Phytochemical Analysis of Centaurea calcitrapa L. Aerial Flowering Parts Serial Solvent Extracts and Its Antibacterial and Antioxidant Activities
Abstract
1. Introduction
2. Material and Methods
2.1. Solvents and Chemicals
2.2. Sample Preparation
2.3. Preparation of Plant Extracts
2.4. Phytochemical Screening Tests
2.5. Total Phenolic Acid Content
2.6. Total Flavonoid Content
2.7. Total Tannin Content
2.8. Biological Studies
2.8.1. Testing Organisms
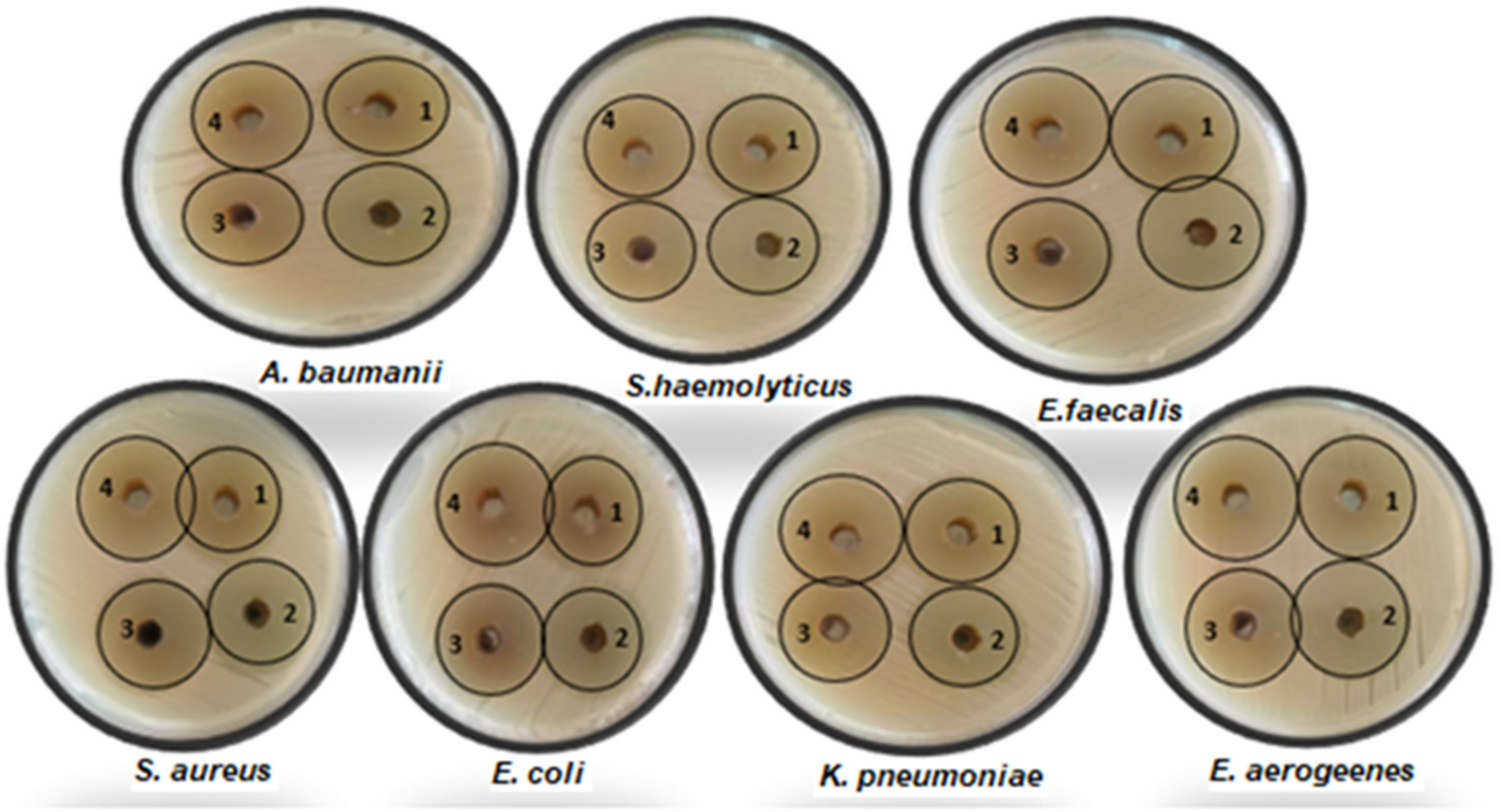
2.8.2. Method for Diffusing Agar Wells
2.8.3. Determination of Minimum Inhibitory Concentrations (MICs)
2.8.4. Resazurin Solution
2.8.5. Estimation of Minimum Lethal Concentrations (MLCs)
2.8.6. Antioxidant Activity
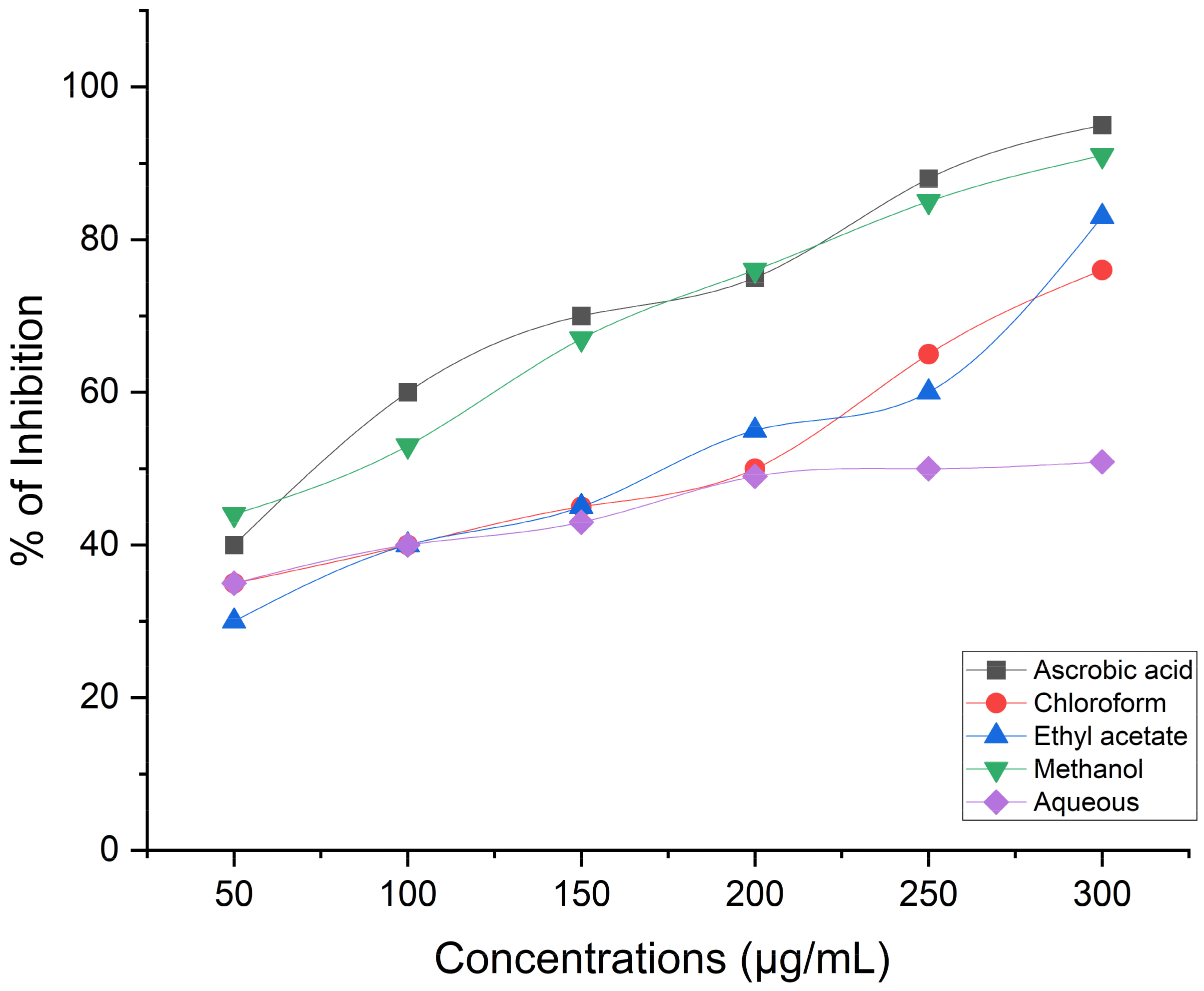
Estimation of Antioxidant Activity Using the DPPH Radical Scavenging Procedure
Ferric Reducing/Antioxidant Power (FRAP) Assay
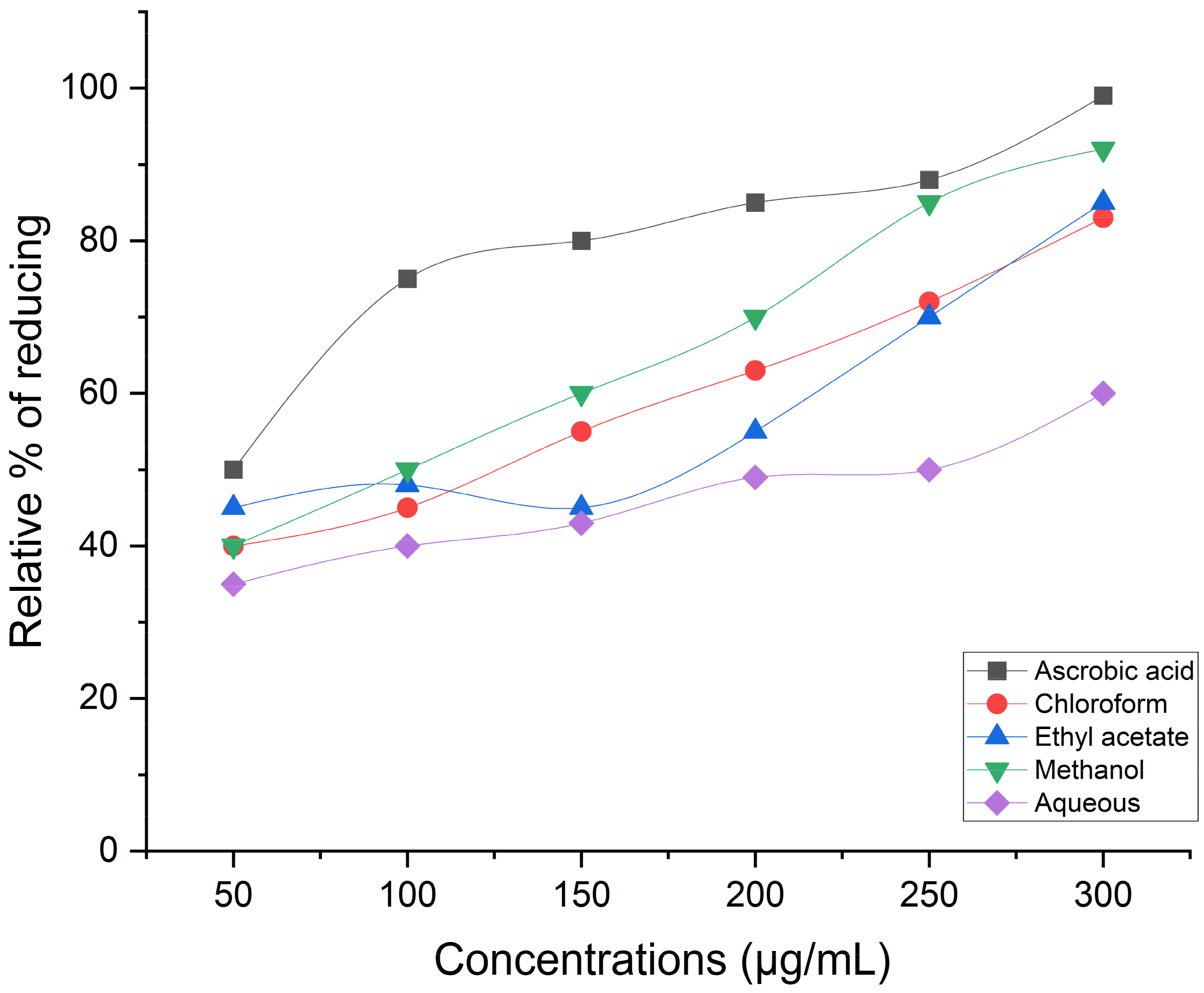
Hydrogen Peroxide Scavenging Activity
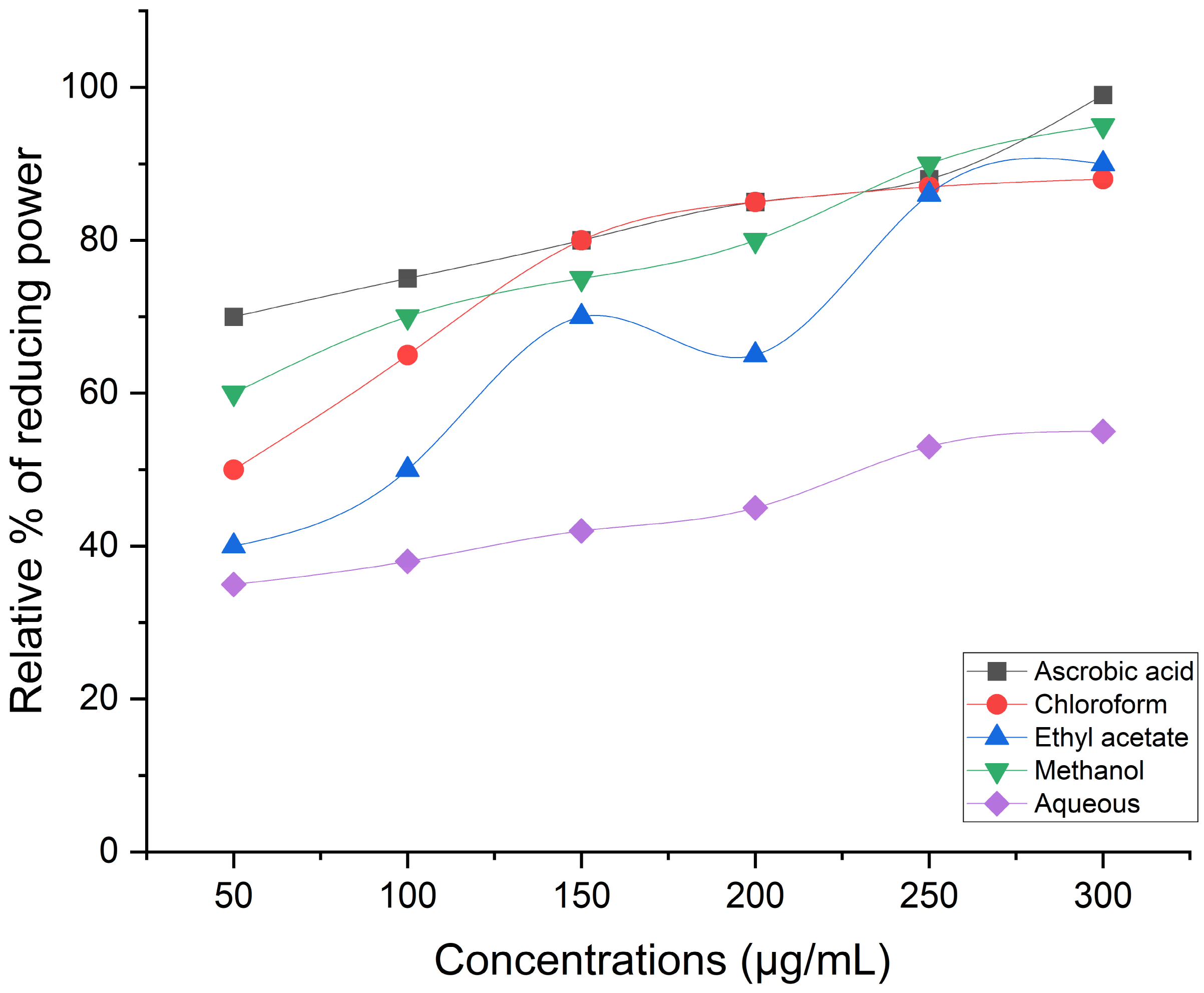
Reducing Power Assay
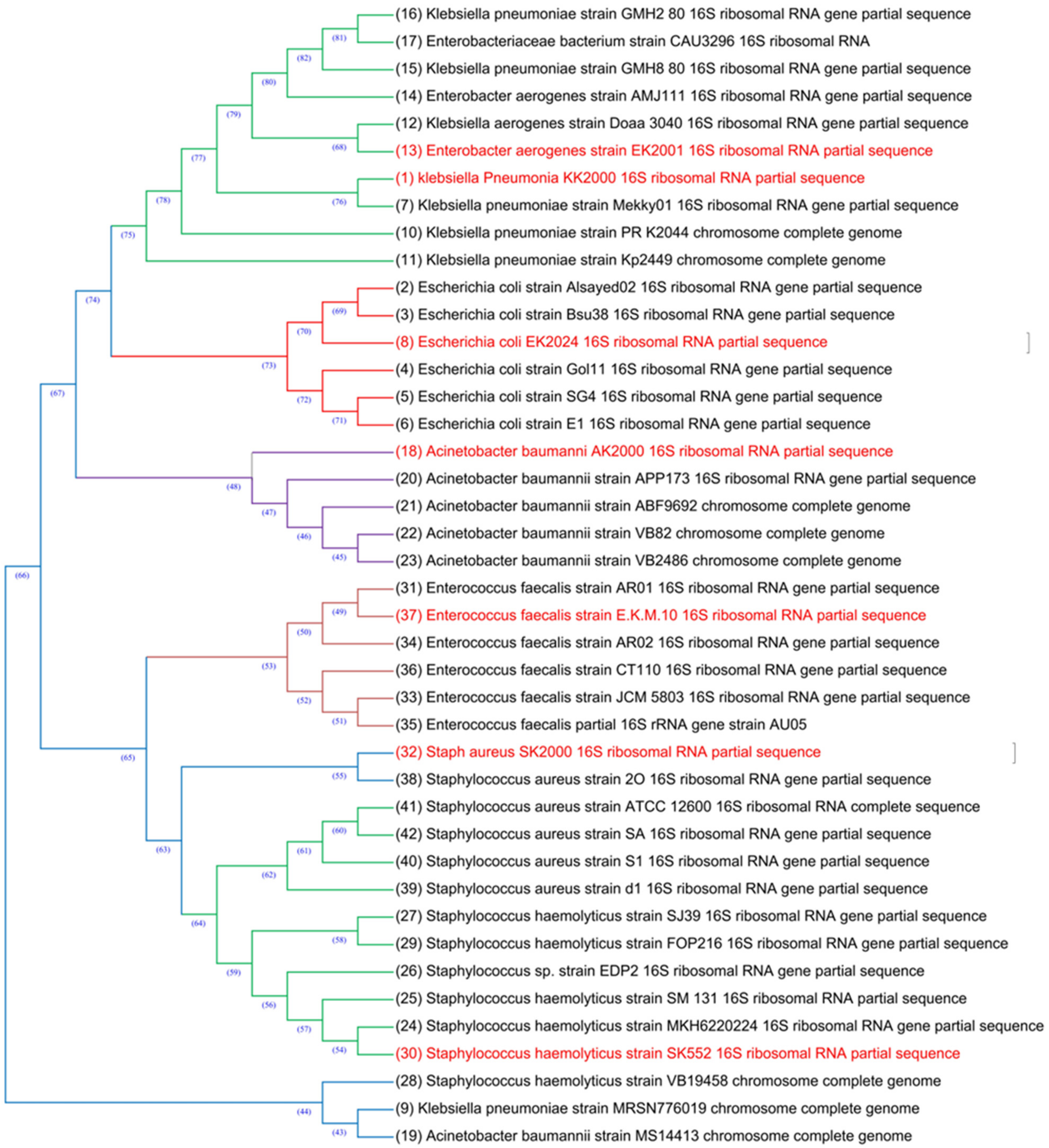
2.8.7. 16S Identification of MDR Bacterial Isolates
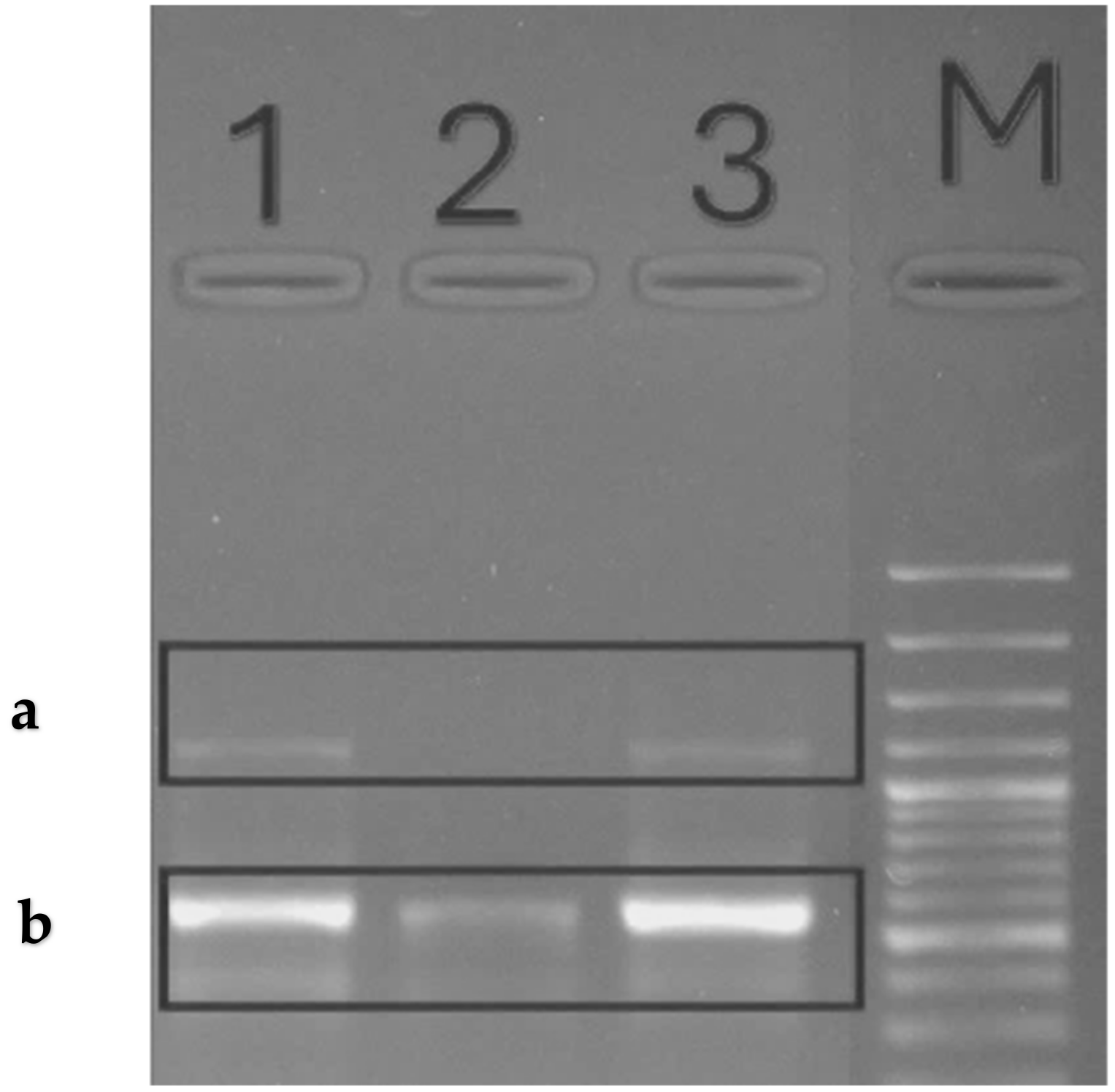
2.8.8. Ribonucleic Acid Isolation
2.8.9. Conventional Reverse Transcription Polymerase
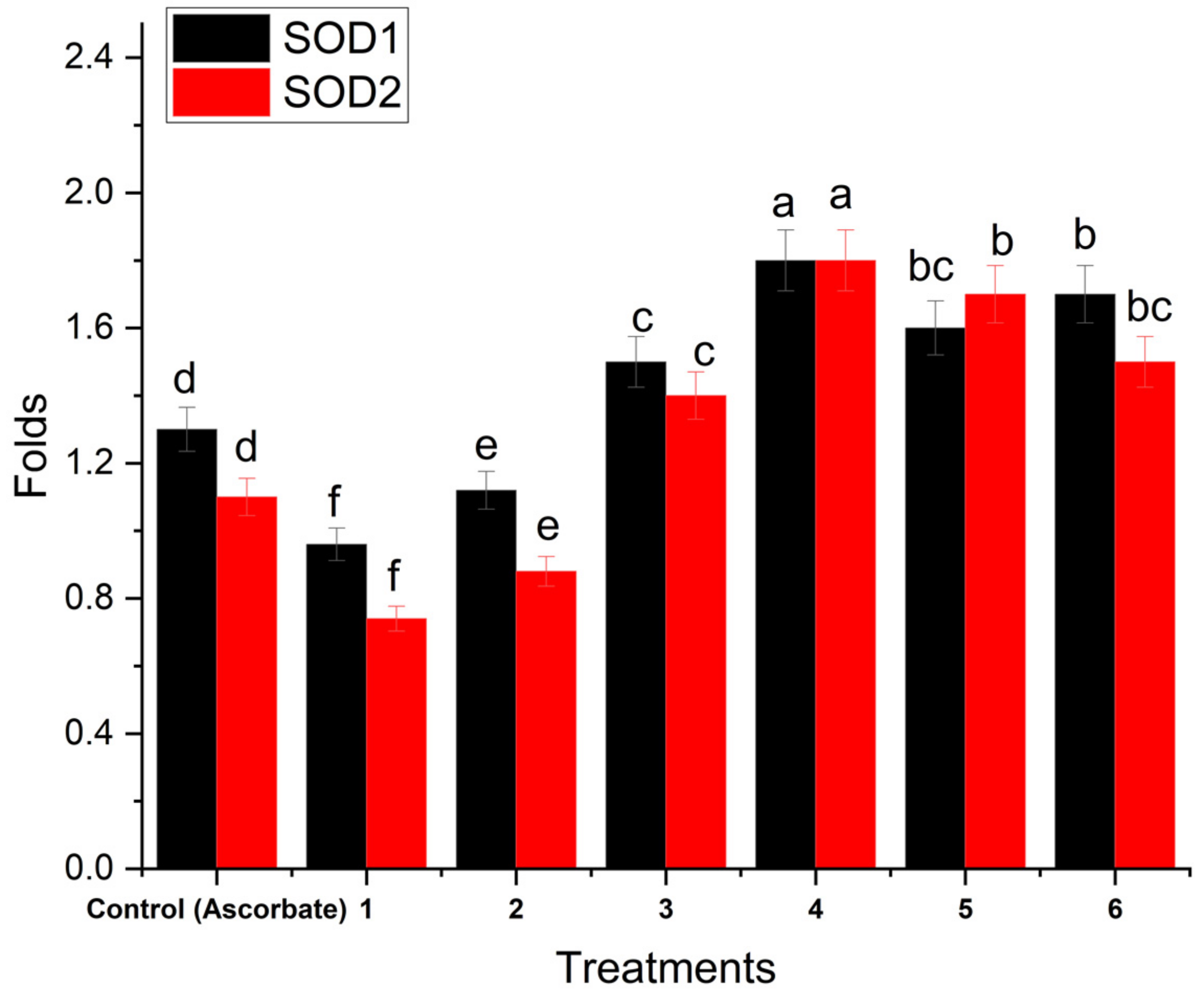
2.8.10. Real-Time PCR Amplification
3. Statistical Analysis
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umaru, I.J.; Shuaibu, S.I.; Adam, R.B.; Habibu, B.; Umaru, K.I.; Haruna, D.E.; David, B.C. Effect of herbal medicine and its biochemical implication. Int. J. Adv. Biochem. Res. 2020, 4, 46–57. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.C. Application of biotechnology in producing plant bio-active compounds. In Natural Bio-Active Compounds: Volume 3: Biotechnology, Bioengineering, and Molecular Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 59–78. [Google Scholar]
- Bruhn, J.G.; Rivier, L. Ethnopharmacology–A journal, a definition and a society. J. Ethnopharmacol. 2019, 242, 112005. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Panda, S.K.; Jouneghani, R.S.; Liu, M.; Parajuli, N.; Leyssen, P.; Neyts, J.; Luyten, W. Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evid.-Based Complement. Altern. Med. 2020, 2020, 1043471. [Google Scholar] [CrossRef] [PubMed]
