Screening and Selection of Antibiotics for Enhanced Production of Astaxanthin by Haematococcus lacustris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Culture Conditions
2.2. Chemicals and Reagents
2.3. Biomass Dry Weight, Cell Number, and Astaxanthin Accumulation Analyses
2.4. Statistical Analyses
3. Results
3.1. Effects of Antibiotics on the Cell Numbers and Astaxanthin Content of H. lacustris on Day 15
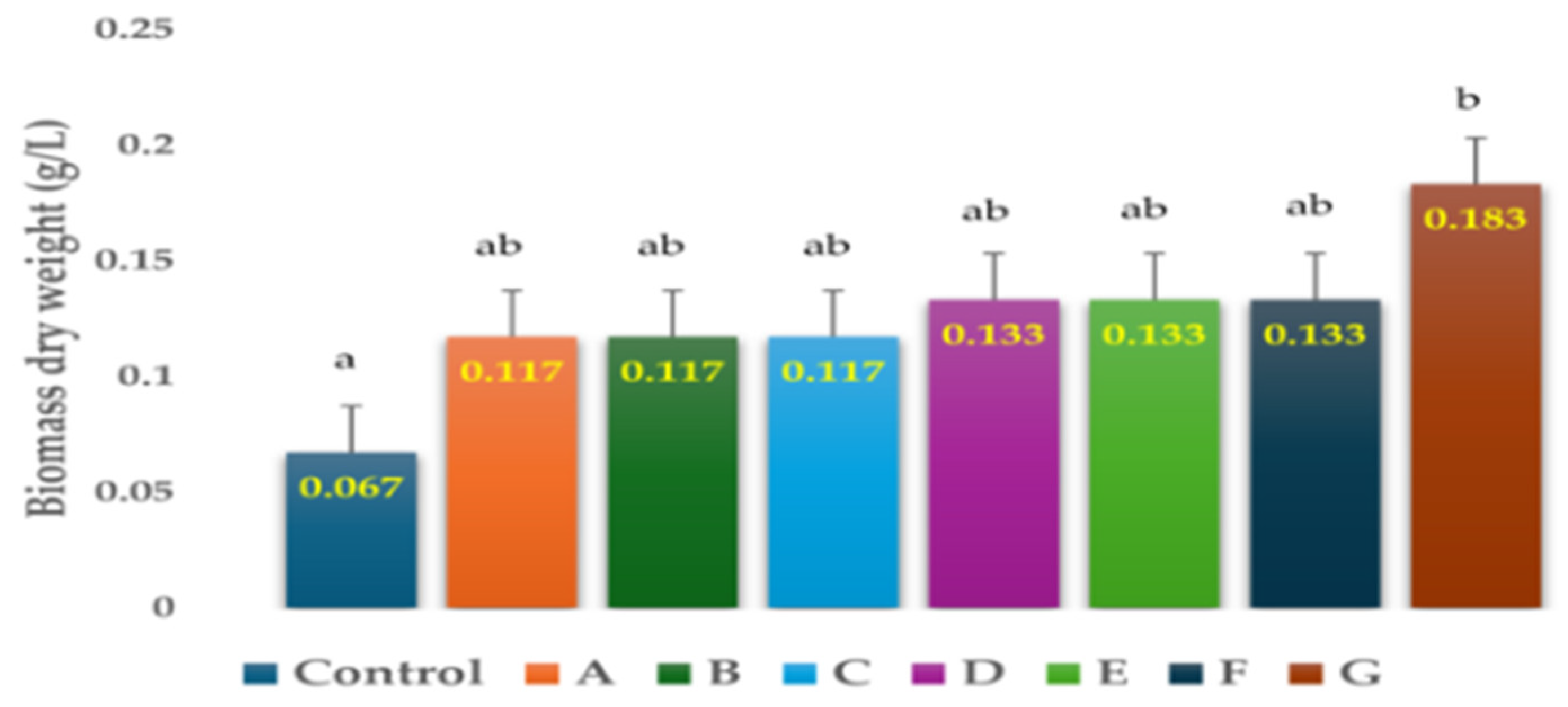
3.2. Effects of Antibiotics on the Biomass Dry Weight of H. lacustris on Day-15
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caprio, F.D. Methods to quantify biological contaminants in microalgae cultures. Algal Res. 2020, 49, 2211–9264. [Google Scholar] [CrossRef]
- Byeon, H.; An, Y.; Kim, T.; Rayamajhi, V.; Lee, J.; Shin, H.; Jung, S. Effects of Four Organic Carbon Sources on the Growth and Astaxanthin Accumulation of Haematococcus lacustris. Life 2024, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.A.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Anandan, R.; Ravishankar, C.N.; Mathew, S. Bioactivities of astaxanthin from natural sources, augmenting its biomedical potential: A review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Fábryová, T.; Tumova, L.; Silva, D.C.; Pereira, D.M.; Andrade, P.B.; Valentão, P.; Hrouzek, P.; Kopecký, J.; Cheel, J. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. Biomass Biofuels Bioprod. 2020, 49, 101947. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- An, Y.; Kim, T.; Byeon, H.; Rayamajhi, V.; Lee, J.; Jung, S.; Shin, H. Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Appl. Sci. 2024, 14, 1104. [Google Scholar] [CrossRef]
- Rizzardi, N.; Pezzolesi, L.; Samorì, C.; Senese, F.; Zalambani, C.; Pitacco, W.; Calonghi, N.; Bergamini, C.; Prata, C.; Fato, R. Natural Astaxanthin Is a Green Antioxidant Able to Counteract Lipid Peroxidation and Ferroptotic Cell Death. Int. J. Mol. Sci. 2022, 23, 15137. [Google Scholar] [CrossRef] [PubMed]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Li, F.; Cai, M.; Wu, Y.; Lian, Q.; Qian, Z.; Luo, J.; Zhang, Y.; Zhang, N.; Li, C.; Huang, X. Effects of Nitrogen and Light Intensity on the Astaxanthin Accumulation in Motile Cells of Haematococcus pluvialis. Front. Mar. Sci. 2022, 9, 909237. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Lam, T.P.; Lee, T.M.; Chen, C.Y.; Chang, J.S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour. Technol. 2018, 252, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.; de Carvalho, J.C.; Júnior, A.I.M.; Faulds, C.; Bertrand, E.; Soccol, C.R. Biological contamination and its chemical control in microalgal mass cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9345–9358. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.N.; Lee, C.G. Antibiotics addition as an alternative sterilization method for axenic cultures in Haematococcus pluvialis. J. Ind. Eng. Chem. 2007, 13, 110–115. [Google Scholar]
- Grzybowska, W.; Banaszczyk-Ruś, M.; Tyski, S. Ocena oddziaływania aminoglikozydu z antybiotykiem innej grupy na wybrane szczepy bakteryjne [Interaction of aminoglycosides and the other antibiotic on selected bacterial strains]. Med. Dosw. Mikrobiol. 2004, 56, 275–285. [Google Scholar] [PubMed]
- Kovacik, A.; Tvrda, E.; Jambor, T.; Fulopova, D.; Kovacikova, E.; Hleba, L.; Kołodziejczyk, Ł.M.; Hlebova, M.; Gren, A.; Massanyi, P. Cytotoxic effect of aminoglycoside antibiotics on the mammalian cell lines. J. Environ. Sci. Health. A. Tox. Hazard Subst. Environ. Eng. 2021, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wray, R.; Iscla, I.; Gao, Y.; Li, H.; Wang, J.; Blount, P. Dihydrostreptomycin Directly Binds to, Modulates, and Passes through the MscL Channel Pore. PLoS Biol. 2016, 14, 1002473. [Google Scholar] [CrossRef]
- Veirup, N.; Kyriakopoulos, C. Neomycin. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shaik, A.B.; Rahman, M. Chapter 7—Antitubercular drugs. In Medicinal Chemistry of Chemotherapeutic Agents; Acharya, P.C., Kurosu, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 217–265. [Google Scholar]
