Abstract
Matrix metalloproteinase (MMP)-2 and -9, which degrade type IV collagen, are linked to cancer invasion and metastasis. Gene polymorphisms in MMP-2 and MMP-9 can influence their function, impacting cancer development and progression. This study analyzed the association between polymorphisms MMP-2 rs243865 (C-1306T), rs2285053 (C-735T), and MMP-9 rs3918242 (C-1562T) with serum concentrations of these enzymes in upper tract urothelial cancer (UTUC) patients. We conducted a case–control study with 218 UTUC patients and 580 healthy individuals in Taiwan. Genotyping was performed using PCR/RFLP on DNA from blood samples, and MMP-2 and MMP-9 serum levels and mRNA expressions in 30 UTUC patients were measured using ELISA and real-time PCR. Statistical analysis showed that MMP-2 rs2285053 and MMP-9 rs3918242 genotypes were differently distributed between UTUC patients and controls (p = 0.0199 and 0.0020). The MMP-2 rs2285053 TT genotype was associated with higher UTUC risk compared to the CC genotype (OR = 2.20, p = 0.0190). Similarly, MMP-9 rs3918242 CT and TT genotypes were linked to increased UTUC risk (OR = 1.51 and 2.92, p = 0.0272 and 0.0054). In UTUC patients, TT carriers of MMP-2 rs2285053 and MMP-9 rs3918242 showed higher mRNA and protein levels (p < 0.01). These findings suggest that MMP-2 rs2285053 and MMP-9 rs3918242 genotypes are significant markers for UTUC risk and metastasis in Taiwan.
1. Introduction
Urothelial carcinoma, one of the most prevalent tumors, can originate in the upper urinary tract (pyelocaliceal cavities and ureter) or lower urinary tract (bladder and urethra). Upper tract urothelial carcinoma (UTUC) is specifically referred to when the carcinoma arises in the upper tract and is often studied separately. In Taiwan, the incidence of urothelial tumors has been steadily increasing over recent years [1]. While UTUC accounts for only 5% of urothelial carcinomas in Western countries [2,3], it constitutes up to 31% in Taiwan [4,5,6], making Taiwan a high-incidence area for UTUC [7]. This discrepancy is possibly linked to environmental risk factors, such as arsenic-contaminated drinking water [8] and aristolochic acid from Chinese herbal medicine [9]. Given the higher UTUC incidence in Taiwan, genomic and proteomic studies in this population could yield valuable insights when compared to Western populations. Although risk factors such as smoking [10], analgesic abuse [11], arsenic contamination [12], and occupational exposure [9] have been associated with UTUC, these have provided limited clinical utility. Recent evidence suggests that genetic variations may predispose individuals to UTUC, potentially serving as predictive indicators [13,14].
Matrix metalloproteinases (MMPs), also known as matrixins, are a group of peptidases integral to inflammation, carcinogenesis, and cancer cell migration through the regulation of extracellular matrix (ECM) components [15,16]. Among these, MMP-2 and MMP-9 are particularly well studied. As gelatinases, MMP-2 and MMP-9 degrade type IV collagen in the basement membrane, contributing to carcinogenic processes such as cell proliferation, angiogenesis, and tumor metastasis when their activity is dysregulated [17,18,19]. These enzymes are crucial for maintaining cellular processes, including growth, inflammation, wound healing, cell remodeling, and angiogenesis. Research indicates that MMP-2 and MMP-9 play significant roles in cancer cell proliferation and invasion, impacting tumor growth, aggressive progression, and patient survival in UTUC [20,21].
In recent years, numerous genetic studies have explored the associations between MMP-2 and MMP-9 polymorphisms and cancer risk across various types of cancer, including head and neck [22,23,24], lung [25,26,27,28], esophageal [29,30,31], breast [32,33,34,35], hepatocellular [36,37], gastric [30,38,39,40], colorectal [41,42,43,44], gallbladder [45,46], cervical [47], bladder [48,49], renal [50], and prostate cancer [51,52,53] in diverse populations. However, no studies have been published on the role of MMP-2 or MMP-9 genotypes in UTUC patients indexed in the MEDLINE (PubMed) database. This gap may be due to the low incidence of UTUC, challenges in sample collection, and limited sample sizes.
Given this context, our study aims to investigate the potential association between MMP-2 rs243865 (C-1306T), rs2285053 (C-735T), and MMP-9 rs3918242 (C-1562T) genotypes and the risk of developing UTUC in a Taiwanese cohort of 218 UTUC patients and 580 healthy controls. Additionally, we will explore the genotype–phenotype correlation of MMP-2 and MMP-9 and assess the metastatic potential related to these MMPs to understand the aggressiveness of UTUC.
2. Materials and Methods
2.1. Recruitment of UTUC Patients and Non-UTUC Control Groups
A total of 218 UTUC patients were recruited at China Medical University, all diagnosed via pathological examination of biopsy or surgical resection specimens. Clinical and histopathological data were meticulously collected from patient charts and pathological reports, subsequently reviewed, and entered into a database. Tumor staging was conducted using the TNM system [54], while pathological grading followed the World Health Organization criteria [55]. For the control group, 580 healthy individuals, matched by age and admitted to the same hospital for health checkups, were selected. These controls had no history of neoplastic urological disease or other malignancies. All participants provided informed consent, and the study was approved by the Human Research Committees (DMR104-IRB-158).
2.2. Genotyping Methodology of MMP-2 rs243865 and rs2285053
In this study, DNA was extracted from the peripheral blood leukocytes of each participant using the QIAamp Blood Mini Kit (Blossom, Taipei, Taiwan), following the methodology outlined in previous publications [56,57,58]. The primers, restriction endonucleases, and PCR conditions for genotyping MMP-2 rs243865 and rs2285053 were consistent with our earlier publications [51,59]. Specifically, PCR fragments were digested overnight with restriction enzymes Xsp I and Hinf I (New England Biolabs, Taipei, Taiwan) for MMP-2 rs243865 and rs2285053, respectively. The genotyping profiles were subsequently analyzed by two independent researchers using 3% agarose gel electrophoresis to ensure accuracy.
2.3. Genotyping Methodology of MMP-9 rs3918242
The primer design, selection of restriction endonucleases, and PCR conditions for genotyping MMP-9 rs3918242 followed the protocols established in our previous studies [60,61]. PCR fragments were digested overnight with the restriction enzyme Sph I (New England Biolabs, Taipei, Taiwan) for MMP-9 rs3918242. The genotyping results, based on the digestibility of C allele and T allele DNA sequences with Sph I, were as follows: the CC genotype produced a 386 bp fragment, the CT genotype produced fragments of 386, 320, and 66 bp, and the TT genotype produced fragments of 320 and 66 bp.