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S.; Research, P. Antibiotic resistance in the environment: A critical insight on its occurrence, fate, and eco-toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24889–24916. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Forootan, M.; Nazer, M.R.; Karimi, E.; Noori, M. Nosocomial Infections, Challenges and Threats: A Review Article. Iran. J. Med. Microbiol. 2020, 14, 162–181. [Google Scholar] [CrossRef]
- Ciabuschi, F.; Baraldi, E.; Lindahl, O.; Callegari, S. Supporting innovation against the threat of antibiotic resistance: Exploring the impact of public incentives on firm performance and entrepreneurial orientation. J. Bus. Res. 2020, 112, 271–280. [Google Scholar] [CrossRef]
- Harikumar, G.; Krishanan, K. The growing menace of drug resistant pathogens and recent strategies to overcome drug resistance: A review. J. King Saud Univ.-Sci. 2022, 34, 101979. [Google Scholar] [CrossRef]
- Shamim, A.; Ali, A.; Iqbal, Z.; Mirza, M.A.; Aqil, M.; Kawish, S.; Siddiqui, A.; Kumar, V.; Naseef, P.P.; Alshadidi, A.A.F.; et al. Natural Medicine a Promising Candidate in Combating Microbial Biofilm. Antibiotics 2023, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Maqsummiya, Z.U.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M. Antioxidant, anti-proliferative activity and chemical fingerprinting of centaurea calcitrapa against breast cancer cells and molecular docking of caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Shams, K.A.; Mohamed, T.A.; Saleh, I.; Elshamy, A.I.; Atia, M.A.; El-Beih, A.A. Comparative study on the essential oils from five wild Egyptian Centaurea species: Effective extraction techniques, antimicrobial activity and in-silico analyses. Antibiotics 2021, 10, 252. [Google Scholar] [CrossRef]