- Kanamycin. Tuberculosis 2008, 88, 117–118. [CrossRef] [PubMed]
- Fitzgerald, K.T.; Newquist, K.L. Chapter 20—Poisonings in the Captive Reptile. In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 229–249. [Google Scholar]
- Pongs, O. Chloramphenicol. In Mechanism of Action of Antibacterial Agents; Hahn, F.E., Ed.; Antibiotics; Springer: Berlin/Heidelberg, Germany, 1979; Volume 5/1. [Google Scholar]
- Yocum, R.R.; Rasmussen, J.R.; Strominger, J.L. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J. Biol. Chem. 1980, 255, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Ioannidou, E.N.; Falagas, M.E. Ampicillin/Sulbactam. Drugs 2007, 67, 1829–1849. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Jiménez, A.; Vázquez, D.; Davies, J.E.; Schindler, D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim. Biophys. Acta 1978, 521, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Burgaña, A.; Abellana, R.; Yordanov, S.Z.; Kazan, R.; Pérez, O.A.M.; Ramos, C.C.; Hernández, C.G.; Rivero, M.M.; Gonçalves, A.Q.; Padilla, E.; et al. Paromomycin is superior to metronidazole in Dientamoeba fragilis treatment. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, V.; An, Y.; Byeon, H.; Lee, J.; Kim, T.; Choi, A.; Lee, J.; Lee, K.; Kim, C.; Shin, H.; et al. A Study on the Effect of Various Media and the Supplementation of Organic Compounds on the Enhanced Production of Astaxanthin from Haematococcus lacustris (Girod—Chantrans) Rostafinski (Chlorophyta). Microorganisms 2024, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Frascaroli, G.; Roberts, J.; Hunter, C.; Escudero, A. Removal efficiencies of seven frequently detected antibiotics and related physiological responses in three microalgae species. Environ. Sci. Pollut. Res. 2024, 31, 14178–14190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Chang, F.; Dang, C.; Fu, J. Combined Effects of Sulfamethoxazole and