Management of Peritoneal Dialysis-Associated Emergencies during the COVID-19 Pandemic: The Experience of a Center of Excellence
Abstract
1. Introduction
2. Materials and Methods
3. Results
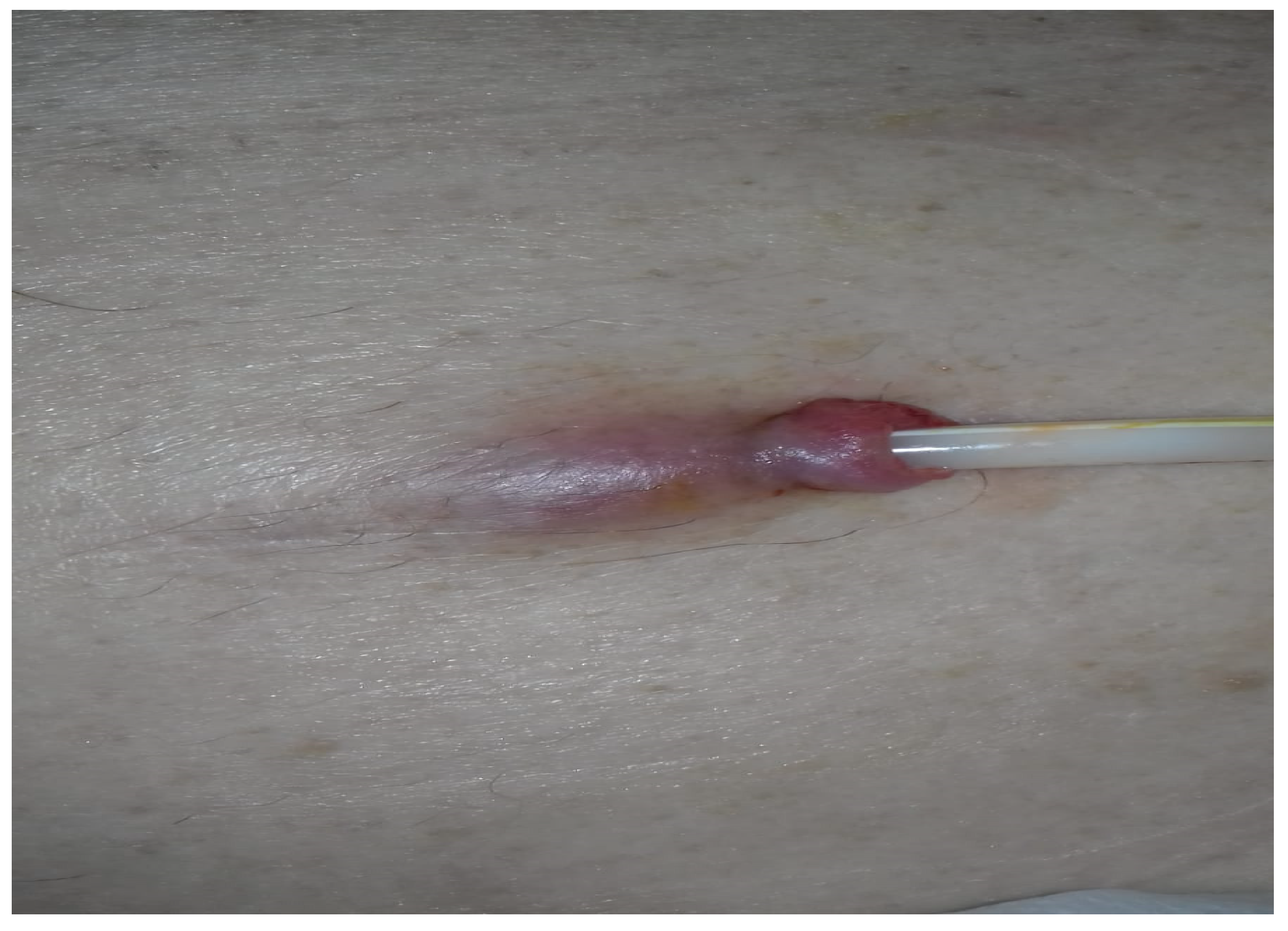
3.1. Case 1
- -
- to interrupt oral anticoagulant and antiplatelet administration one week prior to admission, prevention being ensured by in-house administration of low-molecular-weight heparin;
- -
- to monitor temperature twice a day;
- -
- to monitor the appearance of the peritoneal fluid;
- -
- to monitor the wound from the catheter exteriorization opening;
- -
- to maintain complete isolation at home throughout the period, without outside contact.