2.4. Transcriptional Expression of MMP-2 and MMP-9
To investigate the relationship between mRNA expression and high-risk MMP-2 and MMP-9 genotypes, we collected 30 tissue samples from UTUC patients and extracted RNA using Qiagen RNA extraction kits. Real-time quantitative PCR was performed using an FTC-3000 instrument (Funglyn Biotech Inc., Toronto, ON, Canada). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the internal control for quantitative analysis, as previously described [62,63]. The primer sequences were as follows: for MMP-2 mRNA, forward 5′-GTGCTTACCTAGCACATGCAAT-3′ and reverse 5′-CGCATGGTCTCGATGGTATTC-3′; for MMP-9 mRNA, forward 5′-TTCCTTGGTCTGGTGTCCC-3′ and reverse 5′-CCCACTTCTTGTCGCTGTC-3′; and for GAPDH mRNA, forward 5′-GAAATCCCATCACCATCTTCCAGG-3′ and reverse 5′-GAGCCCCAGCCTTCTCCATG-3′. The results were averaged from three independent tests and normalized to GAPDH expression.
2.5. Translational Expression of MMP-2 and MMP-9
Protein extraction from the 30 UTUC patient tissue samples was conducted as described in previous publications [64,65]. Protein concentrations were determined using the Bradford protein assay reagent (Bio-Rad, Hercules, CA, USA) with BSA as the standard. Protein extracts were prepared in sample buffer (62 mM Tris–HCl, 2% SDS, 10% glycerol, 5% β-mercaptoethanol) and heated at 97 °C for 5 min. Equal amounts (50 µg) of denatured protein were loaded per lane, separated by 8–15% SDS-PAGE, and transferred onto PVDF membranes overnight. Membranes were blocked with 5% non-fat dried milk in PBS containing 1% Tween-20 for 1 h at room temperature, then incubated with primary antibodies against MMP-2 and MMP-9 for 2 h. Subsequently, blots were incubated with HRP-conjugated anti-mouse or anti-rabbit secondary antibodies (1:5000) overnight at room temperature. Detection was performed using the Immobilon Western-HRP Substrate (Millipore, Billerica, MA, USA) with enhanced chemiluminescence.
2.6. Statistical Analysis Methodology
To ensure the control group accurately represented the general population, a Hardy–Weinberg equilibrium assessment was conducted using a goodness-of-fit test to detect deviations in genotype frequencies at polymorphic sites on the MMP-2 and MMP-9 genes. An unpaired Student’s t-test was used to compare various parameters, including age and quantitative mRNA and protein levels, between the case and control groups. Pearson’s chi-square test with Yates’ correction was utilized to compare the distribution of genotypes among subgroups. A p-value of less than 0.05 was considered statistically significant for all tests. Logistic regression analysis was employed to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for genotypes associated with UTUC.
3. Results
3.1. Demographic Characteristics of the UTUC Population
Table 1 displays the frequency distributions of clinical characteristics among participants, including 218 UTUC patients and 580 healthy controls. Epidemiologically, gender (p = 0.4256) and age (p = 0.8518) showed no significant differences, indicating well-matched populations. From a clinical and pathological perspective, tumors were distributed across renal pelvic (38.5%), ureter (34.9%), and multiple sites (26.6%). Among UTUC patients, 18.8% experienced metastasis, 60.6% had high-grade tumors, and 77.1% were at stages lower than pT3 (Table 1).

Table 1.
Demographics of the 218 UTUC patients and 580 healthy controls.
3.2. The Genotyping Outcomes for the UTUC Patients and Non-UTUC Control Groups
Table 2 illustrates the distribution of MMP-2 rs243865, rs2285053, and MMP-9 rs3918242 genotypes among 218 UTUC cases and 580 non-UTUC healthy controls. The analysis revealed differential distribution of MMP-2 rs2285053 and MMP-9 rs3918242 genotypes between the UTUC and non-UTUC control groups (p for trend = 0.0199 and 0.0020, respectively). Specifically, the MMP-2 rs2285053 homozygous variant TT was associated with increased UTUC risk compared to the wild-type CC genotype (OR = 2.20, 95%CI = 1.18–4.10, p = 0.0190). However, the MMP-2 rs2285053 heterozygous variant CT did not show association with UTUC risk (OR = 1.34, 95%CI = 0.97–1.87, p = 0.0946). In the dominant model, MMP-2 rs2285053 CT + TT genotypes were significantly higher in the UTUC group compared to the non-UTUC control group (OR = 1.44, 95%CI = 1.05–1.98, p = 0.0261). Regarding MMP-9 rs3918242, both the heterozygous variant CT and homozygous variant TT were associated with elevated UTUC risk compared to the wild-type CC genotype (OR = 1.51 and 2.92, 95%CI = 1.06–2.15 and 1.41–6.06, p = 0.0272 and 0.0054, respectively). The MMP-9 rs3918242 CT + TT genotypes were significantly higher in the UTUC group compared to the non-UTUC control group (OR = 1.66, 95%CI = 1.19–2.32, p = 0.0035). Conversely, none of the genetic models showed MMP-2 rs243865 to be associated with UTUC risk.

Table 2.
Genotypic frequency distributions of MMP-2 rs243865, rs2285053, and MMP-9 rs3918242 among 218 UTUC patients and 580 healthy controls.
3.3. The Allelic Frequency Distribution Analyzing Outcomes for the UTUC Patients and Non-UTUC Control Groups
In the UTUC group, the frequency of the MMP-2 rs243865 variant allele T was 11.5%, which did not significantly differ from the 10.1% observed in the non-UTUC control group (OR = 1.15, 95%CI = 0.81–1.64, p = 0.4766, Table 3 top part). Consistent with the findings in Table 2, the allelic frequencies of the variant T alleles for MMP-2 rs2285053 and MMP-9 rs3918242 were both significantly higher in UTUC cases than in the non-UTUC control groups (Table 3 middle and bottom parts).

Table 3.
Allelic frequencies for MMP-2 rs243865, rs2285053, and MMP-9 rs3918242 among 218 UTUC patients and 580 healthy controls.
3.4. The mRNA and Protein Expression Levels of MMP-2 and MMP-9
We aimed to explore the correlations between MMP-2 and MMP-9 genotypes and their phenotypes. Firstly, we analyzed genotype-based mRNA expression levels among 30 UTUC patients. At the MMP-2 rs243865 polymorphic site, twenty-one, seven, and two patients were CC, CT, and TT carriers, respectively. No statistically significant difference was observed in MMP-2 mRNA expression levels among CC, CT, or TT carriers (all p > 0.05, Figure 1A). For MMP-2 rs2285053, 16, 10, and 4 patients carried the CC, CT, and TT genotypes. The mRNA transcript level was significantly higher in CT carriers (p = 0.0189) and even higher in TT carriers (p = 0.0011) compared to CC carriers (Figure 1B). Regarding MMP-9 rs3918242, CT carriers exhibited higher mRNA transcript levels than CC carriers (p = 0.0036), while TT carriers had higher levels than both CT (p = 0.0034) and CC (p < 0.0001) carriers (Figure 1C).
