The Role of Brassinosteroids in Plant Cold Stress Response
Abstract
:1. Introduction
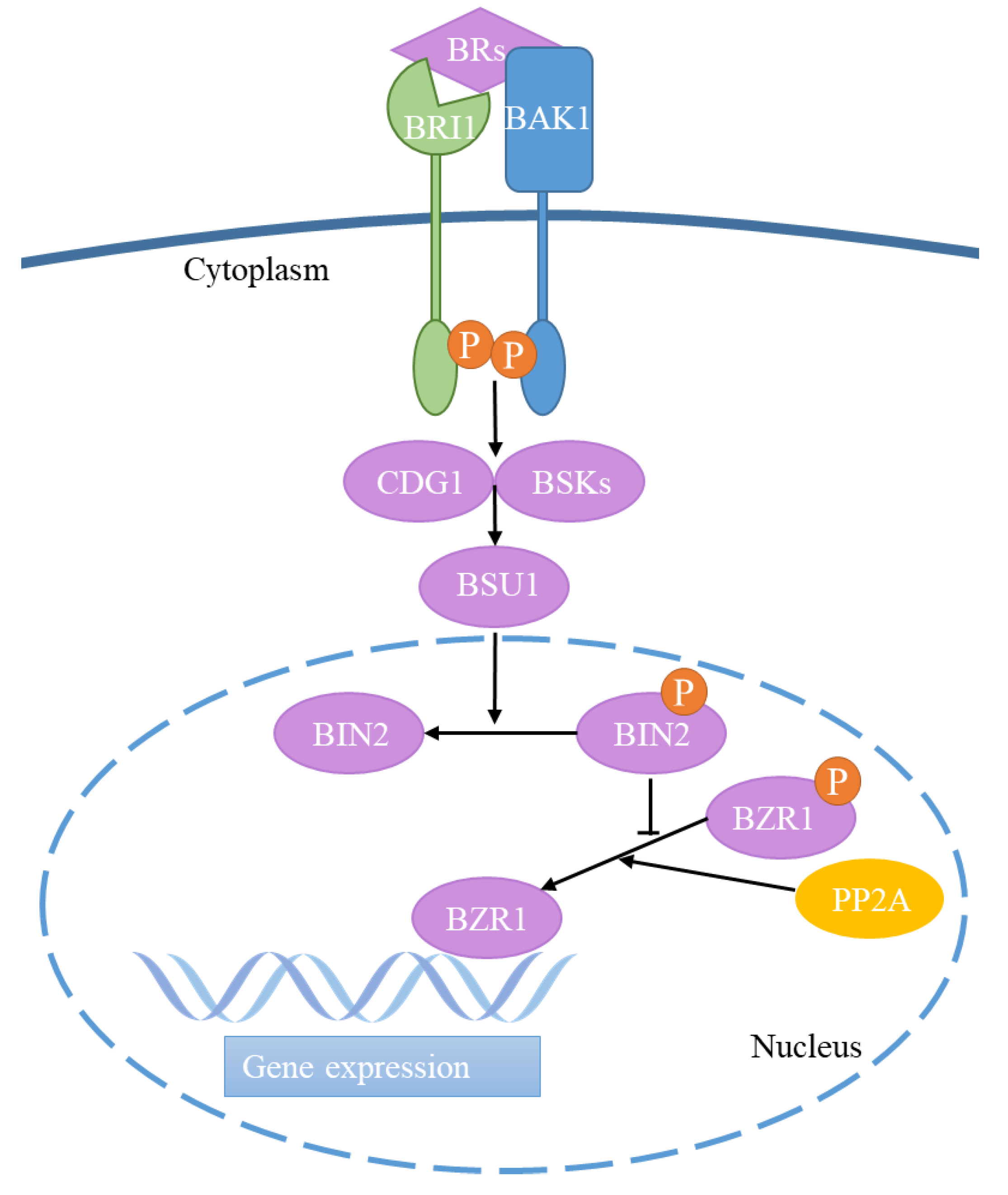
2. Brassinosteroid Signaling
3. Brassinosteroids Enhance Plant Cold Resistance
3.1. BRs’ Impact on CBF Regulation
3.2. BRs in CBF-Independent Signaling Pathways
3.2.1. ROS
3.2.2. Photosynthesis
3.2.3. Autophagy
4. Crosstalk between Brassinosteroids and Other Plant Hormones in the Cold Stress Response
4.1. Crosstalk between BRs and Ethylene
4.2. Crosstalk between BRs and ABA
4.3. Crosstalk between BRs and JA
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold Acclimation of Arabidopsis Thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Shahzad, B.; Im, S.Y.; Lee, D.-J. Brassinosteroids and Cold Stress Tolerance in Plants. In Brassinosteroids in Plant Developmental Biology and Stress Tolerance; Ahammed, G.J., Sharma, A., Yu, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; Chapter 9; pp. 189–199. [Google Scholar]
- Liu, J.; Shi, Y.; Yang, S. Insights into the Regulation of C-Repeat Binding Factors in Plant Cold Signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, Signalling, and Regulatory Mechanism of Cold-Stress Tolerance in Plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, X.; Bürger, M.; Chory, J.; Wang, X. The Role of Ethylene in Plant Temperature Stress Response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Cold-Stress Responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid Signal Transduction: From Receptor Kinase Activation to Transcriptional Networks Regulating Plant Development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of Brossinosteroids Signaling in Biotic and Abiotic Stresses. J. Agric. Food Chem. 2023, 71, 7947–7960. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.Z.; Majee, M.; Datta, A. Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef] [PubMed]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Batool, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and Physiological Responses in Plant Growth and Abiotic Stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Tachibana, R.; Yamagami, A.; Miyagi, S.; Nakazawa-Miklasevica, M.; Matsui, M.; Sakuta, M.; Tanaka, R.; Asami, T.; Nakano, T. BRZ-INSENSITIVE-PALE GREEN 1 Is Encoded by Chlorophyll Biosynthesis Enzyme Gene That Functions in the Downstream of Brassinosteroid Signaling. Biosci. Biotechnol. Biochem. 2022, 86, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Wang, Z.-Y. Brassinosteroid Signal Transduction from Receptor Kinases to Transcription Factors. In Annual Review of Plant Biology; Merchant, S., Briggs, W.R., Ort, D., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 61. [Google Scholar]
- Wang, Z.-Y. Brassinosteroids Modulate Plant Immunity at Multiple Levels. Proc. Natl. Acad. Sci. USA 2012, 109, 7–8. [Google Scholar] [CrossRef]
- Kim, T.-W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.-X.; Sun, Y.; Burlingame, A.L.; Wang, Z.-Y. Brassinosteroid Signal Transduction from Cell-Surface Receptor Kinases to Nuclear Transcription Factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kim, T.-W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. BSKs Mediate Signal Transduction from the Receptor Kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Guan, S.; Burlingame, A.L.; Wang, Z.