Antibiotic-Loaded PMMA Beads for Recurrent Sternocutaneous Fistula: Expanding the Surgical Armamentarium in Post-Sternotomy Osteomyelitis: Case Report and Literature Review
Abstract
1. Introduction
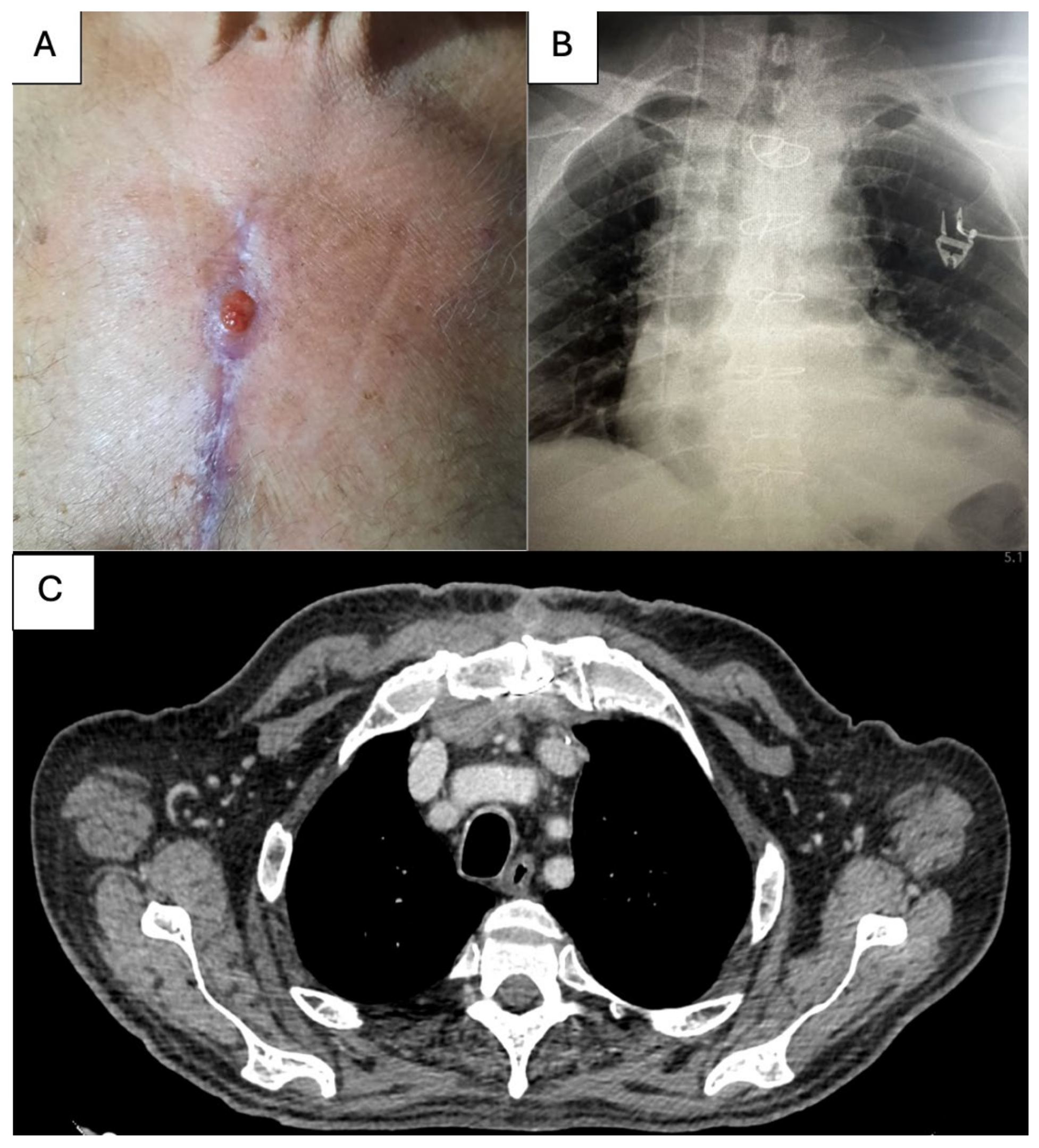
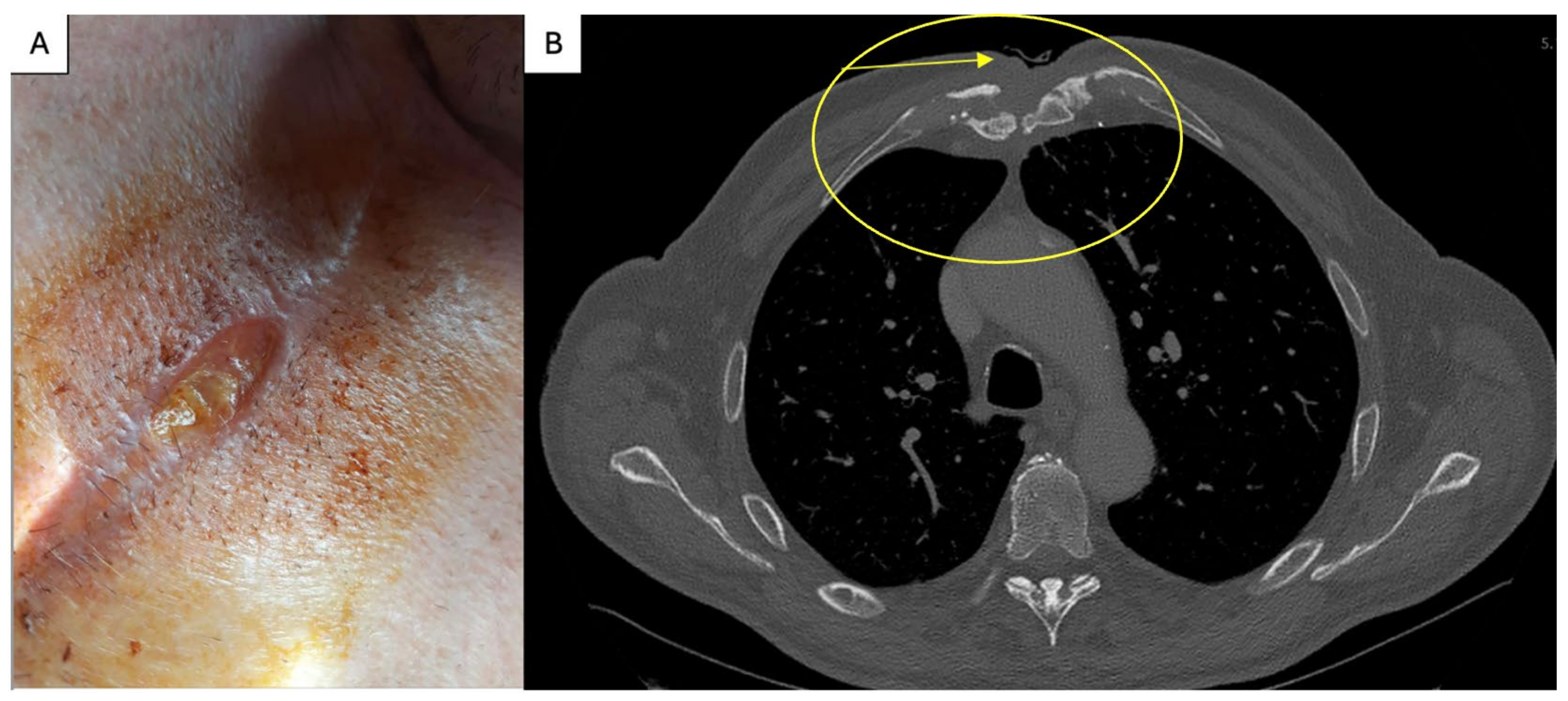
2. Case Report
3. Discussion
3.1. Etiology of CFS
3.2. Risk Factors
3.3. Treatment
3.4. Future Directions
| Author | Year | Type | Cause of Infection | Outcome |
|---|---|---|---|---|
| Coghill et al. [53] | 2018 | Case report (3 year male) | Mediastinitis after cardiac surgery | Successful treatment |
| Genovese et al. [54] | 2016 | Retrospective review (6 adult patients) | prosthetic vascular graft infections | Successful treatment at Mean follow-up 7.3 ± 8.3 months |
| Chiu et al. [12] | 2006 | Case report (2 adult patients) | Mediastinitis | Successful treatment |
| Stone et al. [55] | 2012 | Retrospective review (42 adult patients) | lower extremity vascular surgical site infections | At the median follow-up of 17 months, the limb loss was 21.4% and the recurrent infection rate was 19.4% (seven of 36) |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steingrímsson, S.; Gustafsson, R.; Gudbjartsson, T.; Mokhtari, A.; Ingemansson, R.; Sjögren, J. Sternocutaneous fistulas after cardiac surgery: Incidence and late outcome during a ten-year follow-up. Ann. Thorac. Surg. 2009, 88, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Tabaković, Z.; Marinković, M.; Milačić, P.; Mićović, S.; Živković, I. Treatment of Sternocutaneous Fistula Due to Cardiac Surgery Using Extracellular Matrix Patch. Braz. J. Cardiovasc. Surg. 2025, 40, e20240137. [Google Scholar] [CrossRef]