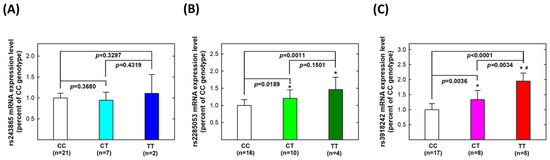
Figure 1.
Expression levels of mRNA transcripts of MMP-2 and MMP-9 genes in tissue samples collected from patients with UTUC according to genotype at MMP-2 rs243865 (A), MMP-2 rs2285053 (B), and MMP-9 rs3918242 (C). The average (fold) expression levels were normalized, applying GAPDH as an internal standard. Each assay was conducted at least three times. * Significantly different from CC genotypes; # significantly different from CT genotypes.
Secondly, we investigated genotype-based protein levels among the same 30 UTUC patients. No statistically significant difference was found in MMP-2 protein expression levels among CC, CT, or TT carriers of MMP-2 rs243865 (all p > 0.05, Figure 2A). For MMP-2 rs2285053, protein expression levels were significantly higher in CT carriers (p = 0.0001) and further higher in TT carriers (p = 0.0054) compared to CC carriers (Figure 2B). Regarding MMP-9 rs3918242, CT carriers exhibited higher protein expression levels than CC carriers (p < 0.0001), while TT carriers had higher levels than both CT (p = 0.0119) and CC (p < 0.0001) carriers (Figure 2C).
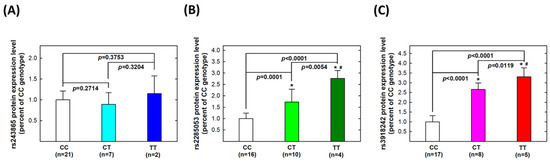
Figure 2.
Expression levels of proteins of MMP-2 and MMP-9 genes in tissue samples collected from patients with UTUC according to genotype at MMP-2 rs243865 (A), MMP-2 rs2285053 (B), and MMP-9 rs3918242 (C). The average (fold) expression levels were normalized, applying β-actin as an internal standard. Each assay was conducted at least three times. * Significantly different from CC genotypes; # significantly different from CT genotypes.
3.5. The Associations of SNPs with the Risks of Metastasis in UTUC Patients
Given the reported predictive role of MMP-2 and/or MMP-9 overexpression in metastatic progression in various cancers, we sought to investigate whether MMP-2 and/or MMP-9 genotypes were linked to metastatic risk in UTUC. We found that patients carrying the variant genotypes (CT + TT) of MMP-2 rs2285053 and MMP-9 rs3918242 were associated with significantly increased risks of metastasis (OR = 2.47, 95% CI = 1.20–4.98; and OR = 3.47, 95% CI = 1.71–7.00, respectively). MMP-2 rs243865 showed no significant association (Table 4).

Table 4.
Associations of MMP-2 rs243865, rs2285053, and MMP-9 rs3918242 with the risks of metastasis among 218 UTUC patients.
4. Discussion
Over the past decade, the Cancer Genome Atlas (TCGA) has been instrumental in linking molecular biomarkers with comprehensive insights into the molecular pathways underlying carcinogenesis, tumor progression, and potential therapeutic targets [66]. Despite this, studies on molecular markers for UTUC have predominantly focused on tissue-based markers like p53 [67], Ki67 [68], and HER-2 [69], often limited by sample size. As early as 2000, Nakanish et al. reported a positive correlation between MMP-2 staining levels and UTUC stage in 102 Japanese cases [70]. Subsequent studies by Miyata et al. provided evidence of MMP-2 expression significantly correlating with tumor stage and grade in 91 UTUC patients, although predictive impacts on survival were insignificant [71]. In 2005, Kamijima et al. found little correlation between p53, Ki-67, MMP-2, and MMP-9 overexpression and survival status among 69 UTUC patients, except for Ki-67 [72]. However, invasive sampling inconveniences and errors from tissue staining hinder precision medicine. In our case–control study, we examined MMP-2 and MMP-9 genotypic profiles in 798 Taiwanese subjects, including 218 UTUC cases and 580 non-UTUC controls (as shown in Table 1). Our study, the first to assess MMP-2 and MMP-9 genotypes’ impact on UTUC risk, highlighted the differential distribution of MMP-2 rs2285053 and MMP-9 rs3918242 genotypes between UTUC and control groups (in Table 2). This supports the potential of MMP-2 rs2285053 and MMP-9 rs3918242 variant genotypes as novel diagnostic predictors for UTUC, with MMP-9 rs3918242 possibly more sensitive, as even the heterozygous variant genotype reached significance (Table 2).
We conducted a genotype–phenotype correlation analysis on tumor tissues from thirty UTUC patients. Our findings revealed significantly higher mRNA transcript and protein levels in variant genotype carriers compared to wild-type genotype carriers for both MMP-2 rs2285053 and MMP-9 rs3918242 polymorphic sites (Figure 1 and Figure 2). Despite studies by Nakanish, Miyata, and Kamijima’s groups not supporting MMP-2 rs2285053 or MMP-9 rs3918242 as effective overall survival predictors [70,71,72], our results provide evidence of their significant roles in determining and predicting metastatic potential (Table 3). The presence of the variant T allele at both MMP-2 rs2285053 and MMP-9 rs3918242 appeared to elevate metastatic risk. Importantly, MMP-2 and MMP-9 emerged as determinants and predictors for UTUC, not only in diagnostic susceptibility but also prognostic metastatic potential. In the existing literature, the only MMP member reported to associate with metastasis is MMP-11. In 2016, MMP-11 overexpression was associated with aggressive tumor phenotype and unfavorable clinical outcomes in UTUC [73].
We hypothesized that MMPs play a pivotal role in ECM remodeling and are implicated in various processes of carcinogenesis. The clinical significance of MMPs, particularly MMP-2 and MMP-9, has been elucidated in numerous dysregulated conditions such as neoplastic, autoimmune, and chronic inflammation disorders [74,75,76]. MMP-mediated degradation of ECM components facilitates tumor cell invasion and metastasis in the microenvironment [77,78]. Among MMP members, MMP-1, -2, -3, -9, and -13 have been exclusively associated with cancer metastasis [79,80]. In the literature, MMP-2 and MMP-9 are frequently implicated in the development and expansion of tumor cells in bone metastasis [81,82,83]. In our study, we found that the MMP-2 rs2285053 and MMP-9 rs3918242 genotypes were associated with metastasis (Table 4). Risky genotypes (CT and TT) of MMP-2 rs2285053 and MMP-9 rs3918242 correlated with elevated transcriptional and translational levels of MMP-2 and MMP-9 (Figure 1 and Figure 2). MMP-9 and MMP-2 belong to gelatinases, one of the five groups in the MMP family, based on structure and substrate specificity [84]. MMP-2 specifically degrades type IV collagen and denatured collagens [85]. Our genotype-based phenotypic results showed that mRNA and protein expression of MMP-2 were highest in TT carriers, followed by CT and CC carriers at both MMP-2 rs2285053 and MMP-9 rs3918242 sites (Figure 1 and Figure 2). MMP-9 enhances tumor cell metastatic capacity by degrading collagen proteins of the ECM after activation by extracellular proteases [86,87]. In our study, mRNA and protein levels of MMP-9 were highest in TT carriers, followed by CT carriers, then CC carriers at both MMP-2 rs2285053 and MMP-9 rs3918242 (Figure 1 and Figure 2). Notably, numerous studies have demonstrated that MMP-2 and MMP-9 inhibition undermines tumor metastasis capability [88,89,90,91]. Several research groups have proposed strategies for enhancing MMP inhibitors’ effectiveness, particularly MMP-2 and MMP-9, in cancer treatments [92,93]. Further investigation is warranted to ascertain whether MMP-2 and/or MMP-9 are significantly more highly expressed in UTUC tissues compared to adjacent non-UTUC tissues and if the suppression of MMP-2 and/or MMP-9 can reduce the metastatic capacity of UTUC primarily cultured cells.
