and thyroid hormones by electrochemiluminescent immunoassays. Clin. Biochem. 1999, 32, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Reinert, S.E.; Baird, G.L.; Quintos, S.B. Comparing HbA1C by POC and HPLC. Rhode Isl. Med. J. 2018, 101, 43–46. [Google Scholar]
- Profaizer, T.; Kumánovics, A. Human Leukocyte Antigen Typing by Next-Generation Sequencing. Clin. Lab. Med. 2018, 38, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Sojeong, K.; Sunho, L.; Jonghee, H.; Yangrae, C.; Joohon, S.; Han-Na, K.; Hyung-Lae, K.; Jongsun, J. HLAscan: Genotyping of the HLA region using next-generation sequencing data. BMC Bioinform. 2017, 18, 258. [Google Scholar]
- Pascanu, I.; Pop, R.; Barbu, C.G.; Dumitrescu, C.P.; Gherlan, I.; Marginean, O.; Preda, C.; Procopiuc, C.; Vulpoi, C.; Hermanussen, M. Development of Synthetic Growth Charts for Romanian Population. Acta Endocrinol. 2016, 12, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valencia, P.; Bougnères, P.; Valleron, A.J. Global epidemiology of type 1 diabetes in young adults and adults: A systematic review. BMC Public. Health 2015, 15, 255. [Google Scholar] [CrossRef]
- James, L.; Georgopoulos, A. Immunogenetic epidemiology of type 1 diabetes in 14 continental western European countries. J. Immunol. Sci. 2021, 5, 29–35. [Google Scholar] [CrossRef]
- Masako, T.; Ichiro, H.; Toshiyuki, I.; Norio, A.; Atsushi, K. Analysis of HLA class II genes associated with susceptibility to type 1 diabetes in Japanese patients with autoimmune thyroid disease. Acta Medica Nagasaki. 2021, 65, 37–43. [Google Scholar] [CrossRef]
- Guja, C.; Guja, L.; Nutland, S.; Rance, H.; Sebastien, M.; Todd, J.A.; Ionescu-Tirgoviste, C. Type 1 diabetes genetic susceptibility encoded by HLA DQB1 genes in Romania. J. Cell Mol. Med. 2004, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tîrgoviste, C.; Guja, C.; Herr, M.; Cucca, E.; Welsh, K.; Bunce, M.; Marshall, S.; Todd, J.A. Low frequency of HLA DRB1*03—DQB1*02 and DQB1*0302 haplotypes in Romania is consistent with the country’s low incidence of Type I diabetes. Diabetologia 2001, 44 (Suppl. S3), B60–B66. [Google Scholar] [CrossRef] [PubMed]
- Orzan, A.; Novac, C.; Mihu, M.; Tirgoviste, C.I.; Balgradean, M. Type 1 diabetes and thyroid autoimmunity in children. Maedica 2016, 11, 308–312. [Google Scholar]
- Reghina, A.D.; Albu, A.; Petre, N.; Mihu, M.; Florea, S.; Fica, S. Thyroid autoimmunity in 72 children with type 1 diabetes mellitus: Relationship with pancreatic autoimmunity and child growth. J. Pediatr. Endocrinol. Metab. 2012, 25, 723–726. [Google Scholar] [CrossRef]
- Kahles, H.; Fain, P.R.; Baker, P.; Eisenbarth, G.; Badenhoop, K. Genetics of autoimmune thyroiditis in type 1 diabetes reveals a novel association with DPB1*0201: Data from the type 1 diabetes genetics consortium. Diabetes Care 2015, 38, 21–28. [Google Scholar] [CrossRef]
- Gorodezky, C.; Alaez, C.; Murguía, A.; Rodríguez, A.; Balladares, S.; Vazquez, M.; Flores, H.; Robles, C. HLA and autoimmune diseases: Type 1 diabetes (T1D) as an example. Autoimmun. Rev. 2006, 5, 187–194. [Google Scholar] [CrossRef]
- Yang, X.; Chai, M.; Lin, M. Proportion of vitamin D deficiency in children/adolescents with type 1 diabetes: A systematic review and meta-analysis. BMC Pediatr 2024, 24, 192. [Google Scholar] [CrossRef]
- Masteller, E.L.; Warner, M.R.; Ferlin, W.; Judkowski, V.; Wilson, D.; Glaichenhaus, N.; Bluestone, J.A. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J. Immunol. 2003, 171, 5587–5595. [Google Scholar] [CrossRef]
- American Diabetes Asociation -Standards of Care in Diabetes—2024. Available online: https://professional.diabetes.org/standards-of-care (accessed on 1 March 2024).