-Y. The CDG1 Kinase Mediates Brassinosteroid Signal Transduction from BRI1 Receptor Kinase to BSU1 Phosphatase and GSK3-like Kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, S.; Mostizky, Y.; Sunitha, S.; Shani, E.; Nahum, H.; Salomon, D.; Hayun, L.B.; Gruetter, C.; Rauh, D.; Ori, N.; et al. BSKs Are Partially Redundant Positive Regulators of Brassinosteroid Signaling in Arabidopsis. Plant J. 2013, 74, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-Localized BZR1 Mediates Brassinosteroid-Induced Growth and Feedback Suppression of Brassinosteroid Biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 Accumulates in the Nucleus in Response to Brassinosteroids to Regulate Gene Expression and Promote Stem Elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.-W.; Zhou, H.-W.; Deng, Z.; Gampala, S.S.; et al. PP2A Activates Brassinosteroid-Responsive Gene Expression and Plant Growth by Dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.; Liu, M.; Yuan, W.; Zhao, Z.; Liu, X.; Peng, Y.; Yang, X.; Sun, Y.; Tang, W. Nucleocytoplasmic Trafficking and Turnover Mechanisms of BRASSINAZOLE RESISTANT1 in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2101838118. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, B.H.; Lim, C.J.; Lim, C.O.; Nam, K.H. Constitutive Activation of Stress-Inducible Genes in a Brassinosteroid-Insensitive 1 (Bri1) Mutant Results in Higher Tolerance to Cold. Physiol. Plant. 2010, 138, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Vafeados, D.; Tao, Y.; Yoshida, S.; Asami, T.; Chory, J. A New Class of Transcription Factors Mediates Brassinosteroid-Regulated Gene Expression in Arabidopsis. Cell 2005, 120, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 Is a Direct Target of BES1 and Cooperates with BES1 to Regulate Brassinosteroid-Induced Gene Expression. Plant J. 2009, 58, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Zhu, J.-Y.; Wang, Z.-Y. Interaction between BZR1 and PIF4 Integrates Brassinosteroid and Environmental Responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Yan, Y.; Gao, L.; He, C.; Wang, J.; Yuan, Q.; Miao, L.; Li, S.; Di, Q.; et al. Proteome and Phosphoproteome Analysis of 2,4-Epibrassinolide-Mediated Cold Stress Response in Cucumber Seedlings. Front. Plant Sci. 2023, 14, 1104036. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.E.; Poppenberger, B. Modes of Brassinosteroid Activity in Cold Stress Tolerance. Front. Plant Sci. 2020, 11, 583666. [Google Scholar] [CrossRef]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids Participate in the Control of Basal and Acquired Freezing Tolerance of Plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Gookin, T.E.; Assmann, S.M. Significant Reduction of BiFC Non-Specific Assembly Facilitates in Planta Assessment of Heterotrimeric G-Protein Interactors. Plant J. 2014, 80, 553–567. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, M.; Li, Y.; Wang, J.; He, C.; Yu, X. The CsGPA1-CsAQPs Module Is Essential for Salt Tolerance of Cucumber Seedlings. Plant Cell Rep. 2020, 39, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, M.; Ma, S.; Feng, Q.; Wang, Y.; Di, Q.; Zhou, M.; He, C.; Li, Y.; Gao, L.; et al. Mechanism of CsGPA1 in Regulating Cold Tolerance of Cucumber. Hortic. Res. 2022, 9, uhac109. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, S.; Meng, D.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 Is a Key Regulator of Root Growth and Development. Physiol. Plant. 2023, 175, e13977. [Google Scholar] [CrossRef]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 Is Essential for Cucumber Survival under Cold Stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef]