Although we have shown that SNPs in MMP2 and MMP9 are associated with increased risks of UTUC and metastasis, the effect of individual SNPs on cancer risks and outcomes is modest and not clinically applicable. Recent studies have shown the potential clinical utility of polygenic risk scores using multiple SNPs in increasing predictive power [94,95]. Furthermore, epigenetic modifications, most notably, DNA methylation, are key players in driving cancer development and have shown great potential as biomarkers for cancer risk prediction, early detection, and prognosis [96,97,98]. Future studies should incorporate genetic, epigenetic, environmental, and clinical factors to build comprehensive models for risk stratification and outcome prediction.
We have encapsulated the entire study into an easily understandable summary (Figure 3). In this investigation, we examined three SNPs known for their functional impact: rs243865 and rs2285053 of the MMP-2 gene in its promoter region and rs3918242 of the MMP-9 gene at position -1562. The rs2285053 SNP of MMP-2, situated at position -735, entails a C-to-T transition known to disrupt the binding site of specificity protein 1 (Sp1) to its mRNA, resulting in reduced transcription levels [99]. Similarly, rs3918242 of the MMP-9 gene at position -1562 also alters its promoter activity [28]. The genotypes of MMP-2 rs2285053 and MMP-9 rs3918242 exhibited strong correlations with the mRNA levels of MMP-2 and MMP-9, suggesting that specific variant genotypes might influence the concentrations of both MMP-2 and MMP-9 proteins. This could potentially elevate the risk of UTUC and enhance its metastatic potential, thereby contributing to a more aggressive UTUC phenotype (Figure 3).
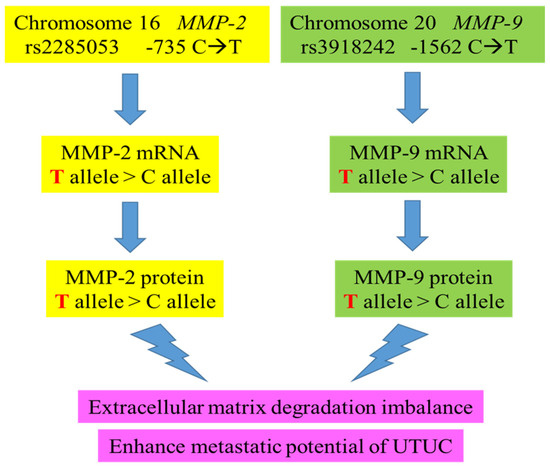
Figure 3.
The proposed genetic influence of MMP-2 and MMP-9 genotypes on their mRNAs, protein levels, and UTUC clinical endpoints of this study.
There are a few limitations to this study. First, this is a single-center study in Taiwan. Multi-center studies in diverse populations can increase the generalizability of our findings to other populations. Second, while the overall sample size was large, the number of tissue samples was limited, which may impact the robustness of the mRNA and protein expression analyses. Third, the clinical utility of SNPs remains limited. Genetic, epigenetic, environmental, and clinical factors are all important for risk prediction. Fourth, this study is based on a case–control design, which limits the ability to establish causality or track the progression of UTUC over time. Fifth, this study controls for some demographic variables but may not account for all the potential confounding factors that could influence UTUC risk and progression. Future studies should address these limitations.
5. Conclusions
In conclusion, our pilot study suggests that MMP-2 rs2285053 and MMP-9 rs3918242 hold promise as pioneering diagnostic indicators for early UTUC detection, supported by compelling phenotypic evidence. Furthermore, their potential as prognostic markers for UTUC metastatic potential adds significant value to their clinical relevance. Urgent efforts are warranted for further investigations across diverse geographical regions to elucidate the intricate involvement of MMPs, particularly MMP-2 and MMP-9, in UTUC etiology.
Author Contributions
Conceptualization, B.-R.W., H.-H.M., D.-T.B., and C.-W.T.; methodology, B.-R.W., H.-H.M., C.-H.C., and C.-H.L.; validation, M.-C.M., Y.-C.Y., and C.-W.T.; formal analysis, W.-S.C., M.-C.M., and Y.-C.Y.; investigation, W.-S.C. and M.-C.M.; resources, B.-R.W., H.-H.M., C.-H.L., and C.-H.C.; data curation, D.-T.B. and C.-W.T.; writing—original draft preparation, C.-W.T., B.-R.W., H.-H.M., and J.G.; writing—review and editing, J.G., D.-T.B., and C.-W.T.; supervision, D.-T.B.; project administration, D.-T.B. and C.-W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Asia University and China Medical University Hospital (CMU112-ASIA-03 and ASIA-112-CMUH-10), Taichung Armed Forces General Hospital (TCAFGH-D-113013), and Taichung Tzu Chi Hospital (TTCRD113-17). The funders had no role in the study design, patient data collection, experiment conduction, statistical analysis, data annotation, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of China Medical University Hospital (DMR104-IRB-158).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The genotyping results and clinical data supporting the findings of this study are available from the corresponding author upon reasonable requests via email at 017891@tool.caaumed.org.tw.