Erythromycin on a Freshwater Microalga, Raphidocelis subcapitata: Toxicity and Oxidative Stress. Antibiotics 2021, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, A.; Gunasekaran, M.; Boussiba, S.; Zarka, A. Establishing and validating axenic cultures of the microalga Haematococcus lacustris (Chlorophyceae). Appl. Phycol. 2022, 3, 82–97. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhang, L.; Yang, G.; Zhao, L.; Pan, K. A method of batch-purifying microalgae with multiple antibiotics at extremely high concentrations. Chin. J. Ocean. Limnol. 2016, 34, 79–85. [Google Scholar] [CrossRef]
- Youn, J.Y.; Sung, B.H. Antibiotics and Their Optimum Concentration for Axenic Culture of Marine Microalgae. Algae 2007, 22, 229–234. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Zhang, L.; Li, X.; He, Z.; Zhou, C.; Han, J. Screening of antibiotics to obtain axenic cell cultures of a marine microalgae Chrysotila roscoffensis. Front. Bioeng. Biotechnol. 2023, 11, 1218031. [Google Scholar]
- Karuppan, R.; Javee, A.; Gopidas, S.K.; Subramani, N. Influence of agriculture fertilizer for the enhanced growth and astaxanthin production from Haematococcus lacustris RRGK isolated from Himachal Pradesh, India. SN Appl. Sci. 2019, 1, 532. [Google Scholar] [CrossRef]
- Lu, W.; Xu, C.; Liu, F.; Su, M.; Cheng, S.; Zhang, Y. Antibiotic removal efficiency by microalgae: A systematic analysis combined with meta-analysis. Process Saf. Environ. Prot. 2023, 174, 912–920. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.H.; Yuan, K.K.; Xu, H.Y.; He, G.H.; Yang, L.; Buhagiar, J.; Yang, W.D.; Zhang, Y.; Lin, C.S.K.; et al. Cytochrome P450-mediated co-metabolism of fluoroquinolones by Haematococcus lacustris for simultaneously promoting astaxanthin and lipid accumulation. Chem. Eng. J. 2023, 465, 142770. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.P.; Sun, Q. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valenzuela, S.; Chávez-Ruvalcaba, F.; Beltrán-Rocha, J.C.; San Claudio, P.M.; Reyna-Martinez, R. Isolation and Culturing Axenic Microalgae: Mini–Review. Open Microbiol. J. 2021, 15, 111–119. [Google Scholar] [CrossRef]