- Xia, X.-J.; Fang, P.-P.; Guo, X.; Qian, X.-J.; Zhou, J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Brassinosteroid-Mediated Apoplastic H2O2-Glutaredoxin 12/14 Cascade Regulates Antioxidant Capacity in Response to Chilling in Tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis Thaliana and Brassica Napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, C.; Cao, B.; Qin, G.; Wang, W.; Tian, S. Brassinolide Enhances Cold Stress Tolerance of Fruit by Regulating Plasma Membrane Proteins and Lipids. Amino Acids. 2012, 43, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, K.; Wei, Y.; Jiang, S.; Ye, J.; Xu, F.; Chen, Y.; Shao, X. PpBZR1, a BES/BZR Transcription Factor, Enhances Cold Stress Tolerance by Suppressing Sucrose Degradation in Peach Fruit. Plant Physiol. Biochem. 2023, 202, 107972. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Shao, Z.; Meng, F.; Chen, H.; Wang, Q.; Zheng, J.; Liu, L. Brassinosteroid Biosynthetic Gene SlCYP90B3 Alleviates Chilling Injury of Tomato (Solanum lycopersicum) Fruits during Cold Storage. Antioxidants 2022, 11, 115. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Wang, Y.; Wang, M.; Wang, F.; Chi, C.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; et al. Crosstalk between Brassinosteroid and Redox Signaling Contributes to the Activation of CBF Expression during Cold Responses in Tomato. Antioxidants 2021, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, H.; Ding, Y.; Shi, Y.; Song, C.; Gong, Z.; Yang, S. BRASSINOSTEROID-INSENSITIVE2 Negatively Regulates the Stability of Transcription Factor ICE1 in Response to Cold Stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Rozhon, W.; Khan, M.; Husar, S.; Adam, G.; Luschnig, C.; Fujioka, S.; Sieberer, T. CESTA, a Positive Regulator of Brassinosteroid Biosynthesis. EMBO J. 2011, 30, 1149–1161. [Google Scholar] [CrossRef]
- Friedrichsen, D.M.; Nemhauser, J.; Muramitsu, T.; Maloof, J.N.; Alonso, J.; Ecker, J.R.; Furuya, M.; Chory, J. Three Redundant Brassinosteroid Early Response Genes Encode Putative bHLH Transcription Factors Required for Normal Growth. Genetics 2002, 162, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, S.; Ruan, X.; Xu, J.; Cao, D. CSN Improves Seed Vigor of Aged Sunflower Seeds by Regulating the Fatty Acid, Glycometabolism, and Abscisic Acid Metabolism. J. Adv. Res. 2021, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, M.; Li, Y.; Feng, X.; Li, M.; Shi, A.; He, C.; Yan, Y.; Wang, J.; Yu, X. Sodium Nitrophenolate Mediates Brassinosteroids Signaling to Enhance Cold Tolerance of Cucumber Seedling. Plant Physiol. Biochem. 2024, 206, 108317. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The Cbfs Triple Mutants Reveal the Essential Functions of CBFs in Cold Acclimation and Allow the Definition of CBF Regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Li, Y.; Li, S.; Shi, A.; Zhou, M.; Ren, H.; Yan, Y.; He, C.; Wang, J.; Sun, M.; et al. Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory. Antioxidants 2022, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive Oxygen Signaling and Abiotic Stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Sauter, M. Epidermal Cell Death in Rice Is Confined to Cells with a Distinct Molecular Identity and Is Mediated by Ethylene and H2O2 through an Autoamplified Signal Pathway. Plant Cell 2009, 21, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.P.; Xu, J.H.; Yin, J.M.; Yang, Z.K.; Tan, W.M. The physiological mechanisms of exogenous brassinolide regulating abiotic stresses in plants: A review. J. Plant Prot. 2023, 50, 22–31, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and Their Role in Response of Plants to Abiotic Stresses. Biol. Plant 2014, 58, 9–17. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, S.; Lin, H.-H. Response of Mitochondrial Alternative Oxidase (AOX) to Light Signals. Plant Signal. Behav. 2011, 6, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-P.; Huang, L.-F.; Cheng, F.; Zhou, Y.-H.; Xia, X.-J.; Mao, W.-H.; Shi, K.; Yu, J.-Q. Brassinosteroids Accelerate Recovery of Photosynthetic Apparatus from Cold Stress by Balancing the Electron Partitioning, Carboxylation and Redox Homeostasis in Cucumber. Physiol. Plant 2013, 148, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderon-Urrea, A.; Lyu, J.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome Analysis of Pepper (Capsicum annuum) Revealed a Role of 24-Epibrassinolide in Response to Chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.; Mishra, K.B.; Kuusisto, I.; Mishra, A.; Novotná, K.; Šebela, D.; Tyystjärvi, E. Effects of Low Temperature on Photoinhibition and Singlet Oxygen Production in Four Natural Accessions of Arabidopsis. Planta 2020, 252, 19. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of Photoprotection to Improve Plant Photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Yu, J.Q.; Huang, L.F.; Hu, W.H.; Zhou, Y.H.; Mao, W.H.; Ye, S.F.; Nogués, S. A Role for Brassinosteroids in the Regulation of Photosynthesis in Cucumis Sativus. J. Exp. Bot. 2004, 55, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.-H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.-Q. Brassinosteroids Promote Photosynthesis and Growth by Enhancing Activation of Rubisco and Expression of Photosynthetic Genes in Cucumis Sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Hashimoto, M.; Endo, T.; Peltier, G.; Tasaka, M.; Shikanai, T. A Nucleus-Encoded Factor, CRR2, Is Essential for the Expression of Chloroplast ndhB in Arabidopsis. Plant J. 2003, 36, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Yamamoto, H.; Okegawa, Y.; Wada, S.; Sato, N.; Taira, Y.; Sugimoto, K.; Makino, A.; Shikanai, T. PGR5-Dependent Cyclic Electron Transport around PSI Contributes to the Redox Homeostasis in Chloroplasts Rather than CO(2) Fixation and Biomass Production in Rice. Plant Cell Physiol. 2012, 53, 2117–2126. [Google Scholar] [CrossRef]
- Takahashi, S.; Milward, S.E.; Fan, D.-Y.; Chow, W.S.; Badger, M.R. How Does Cyclic Electron Flow Alleviate Photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.; Nikolova, D.; Weingarten, R.; Johnson, X.; Richaud, P.; Peltier, G.; Hermann, M.; Magneschi, L.; Hippler, M. Deletion of Proton Gradient Regulation 5 (PGR5) and PGR5-Like 1 (PGRL1) Proteins Promote Sustainable Light-Driven Hydrogen Production in Chlamydomonas Reinhardtii Due to Increased PSII Activity under Sulfur Deprivation. Front. Plant Sci. 2015, 6, 892. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of Chilling Temperatures on Photosynthesis in Warm-Climate Plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Qi, J.; Chi, Y.; Fan, B.; Yu, J.-Q.; Chen, Z. E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses. PLoS Genet. 2014, 10, e1004116. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for Self-Eating in Plant Cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Wang, P.; Yin, Y.; Bassham, D.C. Interactions between Autophagy and Phytohormone Signaling Pathways in Plants. FEBS Lett. 2022, 596, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Harding, T.M.; Morano, K.A.; Scott, S.V.; Klionsky, D.J. Isolation and Characterization of Yeast Mutants in the Cytoplasm to Vacuole Protein Targeting Pathway. J. Cell Biol. 1995, 131, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K. Beginning to Understand Autophagy, an Intracellular Self-Degradation System in Plants. Plant Cell Physiol. 