- Kaur, N.; Singh, J.; Minz, R.W.; Anand, S.; Saikia, B.; Bhadada, S.K.; Dayal, D.; Kumar, M.; Dhanda, S.K. Shared and distinct genetics of pure type 1 diabetes and type 1 diabetes with celiac disease, homology in their auto-antigens and immune dysregulation states: A study from North India. Acta Diabetol. 2024, 61, 791–805. [Google Scholar] [CrossRef]
- Mimbacas, A.; Santos, J.L.; Pisciottano, C.; Grignola, R.; Javiel, G.; Jorge, A.M.; Cardoso, H.; Pérez-Bravo, F. The association between HLA DQ genetic polymorphism and type 1 diabetes in a case-parent study conducted in an admixed population. Eur. J. Epidemiol. 2004, 19, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Chisnoiu, T.; Mihai, C.M.; Balasa, A.L.; Pantazi, A.C.; Andrusca, A.; Constantin, B.M.; Nedelcu, A.D.; Cambrea, S.C. Correlation between vitamin D deficiency and type 1 diabetes in children. Rom. J. Oral Rehabil. 2023, 15, 100–106. [Google Scholar]
| Mean and Std. Error | Median | Normal Values | |
|---|---|---|---|
| AST (U/L) | 23.11 ± 1.12 | 22 | 13–26 |
| ALT (U/L) | 19.5 ± 1.56 | 16.5 | 8 |
| Creatinine (mg/dL) | 0.68 ± 0.02 | 0.64 | 0.57–0.86 |
| Total-cholesterol (mg/dL) | 173.5 ± 8.19 | 164.5 | 140–200 |
| LDL-cholesterol (mg/dL) | 104.22 ± 6.11 | 95.2 | <130 |
| HDL-cholesterol(mg/dL) | 61.74 ± 2.05 | 61 | >40 |
| Triglycerides (mg/dL) | 54.06 ± 3.38 | 50.5 | 35–150 |
| TSH (μUI/mL) | 2.54 ± 0.2 | 2.41 | 0.3–3.6 |
| fT4 (ng/dL) | 1.18 ± 0.02 | 1.2 | 0.8–1.48 |
| 25(OH) vitamin D (ng/mL) | 29.47 ± 2.12 | 24.3 | >30 |
| Total calcium (mg/dL) | 9.48 ± 0.07 | 9.55 | 8.4–10.2 |
| Phosphorus (mg/dL) | 4.74 ± 0.12 | 4.8 | 2.5–4.5 |
| PTH (pg/mL) | 33.37 ± 2.96 | 28.18 | 15–65 |
| ATPO (IU/mL) | 58.85 ± 29.08 | 9 | <16 |
| ATG (IU/mL) | 63.6 ± 26.64 | 10.19 | <20 |
| Anti-transglutaminase antibodies (IU/mL) | 0.882 ± 0326 | 0.45 | <10 |
| Alleles and Haplotypes | Score | p-Value |
|---|---|---|
| A 24:02 | 2.536 | 0.111 |
| A01 | 0.190 | 0.663 |
| A02 | 0.579 | 0.447 |
| A11:01 | 1.381 | 0.240 |
| A32:01 | 0.048 | 0.827 |
| B44:03 | 0.416 | 0.519 |
| B39:06 | 0.435 | 0.510 |
| B18:01 | 1.897 | 0.168 |
| B35:02 | 0.435 | 0.510 |
| C03:03 | 2.132 | 0.144 |
| C07:02 | 0.292 | 0.589 |
| DPB1*03:01 | 0.020 | 0.887 |
| DQB1*03:02 | 0.010 | 0.919 |
| DQB1*02:01 | 1.211 | 0.271 |
| DQB1*05:03 | 0.435 | 0.510 |
| DQB1*05:01 | 0.006 | 0.941 |
| DR3 | 1.211 | 0.271 |
| DQ2 = DQA1*05:01-DQB1*02:01 | 1.211 | 0.271 |
| DR3 + DQ2 | 1.211 | 0.271 |
| DRB1*03:01-DQA1*05:01-DQB1*03:02 | 0.585 | 0.444 |
| DR4-04:01/04:02/04:04/04:05/04:08 | 1.973 | 0.160 |
| DQ8 = DQA1*03-DQB1*03:02 | 0.190 | 0.663 |
| DR4 + DQ8 | 0.045 | 0.832 |
| DQA1*03-DQB1*03:04/DQB1*02 | 1.408 | 0.235 |
| DR4 + DQA1*03-DQB1*03:04/DQB1*02 | 1.408 | 0.235 |
| DR4 + DQA1*03:01-DQB1*04:01 | 2.429 | 0.119 |