- Dimkić, I.; Petrović, M.; Gavrilović, M.; Gašić, U.; Ristivojević, P.; Stanković, S.; Janaćković, P. New perspectives of purple starthistle (Centaurea calcitrapa) leaf extracts: Phytochemical analysis, cytotoxicity and antimicrobial activity. AMB Express 2020, 10, 183. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A. An overview on the recent advances in the treatment of infected wounds: Antibacterial wound dressings. Macromol. Biosci. 2022, 22, 2200014. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Herborne, J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 1973; Volume 2, pp. 5–11. [Google Scholar]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Saied, E.; Mekky, A.E.; Al-Askar, A.A.; Hagag, A.F.; El-bana, A.A.; Ashraf, M.; Walid, A.; Nour, T.; Fawzi, M.M.; Arishi, A.A.; et al. Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals 2023, 13, 450. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Khalifa, R.A.; Nasser, M.S.; Gomaa, A.A.; Osman, N.M.; Salem, H.M. Resazurin Microtiter Assay Plate method for detection of susceptibility of multidrug resistant Mycobacterium tuberculosis to second-line anti-tuberculous drugs. Egypt. J. Chest Dis. Tuberc. 2013, 62, 241–247. [Google Scholar] [CrossRef]
- Mekky, A.E. Study of phytochemical analysis and antimicrobial activity of ethanolic extract of Nigella sativa L. and Matricaria chamomilla L. Al-Azhar J. Agric. Res. 2022, 47, 38–51. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Patel, N. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea linn leaves (Leguminosae). Pharmacogn. Res. 2010, 2, 152. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Cheng, S.-j.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M.J. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Mekky, A.E.; Abdelaziz, A.E.; Youssef, F.S.; Elaskary, S.A.; Shoun, A.A.; Alwaleed, E.A.; Gaber, M.A.; Al-Askar, A.A.; Alsamman, A.M.; Yousef, A. Unravelling the Antimicrobial, Antibiofilm, Suppressing Fibronectin Binding Protein A (fnba) and cna Virulence Genes, Anti-Inflammatory and Antioxidant Potential of Biosynthesized Solanum lycopersicum Silver Nanoparticles. Medicina 2024, 60, 515. [Google Scholar] [CrossRef]
- Szabo, E. Molecular and Clinical Genetic Studies of a Novel Variant of Familial Hypercalcemia. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2002. [Google Scholar]