2012, 53, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Svenning, S.; Lamark, T.; Krause, K.; Johansen, T. Plant NBR1 Is a Selective Autophagy Substrate and a Functional Hybrid of the Mammalian Autophagic Adapters NBR1 and P62/SQSTM1. Autophagy 2011, 7, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Cheng, Y.; Chi, Y.-J.; Fan, B.; Yu, J.-Q.; Chen, Z. NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses. PLoS Genet. 2013, 9, e1003196. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Ma, Z.; Zhang, L.; Xing, S.; Hou, X.; Deng, J.; Liu, J.; Chen, Z.; Qu, L.-J.; Gu, H. Arabidopsis AtBECLIN 1/AtAtg6/AtVps30 Is Essential for Pollen Germination and Plant Development. Cell Res. 2007, 17, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, X.; Fang, P.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Brassinosteroids Act as a Positive Regulator of NBR1-Dependent Selective Autophagy in Response to Chilling Stress in Tomato. J. Exp. Bot. 2020, 71, 1092–1106. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Deng, X.-G.; Zhu, T.; Zheng, T.; Li, P.-X.; Wu, J.-Q.; Zhang, D.-W.; Lin, H.-H. Ethylene Is Involved in Brassinosteroids Induced Alternative Respiratory Pathway in Cucumber (Cucumis sativus L.) Seedlings Response to Abiotic Stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, J.; Liu, J.; Hu, J.; Liu, J.; Chen, Y.; Cai, Z.; Wang, X. Abscisic Acid Signaling Inhibits Brassinosteroid Signaling through Dampening the Dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 2018, 11, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Vannozzi, A.; Palumbo, F.; Barcaccia, G. Exogenous EBR Ameliorates Endogenous Hormone Contents in Tomato Species under Low-Temperature Stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid Signaling Positively Regulates Abscisic Acid Biosynthesis in Response to Chilling Stress in Tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate Regulates the INDUCER OF CBF EXPRESSION–C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 Cascade and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.W.; Cole, M.; Flier, A.; Werr, W. BIM1, a bHLH Protein Involved in Brassinosteroid Signalling, Controls Arabidopsis Embryonic Patterning via Interaction with DORNRÖSCHEN and DORNRÖSCHEN-LIKE. Plant Mol. Biol. 2009, 69, 57–68. [Google Scholar] [CrossRef] [PubMed]
- An, J.-P.; Liu, Z.-Y.; Zhang, X.-W.; Wang, D.-R.; Zeng, F.; You, C.-X.; Han, Y. Brassinosteroid Signaling Regulator BIM1 Integrates Brassinolide and Jasmonic Acid Signaling during Cold Tolerance in Apple. Plant Physiol. 2023, 193, 1652–1674. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhou, M.; Feng, X.; Di, Q.; Meng, D.; Yu, X.; Yan, Y.; Sun, M.; Li, Y. The Role of Brassinosteroids in Plant Cold Stress Response. Life 2024, 14, 1015. https://doi.org/10.3390/life14081015
He Z, Zhou M, Feng X, Di Q, Meng D, Yu X, Yan Y, Sun M, Li Y. The Role of Brassinosteroids in Plant Cold Stress Response. Life. 2024; 14(8):1015. https://doi.org/10.3390/life14081015
Chicago/Turabian StyleHe, Zhiqi, Mengdi Zhou, Xiaojie Feng, Qinghua Di, Di Meng, Xianchang Yu, Yan Yan, Mintao Sun, and Yansu Li. 2024. "The Role of Brassinosteroids in Plant Cold Stress Response" Life 14, no. 8: 1015. https://doi.org/10.3390/life14081015