- Kan, Y.; Pan, J. A one-shot solution to bacterial and fungal contamination in the green alga chlamydomonas reinhardtii culture by using an antibiotic cocktail. J. Phycol. 2010, 46, 1356–1358. [Google Scholar] [CrossRef]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on Microalgal Growth, Metabolism and Microalgae-Based Bioremediation Processes: A Review. Int. J. Mol. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Show, P.L.; Ngo, H.H.; Ho, S.H. Algae-mediated antibiotic wastewater treatment: A critical review. Environ. Sci. Ecotechnol. 2022, 9, 100145. [Google Scholar] [CrossRef] [PubMed]
- Ricky, R.; Chiampo, F.; Shanthakumar, S. Efficacy of Ciprofloxacin and Amoxicillin Removal and the Effect on the Biochemical Composition of Chlorella vulgaris. Bioengineering 2022, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Crofts, T.S.; Wang, B.; Spivak, A.; Gianoulis, T.A.; Forsberg, K.J.; Gibson, M.K.; Johnsky, L.A.; Broomall, S.M.; Rosenzweig, C.N.; Skowronski, E.W.; et al. Shared strategies for β-lactam catabolism in the soil microbiome. Nat. Chem. Biol. 2018, 14, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Feng, W.; Yuan, M.; Li, J.; Xu, H.; Zheng, X.; Zhang, W. Sulfonamides-induced oxidative stress in freshwater microalga Chlorella vulgaris: Evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids. Sci. Rep. 2020, 10, 8243. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Pile, K.D. Tetracyclines. In Compendium of Inflammatory Diseases; Parnham, M.J., Ed.; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Bácsi, I.; Deli, J.; Gonda, S.; Mészáros, I.; Veréb, G.; Dobronoki, D.; Nagy, S.A.; B-Béres, V.; Vasas, G. Non-steroidal anti-inflammatory drugs initiate morphological changes but inhibit carotenoid accumulation in Haematococcus pluvialis. Algal Res. 2018, 31, 1–13. [Google Scholar] [CrossRef]