- Ojha, S.; Yadav, S.; Aggarwal, B.; Gupta, S.K.; Mishra, S. Considering the Conception of Nanotechnology Integrated on Herbal Formulation for the Management of Cancer. Lett. Drug Des. Discov. 2023, 20, 1437–1457. [Google Scholar] [CrossRef]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef]
- Ng, Z.X.; Yong, P.H.; Lim, S.Y. Customized drying treatments increased the extraction of phytochemicals and antioxidant activity from economically viable medicinal plants. Ind. Crop. Prod. 2020, 155, 112815. [Google Scholar] [CrossRef]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of bioactive compounds in human health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and tannin nutraceuticals and their metabolites: How the human gut microbiota influences their properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.R.; Chua, L.S.; Mustaffa, A.A. Ionic liquids as green solvent and their applications in bioactive compounds extraction from plants. Process. Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Bachheti, R.K.; Fikadu, A.; Bachheti, A.; Husen, A. Biogenic fabrication of nanomaterials from flower-based chemical compounds, characterization and their various applications: A review. Saudi J. Biol. Sci. 2020, 27, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Abu Khalta, A. Antibacterial Activity of Plant Extract on Bacteria Species. Palest. Sci. J. Youth 2019, 393. Special Issue 1S1-S55. [Google Scholar]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef] [PubMed]
- Bhovi, V.K. Plants based materials as the antifungal and antibacterial agents. Int. J. Plant Based Pharm. 2022, 2, 98–110. [Google Scholar] [CrossRef]
- Zou, F.; Tan, C.; Shinali, T.S.; Zhang, B.; Zhang, L.; Han, Z.; Shang, N. Plant antimicrobial peptides: A comprehensive review of their classification, production, mode of action, functions, applications, and challenges. Food Funct. 2023, 14, 5492–5515. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Kiruba, S.; Mahesh, M.; Nisha, S.; Miller, P.Z.; Ben, C.; Jeeva, S. Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.) Baker ex Heyne. Asian Pac. J. Trop. Med. 2011, 4, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.M.; Podoprigora, I.; Davares, A.K.; Razan, M.; Das, M.; Senyagin, A. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Veter-World 2021, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Ahmad, M.; Öztürk, M.; Altay, V.; Zafar, M.; Sultana, S.; Malik, K.; Ahmad, M.; Öztürk, M.; Altay, V.J.H.o.A.P.D.; et al. Medicinal Plants Used for Musculoskeletal Disorders. In Herbals of Asia: Prevalent Diseases and Their Treatments; Springer: Berlin/Heidelberg, Germany, 2021; pp. 371–432. [Google Scholar]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Shubharani, R.; Mahesh, M.; Yogananda Murthy, V. Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of Bee Propolis. J. Nanomed. Nanotechnol. 2019, 10, 1000522. [Google Scholar]
- Hashem, A.H.; Saied, E.; Ali, O.M.; Selim, S.; Al Jaouni, S.K.; Elkady, F.M.; El-Sayyad, G.S. Pomegranate peel extract stabilized selenium nanoparticles synthesis: Promising antimicrobial potential, antioxidant activity, biocompatibility, and hemocompatibility. Appl. Biochem. Biotechnol. 2023, 195, 5753–5776. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Anil Kumar, N.V.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Gambogou, B.; Taale, E.; Anani, K.; Kangni-Dossou, M.; Karou, D.S.; Ameyapoh, Y. Microbiological analysis and assessment of the Biotechnological potential of Lactobacillus sp. and Pediococcus sp. strains isolated from Togolese traditional Zea mays fermented food. J. Food Sci. Nutr. Res. 2023, 6, 155–164. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Abd Elkarim, A.S.; Ahmed, A.H.; Taie, H.A.; Elgamal, A.M.; Shabana, M. Synadenium grantii hook f.: HPLC/QTOF-MS/MS tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan J. Chem. 2021, 14, 811–828. [Google Scholar] [CrossRef]
- Bhadra, S.; Pundir, C.; Das, J.; Kar, A.; Chanda, J.; Das, B.; Bhardwaj, P.K.; Mukherjee, P.K. Evaluation of bioactive compounds as AChE inhibitors from medicinal plants. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2022; pp. 349–388. [Google Scholar]