Acknowledgments
The authors are grateful to Yu-Ting Chin and Hou-Yu Shih for their excellent technical assistance. All the participants including those who were not selected into the control group of the study are appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hung, C.F.; Yang, C.K.; Ou, Y.C. Urologic cancer in Taiwan. Jpn. J. Clin. Oncol. 2016, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Cutress, M.L.; Stewart, G.D.; Zakikhani, P.; Phipps, S.; Thomas, B.G.; Tolley, D.A. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): Systematic review. BJU Int. 2012, 110, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Ouzzane, A.; Colin, P. Bladder cancer: Tumour recurrence after radical nephroureterectomy for UTUC. Nat. Rev. Urol. 2014, 11, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Chen, K.K.; Yen, C.C.; Wang, W.S.; Chang, Y.H.; Huang, W.J.; Fan, F.S.; Chiou, T.J.; Liu, J.H.; Chen, P.M. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002, 59, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liao, Y.M.; Tsai, W.M.; Kuo, H.C. Upper urinary tract urothelial carcinoma in eastern Taiwan: High proportion among all urothelial carcinomas and correlation with chronic kidney disease. J. Formos. Med. Assoc. 2007, 106, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Wu, W.J.; Lin, H.H.; Li, W.M.; Huang, C.N.; Hsu, W.C.; Chang, L.L.; Li, C.C.; Yeh, H.C.; Li, C.F.; et al. Prognostic Value of Leptin Receptor Overexpression in Upper Tract Urothelial Carcinomas in Taiwan. Clin. Genitourin. Cancer 2017, 15, e653–e659. [Google Scholar] [CrossRef]
- Shen, C.H.; Chiou, H.Y.; Tung, M.C.; Wu, C.C.; Kao, W.T.; Wang, Y.H.; Juang, G.D. Clinical and demographic characteristics among patients with urothelial carcinomas of the upper urinary tract and bladder in Taiwan. J. Chin. Med. Assoc. 2017, 80, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Roupret, M.; Babjuk, M.; Comperat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Bohle, A.; Van Rhijn, B.W.; Kaasinen, E.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur. Urol. 2015, 68, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Colin, P.; Koenig, P.; Ouzzane, A.; Berthon, N.; Villers, A.; Biserte, J.; Roupret, M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009, 104, 1436–1440. [Google Scholar] [CrossRef]
- Simsir, A.; Sarsik, B.; Cureklibatir, I.; Sen, S.; Gunaydin, G.; Cal, C. Prognostic factors for upper urinary tract urothelial carcinomas: Stage, grade, and smoking status. Int. Urol. Nephrol. 2011, 43, 1039–1045. [Google Scholar] [CrossRef]
- Colin, P.; Koenig, P.; Ballereau, C.; Phe, V.; Berthon, N.; Villers, A.; Biserte, J.; Roupret, M. Sporadic upper urinary tract urothelial cell carcinomas: Identification of interaction between toxic carcinogens and individuals genetic susceptibility. Prog. Urol. 2010, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shao, I.H.; Chang, Y.H.; Pang, S.T. Recent advances in upper tract urothelial carcinomas: From bench to clinics. Int. J. Urol. 2019, 26, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Lin, S.S.; Li, F.J.; Tsai, C.W.; Li, L.Y.; Lien, C.S.; Liao, W.L.; Wu, H.C.; Tsai, C.H.; Shih, T.C.; et al. Significant association of caveolin-1 (CAV1) genotypes with upper urothelial tract cancer. Anticancer. Res. 2013, 33, 4907–4912. [Google Scholar] [PubMed]
- Chang, W.S.; Liao, C.H.; Hsu, C.M.; Huang, C.Y.; Fang, H.Y.; Kao, P.Y.; Tsai, C.W.; Wu, H.C.; Hu, P.S.; Wang, T.C.; et al. Significant Association of Cyclo-oxygenase 2 Genotypes with Upper Tract Urothelial Cancer. Anticancer. Res. 2015, 35, 2725–2730. [Google Scholar] [PubMed]
- Lekstan, A.; Lampe, P.; Lewin-Kowalik, J.; Olakowski, M.; Jablonska, B.; Labuzek, K.; Jedrzejowska-Szypulka, H.; Olakowska, E.; Gorka, D.; Filip, I.; et al. Concentrations and activities of metalloproteinases 2 and 9 and their inhibitors (TIMPS) in chronic pancreatitis and pancreatic adenocarcinoma. J. Physiol. Pharmacol. 2012, 63, 589–599. [Google Scholar] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Butkiewicz, D.; Krzesniak, M.; Drosik, A.; Giglok, M.; Gdowicz-Klosok, A.; Kosarewicz, A.; Rusin, M.; Maslyk, B.; Gawkowska-Suwinska, M.; Suwinski, R. The VEGFR2, COX-2 and MMP-2 polymorphisms are associated with clinical outcome of patients with inoperable non-small cell lung cancer. Int. J. Cancer 2015, 137, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, X.; Qin, X.; Cai, S.; Yu, S. Association of matrix metalloproteinase family gene polymorphisms with lung cancer risk: Logistic regression and generalized odds of published data. Sci. Rep. 2015, 5, 10056. [Google Scholar] [CrossRef] [PubMed]
- Dofara, S.G.; Chang, S.L.; Diorio, C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer. Res. 2020, 40, 3619–3631. [Google Scholar] [CrossRef]
- Miyata, Y.; Mitsunari, K.; Akihiro, A.; Watanabe, S.I.; Mochizuki, Y.; Sakai, H. Smoking-induced changes in cancer-related factors in patients with upper tract urothelial cancer. Mol. Clin. Oncol. 2015, 3, 287–294. [Google Scholar] [CrossRef]
- Su, Y.L.; Luo, H.L.; Huang, C.C.; Liu, T.T.; Huang, E.Y.; Sung, M.T.; Lin, J.J.; Chiang, P.H.; Chen, Y.T.; Kang, C.H.; et al. Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells 2020, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.W.; Gong, C.L.; Hsu, H.M.; Chao, C.C.; Wang, Y.C.; Chang, W.S.; Tsai, Y.T.; Shih, L.C.; Tsai, C.W.; Bau, D.T. Contribution of Matrix Metalloproteinase-2 Promoter Genotypes to Nasopharyngeal Cancer Susceptibility and Metastasis in Taiwan. Cancer Genom. Proteom. 2019, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.; Silva, L.P.D.; Matos, F.R.; Silva, T.A.D.; Medeiros, S.R.B.; Souza, L.B.; Freitas, R.A. Polymorphisms of matrix metalloproteinase-7 and -9 are associated with oral tongue squamous cell carcinoma. Braz. Oral Res. 2020, 35, e019. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Hsu, H.M.; Wang, Y.C.; Chang, W.S.; Shih, L.C.; Sun, K.T.; Hung, Y.W.; Yang, Y.C.; Gong, C.L.; Bau, D.T. Contribution of MMP2 Promoter Genotypes to Oral Cancer Susceptibility, Recurrence and Metastasis in Taiwan. Anticancer. Res. 2018, 38, 6821–6826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, C.; Miao, X.; Wang, Y.; Tan, W.; Sun, T.; Zhang, X.; Xiong, P.; Lin, D. Functional haplotypes in the promoter of matrix metalloproteinase-2 and lung cancer susceptibility. Carcinogenesis 2005, 26, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arriaga, P.; Pascual, T.; Garcia-Alvarez, A.; Fernandez-Somoano, A.; Lopez-Cima, M.F.; Tardon, A. Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer 2012, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Tsai, Y.F.; Wu, W.T.; Chiu, K.L.; Tsai, C.W.; Chang, W.S.; Li, C.H.; Yang, J.S.; Mong, M.C.; Hsia, T.C.; et al. Association of Matrix Metalloproteinase-9 Genotypes With Lung Cancer Risk in Taiwan. Anticancer Res. 2024, 44, 1845–1852. [Google Scholar] [CrossRef]