3.2. The Particularities of The Case
3.3. Case 2
3.4. The Particularities of The Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) 2022. Available online: https://www.who.int/health-topics/coronavirus (accessed on 28 March 2022).
- Ministry of Health. Available online: www.ms.ro (accessed on 20 April 2020).
- Perez-Moran, D.; Perez-Cuevas, R.; Doubova, S.V. Challenges for Peritoneal Dialysis Centers Before and During the COVID-19 Pandemic in Mexico. Arch. Med. Res. 2022, 53, 431–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Dong, J. Operational considerations for peritoneal dialysis management during the COVID-19 pandemic. Clin. Kidney J. 2020, 13, 322–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Htay, H.; Wong, P.M.P.K.; Choo, R.R.; Dawood, U.S.; Foo, M.W.Y.; Jayaballa, M.; Lee, G.; Lee, M.B.; Liu, Y.L.A.; Low, S.; et al. Strategies for Management of Peritoneal Dialysis Patients in Singapore during COVID-19 Pandemic. Ann. Acad. Med. Singap. 2020, 49, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- de Sequera, P.; Quiroga, B.; Goicoechea, M.; En Representación de la Junta Directiva de la Sociedad Española de Nefrología (S.E.N.). Actualización de las recomendaciones de medidas de prevención y aislamiento frente al SARS-CoV-2 en las unidades de diálisis: Un posicionamiento de la Sociedad Española de Nefrología [Update of the prevention and isolation measure recommendations against SARS-CoV-2 in dialysis units of Spain: A position paper of the Spanish Society of Nephrology Council]. Nefrologia 2022, 42, 714–721. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ispd.org/wp-content/uploads/ISPD-PD-management-in-COVID-19_ENG.pdf (accessed on 2 April 2021).
- Ronco, C.; Manani, S.M.; Giuliani, A.; Tantillo, I.; Reis, T.; Brown, E.A. Remote patient management of peritoneal dialysis during COVID-19 pandemic. Perit. Dial. Int. 2020, 40, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Car, J.; Koh, G.C.; Foong, P.S.; Wang, C.J. Video consultations in primary and specialist care during the covid-19 pandemic and beyond. BMJ 2020, 371, m3945. [Google Scholar] [CrossRef] [PubMed]
- El Shamy, O.; Tran, H.; Sharma, S.; Ronco, C.; Narayanan, M.; Uribarri, J. Telenephrology with Remote Peritoneal Dialysis Monitoring during Coronavirus Disease 19. Am. J. Nephrol. 2020, 51, 480–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldonado, M.; Ossorio, M.; Del Peso, G.; Santos, C.; Álvarez, L.; Sánchez-Villanueva, R.; Rivas, B.; Vega, C.; Selgas, R.; Bajo, M.A. COVID-19 incidence and outcomes in a home dialysis unit in Madrid (Spain) at the height of the pandemic. Nefrologia 2021, 41, 329–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfano, G.; Fontana, F.; Giovanella, S.; Morisi, N.; Amurri, A.; Ligabue, G.; Guaraldi, G.; Ferrari, A.; Cappelli, G.; Magistroni, R.; et al. Prevalence, clinical course and outcomes of COVID-19 in peritoneal dialysis (PD) patients: A single-center experience. Clin. Exp. Nephrol. 2023, 27, 171–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geetha, D.; Kronbichler, A.; Rutter, M.; Bajpai, D.; Menez, S.; Weissenbacher, A.; Anand, S.; Lin, E.; Carlson, N.; Sozio, S.; et al. Impact of the COVID-19 pandemic on the kidney community: Lessons learned and future directions. Nat. Rev. Nephrol. 2022, 18, 724–737, Erratum in Nat. Rev. Nephrol. 2022, 18, 738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yavuz, D.; Karagöz Özen, D.S.; Demirağ, M.D. COVID-19: Mortality rates of patients on hemodialysis and peritoneal dialysis. Int. Urol. Nephrol. 2022, 54, 2713–2718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perl, J.; Thomas, D.; Tang, Y.; Yeung, A.; Ip, J.; Oliver, M.J.; Blake, P.G. COVID-19 among Adults Receiving Home versus In-Center Dialysis. CJASN 2021, 16, 1410–1412. [Google Scholar] [CrossRef]
- National Institute of Public Health. Available online: https://cnscbt.ro/ (accessed on 29 November 2020).