- Rosini, E.; Pollegioni, L. Reactive oxygen species as a double-edged sword: The role of oxidative enzymes in antitumor therapy. BioFactors 2022, 48, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Akbaribazm, M.; Khazaei, M.R.; Khazaei, M. Phytochemicals and antioxidant activity of alcoholic/hydroalcoholic extract of Trifolium pratense. Chin. Herb. Med. 2020, 12, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Babu, S.S.; Naroth, S.; Janardanan, D. Antioxidant activity of caffeic acid: Thermodynamic and kinetic aspects on the oxidative degradation pathway. Free. Radic. Res. 2022, 56, 617–630. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5---------<3) | Tm | GC% | Ref |
|---|---|---|---|---|
| SOD1-FW | CGTGGCCTAGCGAGTTATGG | 60.60 | 60 | This Study |
| SOD1-RW | ATAGACACATCGGCCACACC | 59.82 | 55 | |
| SOD2-FW | GGCCTACGTGAACAACCTGA | 59.97 | 55 | |
| SOD2-RW | GCCTGTTGTTCCTTGCAGTG | 59.97 | 55 | |
| GPDH-FW | CCGCATCTTCTTTTGCGTCG | 60.52 | 55 | |
| GPDH-RW | TTCCCGTTCTCAGCCTTGAC | 59.97 | 55 |
| Solvent Used | Sample (Gram) | Boiling Point | Total Hrs of Extraction | Yield (Gram) | Color of Extract |
|---|---|---|---|---|---|
| Chloroform | 100 | 60 °C | 5 | 2.96 | Yellow-brown |
| Ethyl acetate | 95 | 68 °C | 6 | 3.02 | Green |
| Methanol | 90 | 80 °C | 8 | 11.24 | Dark green |
| Aqueous | 74 | 95 °C | 10 | 9.09 | Dark brown |
| Constituents | Tests | Extracts | |||
|---|---|---|---|---|---|
| Chloroform | Ethyl Acetate | Methanol | Aqueous | ||
| Carbohydrates | Molisch’s test | + | + | + | + |
| Fixed Oils and Fats | Saponification test | − | − | − | − |
| Phenol | Ferric chloride test | + | + | + | + |
| Tannins | Ferric chloride test | + | + | + | + |
| Phlobatannins | HCL test | − | − | − | − |
| Flavonoids | Lead acetate test | + | + | + | + |
| AlCL3 test | + | + | + | + | |
| Saponins | Froth test | − | − | + | + |
| Glycosides | Glycosides test | − | + | + | + |
| Conc. H2SO4 test | − | + | + | + | |
| Alkaloids | Dragendroff’s test | − | − | + | − |
| Wagner’s test | − | − | + | − | |
| Hager’s test | − | − | + | − | |
| Quinone | KOH test | − | − | + | + |
| Resins | Resins test | − | − | − | − |
| Sterols and terpenes | Salkowski test | + | + | − | − |