- Wadowska, K.; Blasiak, P.; Rzechonek, A.; Sliwinska-Mosson, M. Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer. Int. J. Mol. Sci. 2023, 24, 10576. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhou, Y.; Miao, X.; Xiong, P.; Tan, W.; Lin, D. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res. 2004, 64, 7622–7628. [Google Scholar] [CrossRef]
- Li, Y.; Sun, D.L.; Duan, Y.N.; Zhang, X.J.; Wang, N.; Zhou, R.M.; Chen, Z.F.; Wang, S.J. Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North China. Mol. Biol. Rep. 2010, 37, 197–205. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Wang, S.; Yu, Q.; Lu, J. Association of Smoking and XPG, CYP1A1, OGG1, ERCC5, ERCC1, MMP2, and MMP9 Gene Polymorphisms with the early detection and occurrence of Laryngeal Squamous Carcinoma. J. Cancer 2018, 9, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Yari, K.; Rahimi, Z.; Moradi, M.T.; Rahimi, Z. The MMP-2 -735 C allele is a risk factor for susceptibility to breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 6199–6203. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.; Demin, S.; Churnosov, M.; Reshetnikov, E.; Aristova, I.; Churnosova, M.; Ponomarenko, I. Matrix Metalloproteinase Gene Polymorphisms Are Associated with Breast Cancer in the Caucasian Women of Russia. Int. J. Mol. Sci. 2022, 23, 12638. [Google Scholar] [CrossRef] [PubMed]
- Bartnykaite, A.; Savukaityte, A.; Bekampyte, J.; Ugenskiene, R.; Laukaitiene, D.; Korobeinikova, E.; Gudaitiene, J.; Juozaityte, E. The Role of Matrix Metalloproteinase Single-Nucleotide Polymorphisms in the Clinicopathological Properties of Breast Cancer. Biomedicines 2022, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Kohan, L.; Mirhosseini, M.; Mortazavizadeh, S.M. The risk of relapse in breast cancer patients is associated with MMP-9 gene polymorphism: A prospective study in a sample of the Iranian population. Nucleosides Nucleotides Nucleic Acids 2022, 41, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Qiu, W.; Dong, X.J.; Zhang, X.M.; Xie, W.M.; Zhang, H.X.; Yuan, X.Y.; Zhou, G.Q.; He, F.C. Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut 2007, 56, 445–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barakat, L.A.; Elsergany, A.R.; Ghattas, M.H.; Mahsoub, N.; Bondok, R.M. Relationship between interferon-induced transmembrane protein 3 and matrix metalloproteinase-9 gene polymorphisms in patients with hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102110. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.K.; Chang, W.S.; Tsai, C.W.; Wang, Y.C.; Yang, M.D.; Hsu, H.S.; Chao, C.Y.; Yu, C.C.; Chen, J.C.; Pei, J.S.; et al. The Association of MMP9 Promoter Rs3918242 Genotype With Gastric Cancer. Anticancer. Res. 2021, 41, 3309–3315. [Google Scholar] [CrossRef]
- Fu, C.K.; Mong, M.C.; Yu, C.C.; Yang, M.D.; Wang, Z.H.; Yang, Y.C.; Chen, J.C.; Pei, J.S.; Hsia, N.Y.; Tsai, C.W.; et al. Association of Matrix Metallopeptidase-2 Genotypes With Risk of Gastric Cancer in Taiwan. Anticancer. Res. 2022, 42, 1749–1755. [Google Scholar] [CrossRef]
- Okada, R.; Naito, M.; Hattori, Y.; Seiki, T.; Wakai, K.; Nanri, H.; Watanabe, M.; Suzuki, S.; Kairupan, T.S.; Takashima, N.; et al. Matrix metalloproteinase 9 gene polymorphisms are associated with a multiple family history of gastric cancer. Gastric Cancer 2017, 20, 246–253. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S.J.; Kim, K.H.; Kim, J.C. Clinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancer. J. Gastroenterol. Hepatol. 2011, 26, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gaibar, M.; Galan, M.; Romero-Lorca, A.; Anton, B.; Malon, D.; Moreno, A.; Fernandez-Santander, A.; Novillo, A. Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 1381. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.Z.; Sameer, A.S.; Mir, A.H.; Mokhdomi, T.A.; Chowdri, N.A.; Haq, E. Matrix metalloproteinase (MMP) -2, -7 and -9 promoter polymorphisms in colorectal cancer in ethnic Kashmiri population—A case-control study and a mini review. Gene 2016, 589, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yueh, T.C.; Hung, Y.C.; Lee, H.T.; Yang, M.D.; Wang, Z.H.; Yang, Y.C.; Ke, T.W.; Pei, J.S.; Tsai, C.W.; Bau, D.T.; et al. Role of Matrix Metallopeptidase-2 Genotypes in Taiwanese Patients With Colorectal Cancer. Anticancer. Res. 2022, 42, 5335–5342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.L.; Misra, S.; Kumar, A.; Mittal, B. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP-2) genetic variants to gallbladder cancer. Liver Int. 2012, 32, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Jeeyar, V.; Prasad Singh, S.; Dixit, M. Functional relevance of MMP2 promoter variants in gallbladder cancer: A case-control study in an Eastern Indian Population. Gene 2024, 913, 148372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, G.; Zhang, Z.; Wang, S.; Zhang, S. MMP-2 and MMP-9 gene polymorphisms associated with cervical cancer risk. Int. J. Clin. Exp. Pathol. 2017, 10, 11760–11765. [Google Scholar] [PubMed]