- Naljayan, M.V.; Schiller, B.; Watnick, S.; Weinhandl, E.D.; Perl, J. How the COVID-19 Pandemic Hit Home in North America Lessons Learned in Improving Home Dialysis Utilization and Outcomes. Clin. J. Am. Soc. Nephrol. 2023, 18, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Yeter, H.H.; Gok Oguz, E.; Akcay, O.F.; Karaer, R.; Yasar, E.; Duranay, M.; Ayli, M.D.; Guz, G. The reliability and success of peritoneal dialysis during the COVID-19 pandemic. Semin. Dial. 2021, 34, 147–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rombolà, G.; Brunini, F. COVID-19 and dialysis: Why we should be worried. J. Nephrol. 2020, 33, 401–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romanian Renal Registry. Available online: www.registrulrenal.ro (accessed on 14 February 2023).
- Milan Manani, S.; Rosner, M.H.; Virzì, G.M.; Giuliani, A.; Berti, S.; Crepaldi, C.; Ronco, C. Longitudinal Experience with Remote Monitoring for Automated Peritoneal Dialysis Patients. Nephron 2019, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.M.; Morisi, N.; Milan Manani, S.; Tantillo, I.; Gonzàlez Barajas, J.D.; Villavicencio, B.D.; Castiglione, C.; Alfano, G.; Donati, G.; Zanella, M. Scheduling of Remote Monitoring for Peritoneal Dialysis Patients. J. Clin. Med. 2024, 13, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centellas-Pérez, F.J.; Ortega-Cerrato, A.; Vera, M.; Devesa-Buch, R.J.; Muñoz-de-Bustillo, E.; Prats, M.; Alonso-Valente, R.; Morais, J.P.; Cara-Espada, P.J.; Yuste-Lozano, C.; et al. Impact of Remote Monitoring on Standardized Outcomes in Nephrology-Peritoneal Dialysis. Kidney Int. Rep. 2023, 9, 266–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Health Topics. Available online: www.who.int/health-topics/digital-health (accessed on 7 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorga, C.; Iorga, C.R.; Andreiana, I.; Stancu, S.H.; Bengulescu, I.; Constantin, T.; Strambu, V. Management of Peritoneal Dialysis-Associated Emergencies during the COVID-19 Pandemic: The Experience of a Center of Excellence. Life 2024, 14, 805. https://doi.org/10.3390/life14070805
Iorga C, Iorga CR, Andreiana I, Stancu SH, Bengulescu I, Constantin T, Strambu V. Management of Peritoneal Dialysis-Associated Emergencies during the COVID-19 Pandemic: The Experience of a Center of Excellence. Life. 2024; 14(7):805. https://doi.org/10.3390/life14070805
Chicago/Turabian StyleIorga, Cristian, Cristina Raluca Iorga, Iuliana Andreiana, Simona Hildegard Stancu, Iustinian Bengulescu, Traian Constantin, and Victor Strambu. 2024. "Management of Peritoneal Dialysis-Associated Emergencies during the COVID-19 Pandemic: The Experience of a Center of Excellence" Life 14, no. 7: 805. https://doi.org/10.3390/life14070805
APA StyleIorga, C., Iorga, C. R., Andreiana, I., Stancu, S. H., Bengulescu, I., Constantin, T., & Strambu, V. (2024). Management of Peritoneal Dialysis-Associated Emergencies during the COVID-19 Pandemic: The Experience of a Center of Excellence. Life, 14(7), 805. https://doi.org/10.3390/life14070805