| Cardiac glycosides | Legal’s test | − | − | − | − |
| Keller–Killiani test | − | − | − | − | |
| Anthraquinone | Borntrager’s Test | − | − | − | − |
| Modified Born. Test | − | − | − | − | |
| Extracts | Total Flavonoids (mg RTE/g) | Total Phenolic Acids (mg GAE/g) | Total Tannins (mg TAE/g) |
|---|---|---|---|
| Chloroform | 305.27 ± 1.88 | 27.42 ± 0.29 | 12.02 ± 0.55 |
| Ethyl acetate | 414.43 ± 2.15 | 64.25 ± 0.96 | 26.01 ± 0.81 |
| Methanol | 425.93 ± 1.27 | 97.25 ± 0.73 | 27.52 ± 0.53 |
| Aqueous | 141.10 ± 1.31 | 17.25 ± 0.73 | 7.35 ± 0.56 |
| No. | Isolate Name | Diameter of Inhibition Zone (mm) by 100 µL/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | Aqueous | Methanol | Ethyl Acetate | Chloroform | Aqueous Ext. 1 | Chloroform Ext. 2 | Ethyl Acetate Ext. 3 | Methanol Ext. 4 | ||
| 1 | S. aureus | 10 | ND | ND | ND | ND | 27 ± 0.34 a | 31 ± 0.58 b | 32 ± 0.58 c | 33 ± 0.44 d |
| 2 | S. haemolyticus | 8 | ND | ND | ND | ND | 27 ± 0.35 a | 31 ± 0.56 b | 32 ± 0.59 c | 32 ± 0.46 c |
| 3 | E. faecalis | ND | ND | ND | ND | ND | 28 ± 0.22 a | 30 ± 0.25 b | 30 ± 0.21 b | 31 ± 0.31 c |
| 4 | E. coli | ND | ND | ND | ND | ND | 28 ± 0.21 a | 30 ± 0.27 b | 31 ± 0.34 c | 32 ± 0.43 d |
| 5 | K. pneumoniae | 11 | ND | ND | ND | ND | 28 ± 0.23 a | 31 ± 0.57 b | 31 ± 0.35 b | 32 ± 0.43 c |
| 6 | E. aerogeenes | ND | ND | ND | ND | ND | 28 ± 0.21 a | 29 ± 0.73 b | 30 ± 0.3 c | 31 ± 0.33 d |
| 7 | A. baumanii | ND | ND | ND | ND | ND | 28 ± 0.27 a | 29 ± 0.74 b | 30 ± 0.22 c | 31 ± 0.34 d |
| No. | MICs and MLCs of Aqueous Ext. by μg/mL | MICs and MLCs of Chloroform Ext. by μg/mL | ||
| MICs | MLCs | MICs | MLCs | |
| 1 | 25 ± 0.43 b | 50 ± 0.52 a | 25 ± 0.42 a | 50 ± 0.5 a |
| 2 | 25 ± 0.44 b | 50 ± 0.51 a | 25 ± 0.4 a | 50 ± 0.52 a |
| 3 | 25 ± 0.41 b | 50 ± 0.53 a | 25 ± 0.42 a | 25 ± 0.72 b |
| 4 | 12.5 ± 0.75 c | 25 ± 0.73 b | 12.5 ± 0.74 b | 50 ± 0.51 a |
| 5 | 25 ± 0.42 b | 50 ± 0.55 a | 25 ± 0.41 a | 50 ± 0.52 a |
| 6 | 50 ± 0.53 a | 50 ± 0.53 a | 25 ± 0.43 a | 50 ± 0.51 a |
| 7 | 50 ± 0.55 a | 50 ± 0.52 a | 25 ± 0.41 a | 50 ± 0.53 a |
| MICs and MLCs of Ethyl Acetate Ext. by μg/mL | MICs and MLCs of Methanol Ext. by μg/mL | |||