- Srivastava, P.; Kapoor, R.; Mittal, R.D. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in Northern India. Urol. Oncol. 2013, 31, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, E.; Reszka, E.; Jablonowski, Z.; Jablonska, E.; Krol, M.B.; Grzegorczyk, A.; Gromadzinska, J.; Sosnowski, M.; Wasowicz, W. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013, 112, 1207–1214. [Google Scholar] [CrossRef]
- Liao, C.H.; Tsai, C.L.; Chang, S.Y.; Lin, Y.H.; Wang, Y.C.; Huang, W.C.; Mong, M.C.; Yang, Y.C.; Wu, W.T.; Chen, J.C.; et al. Impacts of Matrix Metalloproteinase 9 Genotypes on Renal Cell Carcinoma. In Vivo 2023, 37, 2452–2458. [Google Scholar] [CrossRef]
- Li, P.H.; Liao, C.H.; Huang, W.C.; Chang, W.S.; Wu, H.C.; Hsu, S.W.; Chen, K.Y.; Wang, Z.H.; Hsia, T.C.; Bau, D.T.; et al. Association of Matrix Metalloproteinase-2 Genotypes With Prostate Cancer Risk. Anticancer Res. 2023, 43, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Kamankesh, M.; Vaisi-Raygani, A.; Moradi, M.R.; Tanhapour, M.; Rahimi, Z.; Elahi-Rad, S.; Bahrehmand, F.; Aliyari, M.; Aghaz, F.; et al. Activities and polymorphisms of MMP-2 and MMP-9, smoking, diabetes and risk of prostate cancer. Mol. Biol. Rep. 2020, 47, 9373–9383. [Google Scholar] [CrossRef] [PubMed]
- Schveigert, D.; Valuckas, K.P.; Kovalcis, V.; Ulys, A.; Chvatovic, G.; Didziapetriene, J. Significance of MMP-9 expression and MMP-9 polymorphism in prostate cancer. Tumori J. 2013, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Greene, F.L.; Page, D.L.; Fleming, I.D.; Fritz, A.G.; Balch, C.M.; Haller, D.G.; Morrow, M. (Eds.) AJCC Cancer Staging Manual, 6th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Epstein, J.I.; Amin, M.B.; Reuter, V.R.; Mostofi, F.K. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am. J. Surg. Pathol. 1998, 22, 1435–1448. [Google Scholar] [CrossRef]
- Yueh, T.C.; Wang, Y.C.; Chin, Y.T.; Hung, Y.C.; Mong, M.C.; Yang, Y.C.; Pei, J.S.; Gu, J.; Tsai, C.W.; Bau, D.T.; et al. Impact of Mir196a-2 Genotypes on Colorectal Cancer Risk in Taiwan. Int. J. Mol. Sci. 2023, 24, 11613. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.D.; Lin, K.C.; Lu, M.C.; Jeng, L.B.; Hsiao, C.L.; Yueh, T.C.; Fu, C.K.; Li, H.T.; Yen, S.T.; Lin, C.W.; et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine 2017, 7, 10. [Google Scholar] [CrossRef]
- Tsai, C.W.; Chang, W.S.; Yueh, T.C.; Wang, Y.C.; Chin, Y.T.; Yang, M.D.; Hung, Y.C.; Mong, M.C.; Yang, Y.C.; Gu, J.; et al. The Significant Impacts of Interleukin-8 Genotypes on the Risk of Colorectal Cancer in Taiwan. Cancers 2023, 15, 4921. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.S.; Hsia, N.Y.; Wang, Z.H.; Chen, H.C.; Hsia, T.C.; Lin, M.L.; Wang, Y.C.; Chang, W.S.; Bau, D.T.; Tsai, C.W. Contribution of Matrix Metalloproteinase-2 Genotypes to Taiwan Pterygium Risk. In Vivo 2024, 38, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.B.; Hsia, N.Y.; Wang, Z.H.; Yang, J.S.; Hsu, Y.M.; Wang, Y.C.; Chang, W.S.; Bau, D.T.; Yin, M.C.; Tsai, C.W. The Contribution of MMP-9 Genotypes to Pterygium in Taiwan. Anticancer Res. 2020, 40, 4523–4527. [Google Scholar] [CrossRef]
- Kuo, C.C.; Tsai, C.W.; Chang, W.S.; Shen, T.C.; Tzeng, H.E.; Li, C.H.; Wang, Y.C.; Tsai, F.J.; Bau, D.T. Contribution of Matrix Metalloproteinase-9 rs3918242 Genotypes to Childhood Leukemia Risk. Anticancer Res. 2020, 40, 5751–5756. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, W.S.; Pei, J.S.; Kuo, C.C.; Wang, C.H.; Wang, Y.C.; Hsu, P.C.; He, J.L.; Gu, J.; Bau, D.T.; et al. Non-homologous End-joining Genotype, mRNA Expression, and DNA Repair Capacity in Childhood Acute Lymphocytic Leukemia. Cancer Genom. Proteom. 2024, 21, 144–157. [Google Scholar] [CrossRef]
- Tsai, C.W.; Shih, L.C.; Chang, W.S.; Hsu, C.L.; He, J.L.; Hsia, T.C.; Wang, Y.C.; Gu, J.; Bau, D.T. Non-Homologous End-Joining Pathway Genotypes Significantly Associated with Nasopharyngeal Carcinoma Susceptibility. Biomedicines 2023, 11, 1648. [Google Scholar] [CrossRef]
- Hung, K.C.; Tien, N.; Bau, D.T.; Yao, C.H.; Chen, C.H.; Yang, J.L.; Lin, M.L.; Chen, S.S. Let-7g Upregulation Attenuated the KRAS-PI3K-Rac1-Akt Axis-Mediated Bioenergetic Functions. Cells 2023, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Hsu, W.L.; Hsu, S.W.; Chen, C.H.; Hong, K.T.; Tsai, C.W.; Chang, W.S.; Chen, C.C.; Pei, J.S.; Lee, H.T.; et al. Involvement of Mitochondrial Damage and Oxidative Stress in Apoptosis Induced by Betulin Plus Arsenic Trioxide in Neuroblastoma Cells. Anticancer Res. 2023, 43, 2467–2476. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.H.; Byun, S.S.; Jeong, H.; Kwak, C.; Kim, H.H.; Lee, S.E. The role of p53 on survival of upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Clin. Genitourin. Cancer 2013, 11, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.M.; Bagrodia, A.; Haddad, A.Q.; Kapur, P.; Khalil, D.; Hynan, L.S.; Wood, C.G.; Karam, J.A.; Weizer, A.Z.; Raman, J.D.; et al. Multi-institutional validation of the predictive value of Ki-67 in patients with high grade urothelial carcinoma of the upper urinary tract. J. Urol. 2015, 193, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Moschini, M.; Haitel, A.; Wirth, G.J.; Karam, J.A.; Wood, C.G.; Roupret, M.; Margulis, V.; Karakiewicz, P.I.; Briganti, A.; et al. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 251–259. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kawai, T.; Sato, H.; Aida, S.; Kasamatsu, H.; Aurues, T.; Ikeda, T. Expression of matrix metalloproteinase-2 (MMP-2) and of membrane-type-1-matrix metalloproteinase (MT1-MMP) in transitional cell carcinoma of the upper urinary tract. Hum. Pathol. 2000, 31, 193–200. [Google Scholar] [CrossRef]
- Miyata, Y.; Kanda, S.; Nomata, K.; Hayashida, Y.; Kanetake, H. Expression of metalloproteinase-2, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in transitional cell carcinoma of upper urinary tract: Correlation with tumor stage and survival. Urology 2004, 63, 602–608. [Google Scholar] [CrossRef]
- Kamijima, S.; Tobe, T.; Suyama, T.; Ueda, T.; Igarashi, T.; Ichikawa, T.; Ito, H. The prognostic value of p53, Ki-67 and matrix metalloproteinases MMP-2 and MMP-9 in transitional cell carcinoma of the renal pelvis and ureter. Int. J. Urol. 2005, 12, 941–947. [Google Scholar] [CrossRef]