| DR4 + DQA1*03:01-DQB1*02:01 | 0.262 | 0.609 |
| DRB1*04:01-DQA1*-DQB1*03:01 | 2.429 | 0.119 |
| Absence of DQA1*03:01-DQB1*03:02-DRB1*04:03 | 0.435 | 0.510 |
| Absence of DQA1*01:03-DQB1*06:03-DRB1*13:01 | 2.429 | 0.119 |
| Absence of DQ7 = DQA1*03-DQB1*03:01 | 0.416 | 0.519 |
| Absence of DQ7 + DRB1*04 | 0.435 | 0.510 |
| Absence of DPB1*04:02 | 4.400 | 0.036 |
| Absence of DPB1*04:01 | 9.491 | 0.002 |
| Alleles and Haplotypes | Score | p-Value |
|---|---|---|
| A 24:02 | 1.062 | 0.303 |
| A01 | 0.08 | 0.777 |
| A02 | 0.39 | 0.532 |
| A11:01 | 0.935 | 0.334 |
| A32:01 | 0.02 | 0.887 |
| B44:03 | 0.945 | 0.331 |
| B39:06 | 0.294 | 0.588 |
| B18:01 | 1.286 | 0.257 |
| B35:02 | 0.294 | 0.588 |
| C03:03 | 3.74 | 0.053 |
| C07:02 | 0.017 | 0.898 |
| DPB1*03:01 | 0.234 | 0.629 |
| DQB1*03*02 | 0.009 | 0.926 |
| DQB1*02*01 | 1.286 | 0.257 |
| DQB1*05*03 | 0.294 | 0.588 |
| DQB1*05*01 | 0.129 | 0.72 |
| DR3 | 1.286 | 0.257 |
| DQ2 = DQA1*05*01-DQB1*02*01 | 1.286 | 0.257 |
| DR3 + DQ2 | 1.286 | 0.257 |
| DRB1*03:01-DQA1*05:01-DQB1*03:02 | 0.129 | 0.72 |
| DR4-04:01/04:02/04:04/04:05/04:08 | 0.963 | 0.326 |
| DQ8 = DQA1*03-DQB1*03:02 | 0.321 | 0.571 |
| DR4 + DQ8 | 0.15 | 0.699 |
| DQA1*03-DQB1*03:04/DQB1*02 | 0.514 | 0.473 |
| DR4 + DQA1*03-DQB1*03:04/DQB1*02 | 0.514 | 0.473 |
| DR4 + DQA1*03:01-DQB1*04:01 | 0.294 | 0.588 |
| DR4 + DQA1*03:01-DQB1*02:01 | 0.017 | 0.898 |
| DR4 + DQA1*05:01-DQB1*03:02 | 0.129 | 0.72 |
| DRB1*04:01-DQA1*03-DQB1*03:01 | 3.6 | 0.058 |
| Absence of DQA1*01:03-DQB1*06:03-DRB1*13:01 | 3.6 | 0.058 |
| Absence of DQ7 = DQA1*03-DQB1*03:01 | 0.945 | 0.331 |
| Absence of DQ7 + DRB1*04 | 0.294 | 0.588 |
| Absence of DPB1*04:02 | 0.02 | 0.887 |
| Absence of DPB1*04:01 | 4.702 | 0.03 |
| Alleles and Haplotypes | Score | p-Value |
|---|---|---|
| A 24:02 | 2.408 | 0.121 |
| A01 | 1.015 | 0.314 |
| A02 | 1.797 | 0.18 |
| A11:01 | 0.187 | 0.666 |
| A32:01 | 0.256 | 0.613 |
| B44:03 | 0.121 | 0.728 |
| B39:06 | 0.059 | 0.809 |
| B18:01 | 0.256 | 0.613 |
| B35:02 | 0.059 | 0.809 |
| C03:03 | 4.98 | 0.026 |
| C07:02 | 2.408 | 0.121 |
| DPB1*03:01 | 0.187 | 0.666 |
| DQB1*03:02 | 1.145 | 0.285 |
| DQB1*02:01 | 0.298 | 0.585 |
| DQB1*05:03 | 0.059 | 0.809 |
| DQB1*05:01 | 0.493 | 0.482 |
| DR3 | 0.298 | 0.585 |
| DQ2 = DQA1*05:01-DQB1*02:01 | 0.298 | 0.585 |
| DR3 + DQ2 | 0.298 | 0.585 |
| DRB1*03:01-DQA1*05:01-DQB1*03:02 | 0.409 | 0.522 |
| DR4-04:01/04:02/04:04/04:05/04:08 | 0.014 | 0.906 |
| DQ8 = DQA1*03-DQB1*03:02 | 1.015 | 0.314 |
| DR4 + DQ8 | 0.895 | 0.344 |
| DQA1*03-DQB1*03:04/DQB1*02 | 0.409 | 0.522 |
| DR4 + DQA1*03-DQB1*03:04/DQB1*02 | 0.409 | 0.522 |
| DR4 + DQA1*03:01-DQB1*04:01 | 0.059 | 0.809 |
| DR4 + DQA1*03:01-DQB1*02:01 | 0.33 | 0.565 |
| DR4 + DQA1*05:01-DQB1*03:02 | 0.409 | 0.522 |