- Steingrímsson, S.; Sjögren, J.; Gudbjartsson, T. Incidence of sternocutaneous fistulas following open heart surgery in a nationwide cohort. Scand. J. Infect. Dis. 2012, 44, 623–625. [Google Scholar] [CrossRef]
- Sjögren, J.; Nilsson, J.; Gustafsson, R.; Malmsjö, M.; Ingemansson, R. The impact of vacuum-assisted closure on long-term survival after post-sternotomy mediastinitis. Ann. Thorac. Surg. 2005, 80, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tocco, M.P.; Ballardini, M.; Masala, M.; Perozzi, A. Post-sternotomy chronic osteomyelitis: Is sternal resection always necessary? Eur. J. Cardiothorac. Surg. 2013, 43, 715–721. [Google Scholar] [CrossRef]
- Klemm, K. Gentamycin-PMMA-Kugeln in der Behandlung abszedierender Knochen- und Weichteilinfektionen [Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl)]. Zentralbl. Chir. 1979, 104, 934–942. [Google Scholar] [PubMed]
- Majid, S.A.; Lindberg, L.T.; Gunterberg, B.; Siddiki, M.S. Gentamicin-PMMA beads in the treatment of chronic osteomyelitis. Acta Orthop. Scand. 1985, 56, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Jerosch, J.; Lindner, N.; Fuchs, S. Langfristige Therapieergebnisse der chronischen, posttraumatischen Osteomyelitis mit Gentamycin-PMMA-Ketten [Results of long-term therapy of chronic, post-traumatic osteomyelitis with gentamycin PMMA chains]. Unfallchirurg 1995, 98, 338–343. [Google Scholar] [PubMed]
- Walenkamp, G.H.; Kleijn, L.L.; de Leeuw, M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop. Scand. 1998, 69, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Lin, T.Y.; Chu, S.H.; Lu, C.W. Managing sternal osteomyelitis with antibiotic bead implantation. Asian Cardiovasc. Thorac. Ann. 2006, 14, e41–e42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Frederiksen, J.W.; Sanders, J.H.; Lewis, V.; Michaelis, L.L. Management of