- Li, W.M.; Wei, Y.C.; Huang, C.N.; Ke, H.L.; Li, C.C.; Yeh, H.C.; Chang, L.L.; Huang, C.H.; Li, C.F.; Wu, W.J. Matrix metalloproteinase-11 as a marker of metastasis and predictor of poor survival in urothelial carcinomas. J. Surg. Oncol. 2016, 113, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Prasad, D.; Mukherjee, S. Matrix Metalloproteinases in Oral Cancer Pathogenesis and their Use in Therapy. Anticancer Agents Med. Chem. 2024, 24, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.C.L.; Pereira, E.R.; Francelino, A.L.; Guembarovski, A.; Fuganti, P.E.; de Oliveira, K.B.; Miqueloto, C.A.; Serpeloni, J.M.; Guembarovski, R.L. Metalloproteinase 9 immunostaining profile is positively correlated with tumor grade, extraprostatic extension and biochemical recurrence in prostate cancer. Pathol. Res. Pract. 2024, 253, 155024. [Google Scholar] [CrossRef] [PubMed]
- Nannuru, K.C.; Futakuchi, M.; Varney, M.L.; Vincent, T.M.; Marcusson, E.G.; Singh, R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010, 70, 3494–3504. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Gligorijevic, B. Proteolytic and mechanical remodeling of the extracellular matrix by invadopodia in cancer. Phys. Biol. 2022, 20, 015001. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Guo, J.; Song, Z.; Muming, A.; Zhang, H.; Awut, E. Cysteine protease inhibitor S promotes lymph node metastasis of esophageal cancer cells via VEGF-MAPK/ERK-MMP9/2 pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 1–9. [Google Scholar] [CrossRef]
- Choi, E.K.; Kim, H.D.; Park, E.J.; Song, S.Y.; Phan, T.T.; Nam, M.; Kim, M.; Kim, D.U.; Hoe, K.L. 8-Methoxypsoralen Induces Apoptosis by Upregulating p53 and Inhibits Metastasis by Downregulating MMP-2 and MMP-9 in Human Gastric Cancer Cells. Biomol. Ther. 2023, 31, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, S.; Chen, Y.; Zheng, L.; Chen, L.; Ding, H.; Ding, J.; Lou, D.; Liu, F.; Zheng, B. AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-kappaB, HIF1alpha, MMP2, and MMP9 upregulation. Cell Signal. 2019, 58, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, B.; Vandooren, J.; Locatelli, E.; Fiten, P.; Opdenakker, G.; Proost, P.; Kruger, A.; Lellouche, J.P.; Israel, L.L.; Shenkman, L.; et al. Matrix metalloproteinase-9 (MMP-9) as an activator of nanosystems for targeted drug delivery in pancreatic cancer. J. Control Release 2016, 239, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Yang, X.Q.; Wang, B.C.; Liu, S.P.; Wang, F.B. Overexpression of twist and matrix metalloproteinase-9 with metastasis and prognosis in gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5055–5060. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Platt-Higgins, A.; Lee, Y.F.; Al Khatib, A.O.; Haggag, Y.; Sutherland, M.; Zhang, S.D.; Aljabali, A.A.A.; Mishra, V.; Serrano-Aroca, A.; et al. Matrix metalloproteinase 2 is a target of the RAN-GTP pathway and mediates migration, invasion and metastasis in human breast cancer. Life Sci. 2022, 310, 121046. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Chin, M.C.; Lin, C.C.; His, Y.T.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Husain, A.; Sze, K.M.; Ho, D.W.; Suarez, E.M.S.; Wang, X.; Lee, E.; Ma, H.T.; Lee, J.M.; Chan, L.K.; et al. Midline 1 interacting protein 1 promotes cancer metastasis through FOS-like 1-mediated matrix metalloproteinase 9 signaling in HCC. Hepatology 2023, 78, 1368–1383. [Google Scholar] [CrossRef]
- Gautam, J.; Banskota, S.; Lee, H.; Lee, Y.J.; Jeon, Y.H.; Kim, J.A.; Jeong, B.S. Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Tauro, M.; Shay, G.; Sansil, S.S.; Laghezza, A.; Tortorella, P.; Neuger, A.M.; Soliman, H.; Lynch, C.C. Bone-Seeking Matrix Metalloproteinase-2 Inhibitors Prevent Bone Metastatic Breast Cancer Growth. Mol. Cancer Ther. 2017, 16, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Hames, R.A.; Mastroianni, N.M.; Greenstein, A.E.; Weed, S.A. Evaluation of the matrix metalloproteinase 9 (MMP9) inhibitor Andecaliximab as an Anti-invasive therapeutic in Head and neck squamous cell carcinoma. Oral Oncol. 2022, 132, 106008. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Kiemeney, L.A.; Pal Choudhury, P.; Milne, R.L.; Lopez de Maturana, E.; Ye, Y.; Joseph, V.; Florez-Vargas, O.; Dyrskjot, L.; Figueroa, J.; et al. Genome-wide Association Study of Bladder Cancer Reveals New Biological and Translational Insights. Eur. Urol. 2023, 84, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Bau, D.T.; Tsai, C.W.; Chang, W.S.; Yang, J.S.; Liu, T.Y.; Lu, H.F.; Wang, Y.W.; Tsai, F.J. Genetic susceptibility to prostate cancer in Taiwan: A genome-wide association study. Mol. Carcinog. 2024, 63, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.Q.; Xiong, G.Y.; Yang, K.W.; Zhang, L.; He, S.M.; Gong, Y.Q.; He, Q.; Li, X.Y.; Wang, Z.C.; Bao, Z.Q.; et al. Detection of urothelial carcinoma, upper tract urothelial carcinoma, bladder carcinoma, and urothelial carcinoma with gross hematuria using selected urine-DNA methylation biomarkers: A prospective, single-center study. Urol. Oncol. 2018, 36, 342.e15–342.e23. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.M.; Chihara, Y.; Pan, F.; Weisenberger, D.J.; Siegmund, K.D.; Sugano, K.; Kawashima, K.; Laird, P.W.; Jones, P.A.; Liang, G. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010, 70, 8169–8178. [Google Scholar] [CrossRef] [PubMed]
- Grelus, A.; Nica, D.V.; Miklos, I.; Belengeanu, V.; Ioiart, I.; Popescu, C. Clinical Significance of Measuring Global Hydroxymethylation of White Blood Cell DNA in Prostate Cancer: Comparison to PSA in a Pilot Exploratory Study. Int. J. Mol. Sci. 2017, 18, 2465. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, S.C.; Shen, T.C.; Tsai, C.W.; Chang, W.S.; Li, H.T.; Wu, C.N.; Chao, C.Y.; Hsia, T.C.; Bau, D.T. The association of matrix metalloproteinas-2 promoter polymorphisms with lung cancer susceptibility in Taiwan. Chin. J. Physiol. 2019, 62, 210–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).