| MICs | MLCs | MICs | MLCs | |
| 1 | 25 ± 0.43 a | 25 ± 0.4 a | 6.25 ± 0.9 b | 12.5 ± 0.7 b |
| 2 | 12.5 ± 0.72 b | 25 ± 0.43 a | 6.25 ± 0.92 b | 12.5 ± 0.69 b |
| 3 | 25 ± 0.41 a | 25 ± 0.41 a | 6.25 ± 0.93 b | 12.5 ± 0.7 b |
| 4 | 12.5 ± 0.7 b | 25 ± 0.4 a | 12.5 ± 0.72 a | 25 ± 0.44 a |
| 5 | 25 ± 0.4 a | 25 ± 0.4 a | 12.5 ± 0.7 a | 25 ± 0.41 a |
| 6 | 25 ± 0.42 a | 25 ± 0.43 a | 12.5 ± 0.7 a | 25 ± 0.42 a |
| 7 | 12.5 ± 0.71 b | 25 ± 0.41 a | 12.5 ± 0.72 a | 25 ± 0.43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekky, A.E.; Saied, E.; Abdelmouty, E.S.; Haggag, M.I.; Khedr, M.; Khalel, A.F.; Al-Habibi, M.M.; Metwally, S.A.; El Askary, A.; Mohammad, A.M.; et al. Phytochemical Analysis of Centaurea calcitrapa L. Aerial Flowering Parts Serial Solvent Extracts and Its Antibacterial and Antioxidant Activities. Life 2024, 14, 900. https://doi.org/10.3390/life14070900
Mekky AE, Saied E, Abdelmouty ES, Haggag MI, Khedr M, Khalel AF, Al-Habibi MM, Metwally SA, El Askary A, Mohammad AM, et al. Phytochemical Analysis of Centaurea calcitrapa L. Aerial Flowering Parts Serial Solvent Extracts and Its Antibacterial and Antioxidant Activities. Life. 2024; 14(7):900. https://doi.org/10.3390/life14070900
Chicago/Turabian StyleMekky, Alsayed E., Ebrahim Saied, Eslam S. Abdelmouty, Muhammad I. Haggag, Mohamed Khedr, Ashjan F. Khalel, Mahmoud M. Al-Habibi, Shimaa A. Metwally, Ahmad El Askary, Abeer Mahmoud Mohammad, and et al. 2024. "Phytochemical Analysis of Centaurea calcitrapa L. Aerial Flowering Parts Serial Solvent Extracts and Its Antibacterial and Antioxidant Activities" Life 14, no. 7: 900. https://doi.org/10.3390/life14070900
APA StyleMekky, A. E., Saied, E., Abdelmouty, E. S., Haggag, M. I., Khedr, M., Khalel, A. F., Al-Habibi, M. M., Metwally, S. A., El Askary, A., Mohammad, A. M., Alshehri, W. A., Sharahili, A. I., Khairy, N. M., Abdelaziz, A. E. M., & Mahmoud, N. N. (2024). Phytochemical Analysis of Centaurea calcitrapa L. Aerial Flowering Parts Serial Solvent Extracts and Its Antibacterial and Antioxidant Activities. Life, 14(7), 900. https://doi.org/10.3390/life14070900