chronic sternal osteomyelitis. Ann. Thorac. Surg. 1985, 40, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Pairolero, P.C.; Arnold, P.G.; Harris, J.B. Long-term results of pectoralis major muscle transposition for infected sternotomy wounds. Ann. Surg. 1991, 213, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Sabatakou, H.; Antoniadou, A.; Dosios, T.; Giamarellou, H. Case report. Successful treatment of two cases of post-surgical sternal osteomyelitis, due to Candida krusei and Candida albicans, respectively, with high doses of triazoles (fluconazole, itraconazole). Mycoses 2001, 44, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Stoney, W.S.; Alford, W.C., Jr.; Burrus, G.R.; Frist, R.A.; Thomas, C.S., Jr. Median sternotomy dehiscence. Ann. Thorac. Surg. 1978, 26, 421–426. [Google Scholar] [CrossRef]
- Siegman-Igra, Y.; Shafir, R.; Weiss, J.; Herman, O.; Schwartz, D.; Konforti, N. Serious infectious complications of midsternotomy: A review of bacteriology and antimicrobial therapy. Scand. J. Infect. Dis. 1990, 22, 633–643. [Google Scholar] [CrossRef]
- Peivandi, A.A.; Kasper-König, W.; Quinkenstein, E.; Loos, A.H.; Dahm, M. Risk factors influencing the outcome after surgical treatment of complicated deep sternal wound complications. Cardiovasc. Surg. 2003, 11, 207–212. [Google Scholar] [CrossRef]
- Gudbjartsson, T.; Jeppsson, A.; Sjögren, J.; Steingrimsson, S.; Geirsson, A.; Friberg, O.; Dunning, J. Sternal wound infections following open heart surgery—A review. Scand. Cardiovasc. J. 2016, 50, 341–348. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsien, J.H.; Tang, Y.B.; Chen, H.C. Reconstruction for sternal osteomyelitis at the lower third of sternum. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 633–641. [Google Scholar] [CrossRef]