| DRB1*04:01-DQA1*03-DQB1*03:01 | 17.986 | <0.001 |
| Absence of DQA1*0301-DQB1*03:02-DRB1*04:03 | 0.059 | 0.809 |
| Absence of DQA1*0103-DQB1*06:03-DRB1*13:01 | 17.986 | <0.01 |
| Absence of DQ7 = DQA1*03-DQB1*03:01 | 0.121 | 0.728 |
| Absence of DQ7 + DRB1*04 | 0.059 | 0.809 |
| Absence of DPB1*04:02 | 0.256 | 0.613 |
| Alleles and Haplotypes | Score | p-Value |
|---|---|---|
| A 24:02 | 1.207 | 0.272 |
| A01 | 1.117 | 0.291 |
| A02 | 1.137 | 0.286 |
| A11:01 | 1.987 | .0159 |
| A32:01 | 0.282 | 0.595 |
| B44:03 | 3.473 | 0.062 |
| B39:06 | 0.626 | 0.429 |
| B18:01 | 0.282 | 0.595 |
| B35:02 | 0.626 | 0.429 |
| C03:03 | 0.028 | 0.867 |
| C07:02 | 1.207 | 0.272 |
| DPB1*03:01 | 5.363 | 0.021 |
| DQB1*03:02 | 0.426 | 0.514 |
| DQB1*02:01 | 1.244 | 0.265 |
| DQB1*05:03 | 0.626 | 0.429 |
| DQB1*05:01 | 0.092 | 0.761 |
| DR3 | 1.244 | 0.265 |
| DQ2 = DQA1*05:01-DQB1*02:01 | 1.244 | 0.265 |
| DR3 + DQ2 | 1.244 | 0.265 |
| DRB1*03:01-DQA1*05:01-DQB1*03:02 | 0.450 | 0.502 |
| DR4-04:01/04:02/04:04/04:05/04:08 | 0.087 | 0.769 |
| DQ8 = DQA1*03-DQB1*03:02 | 0.153 | 0.695 |
| DR4 + DQ8 | 0.014 | 0.904 |
| DQA1*03-DQB1*03:04/DQB1*02 | 1.365 | 0.243 |
| DR4 + DQA1*03-DQB1*03:04/DQB1*02 | 1.365 | 0.243 |
| DR4 + DQA1*03:01-DQB1*04:01 | 0.626 | 0.429 |
| DR4 + DQA1*03:01-DQB1*02:01 | 0.011 | 0.915 |
| DR4 + DQA1*05:01-DQB1*03:02 | 0.450 | 0.502 |
| DRB1*04:01- DQA1*03-DQB1*03:01 | 1.688 | 0.194 |
| Lack of DQA1*03:01-DQB1*03:02- DRB1*04:03 | 0.626 | 0.429 |
| Lack of DQA1*01:03-DQB1*06:03- DRB1*13:01 | 1.688 | 0.194 |
| Absence of DQ7 = DQA1*03- DQB1*03:01 | 0.133 | 0.715 |
| Absence of DQ7 + DRB1*04 | 0.626 | 0.429 |
| Absence of DPB1*04:02 | 2.633 | 0.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arhire, A.I.; Ioacara, S.; Papuc, T.; Chiper, M.S.; Dutescu, I.M.; Moise, A.; Badea, I.R.; Florea, S.; Vlad, A.; Fica, S. Association of HLA Haplotypes with Autoimmune Pathogenesis in Newly Diagnosed Type 1 Romanian Diabetic Children: A Pilot, Single-Center Cross-Sectional Study. Life 2024, 14, 781. https://doi.org/10.3390/life14060781
Arhire AI, Ioacara S, Papuc T, Chiper MS, Dutescu IM, Moise A, Badea IR, Florea S, Vlad A, Fica S. Association of HLA Haplotypes with Autoimmune Pathogenesis in Newly Diagnosed Type 1 Romanian Diabetic Children: A Pilot, Single-Center Cross-Sectional Study. Life. 2024; 14(6):781. https://doi.org/10.3390/life14060781
Chicago/Turabian StyleArhire, Amalia Ioana, Sorin Ioacara, Teodora Papuc, Miruna Sânziana Chiper, Irina Monica Dutescu, Ana Moise, Ioana Roxana Badea, Suzana Florea, Adelina Vlad, and Simona Fica. 2024. "Association of HLA Haplotypes with Autoimmune Pathogenesis in Newly Diagnosed Type 1 Romanian Diabetic Children: A Pilot, Single-Center Cross-Sectional Study" Life 14, no. 6: 781. https://doi.org/10.3390/life14060781