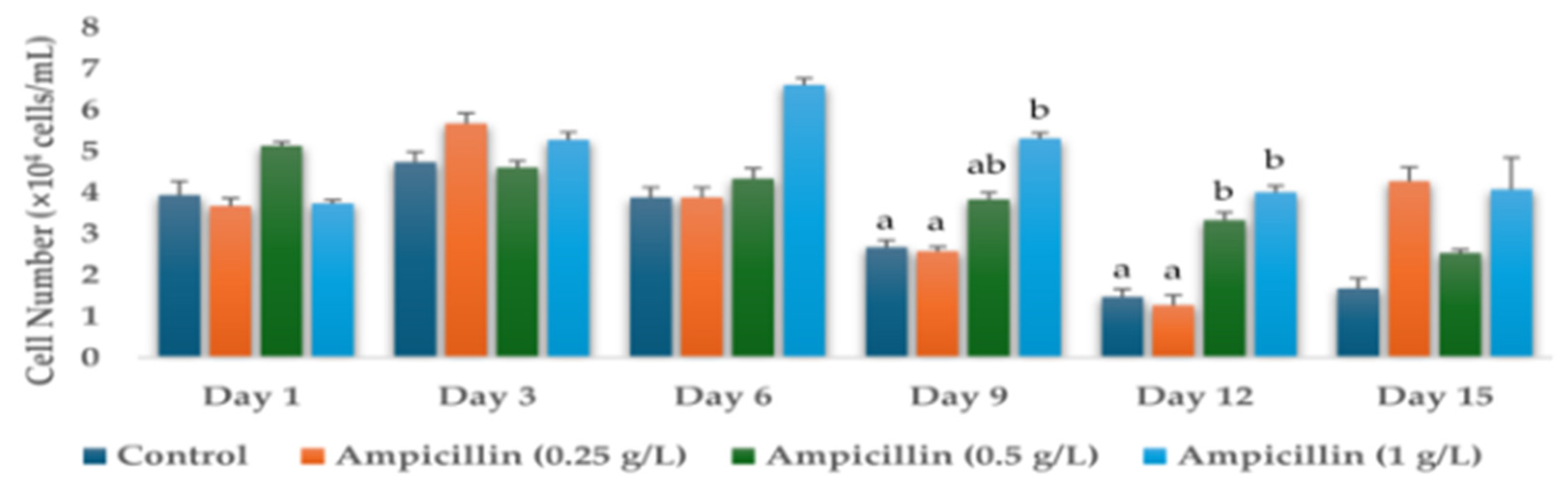
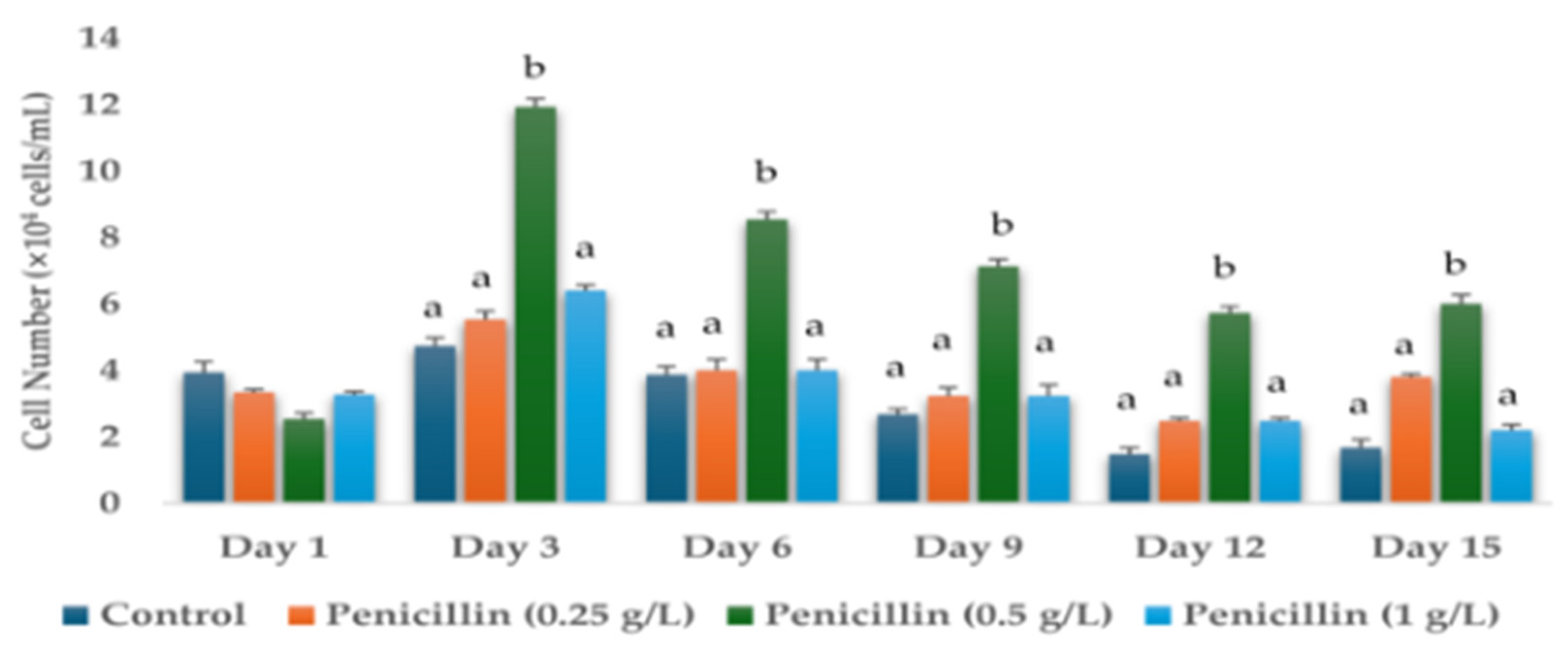

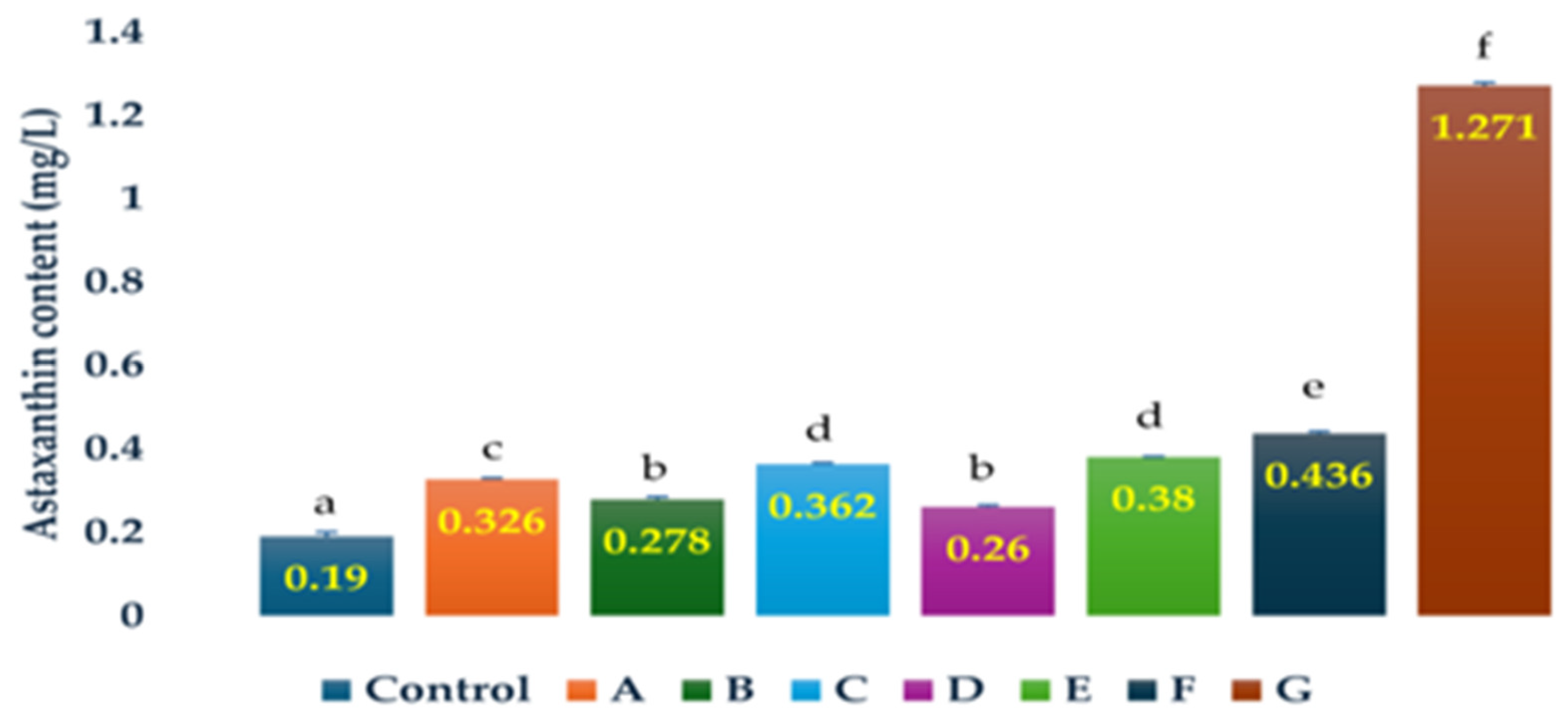
| H. lacustris Culture | Day 15 Cell Number (×104 Cells/mL) | Day 15 Astaxanthin Content (mg/L) |
|---|---|---|
| Control | 1.67 ± 0.25 a | 0.190 ± 0.010 a |
| Penicillin (1 g/L) | 2.20 ± 0.16 a | 0.326 ± 0.004 c |
| Chloramphenicol (0.25 g/L) | 2.33 ± 0.19 a | 0.278 ± 0.006 b |
| Ampicillin (0.5 g/L) | 2.53 ± 0.09 a | 0.362 ± 0.003 d |
| Penicllin (0.25 g/L) | 3.8 ± 0.09 a | 0.26 ± 0.003 b |
| Ampicillin (1 g/L) | 4.07 ± 0.77 b | 0.380 ± 0.001 d |
| Ampicillin (0.25 g/L) | 4.27 ± 0.34 b | 0.436 ± 0.005 e |
| Penicillin (0.5 g/L) | 6.00 ± 0.28 c | 1.271 ± 0.007 f |
| H. Lacustris Culture | Day 15 Biomass Dry Weight (g/L) |
|---|---|
| Control | 0.067 ± 0.02 a |
| Penicillin (1 g/L) | 0.117 ± 0.02 ab |
| Chloramphenicol (0.25 g/L) | 0.117 ± 0.02 ab |
| Ampicillin (0.5 g/L) | 0.117 ± 0.02 ab |
| Penicillin (0.25 g/L) | 0.133 ± 0.02 ab |
| Ampicillin (1 g/L) | 0.133 ± 0.02 ab |
| Ampicillin (0.25 g/L) | 0.133 ± 0.02 ab |
| Penicillin (0.5 g/L) | 0.183 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayamajhi, V.; Byeon, H.; An, Y.; Kim, T.; Lee, J.; Lee, J.; Lee, K.; Kim, C.; Shin, H.; Jung, S. Screening and Selection of Antibiotics for Enhanced Production of Astaxanthin by Haematococcus lacustris. Life 2024, 14, 977. https://doi.org/10.3390/life14080977
Rayamajhi V, Byeon H, An Y, Kim T, Lee J, Lee J, Lee K, Kim C, Shin H, Jung S. Screening and Selection of Antibiotics for Enhanced Production of Astaxanthin by Haematococcus lacustris. Life. 2024; 14(8):977. https://doi.org/10.3390/life14080977
Chicago/Turabian StyleRayamajhi, Vijay, Huijeong Byeon, Yunji An, Taesoo Kim, Jihyun Lee, JongDae Lee, KwangSoo Lee, ChulHyun Kim, HyunWoung Shin, and SangMok Jung. 2024. "Screening and Selection of Antibiotics for Enhanced Production of Astaxanthin by Haematococcus lacustris" Life 14, no. 8: 977. https://doi.org/10.3390/life14080977