- Borger, M.A.; Rao, V.; Weisel, R.D.; Ivanov, J.; Cohen, G.; E Scully, H.; E David, T. Deep sternal wound infection: Risk factors and outcomes. Ann. Thorac. Surg. 1998, 65, 1050–1056. [Google Scholar] [CrossRef]
- Jones, G.; Jurkiewicz, M.J.; Bostwick, J.; Wood, R.; Bried, J.T.; Culbertson, J.; Howell, R.; Eaves, F.; Carlson, G.; Nahai, F. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann. Surg. 1997, 225, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Gentry, L.O. Management of osteomyelitis. Int. J. Antimicrob. Agents 1997, 9, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Thijssens, K.; Rodrigus, I.; Amsel, B.J.; Moulijn, A.C. Chronic osteomyelitis after sternotomy. Acta Chir. Belg. 2001, 101, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Neale, H.W.; Kreilein, J.G.; Schreiber, J.T.; Gregory, R.O. Complete sternectomy for chronic osteomyelitis with reconstruction using a rectus abdominis myocutaneous island flap. Ann. Plast. Surg. 1981, 6, 305–314. [Google Scholar] [CrossRef]
- Lee, A.B., Jr.; Schimert, G.; Shaktin, S.; Seigel, J.H. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery 1976, 80, 433–436. [Google Scholar]
- Jurkiewicz, M.J.; Bostwick, J., 3rd; Hester, T.R.; Bishop, J.B.; Craver, J. Infected median sternotomy wound successful treatment by muscle flaps. Ann. Surg. 1980, 191, 738–744. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rosmarakis, E.S. Recurrent post-sternotomy mediastinitis. J. Infect. 2006, 52, e151–e154. [Google Scholar] [CrossRef]
- Boulemden, A.; Speggiorin, S.; Pelella, G.; Lotto, A.A. Use of an Extracellular Matrix Patch for Sternal Wound Dehiscence after Cardiac Surgery in a Neonate. Tex. Heart Inst. J. 2018, 45, 176–178. [Google Scholar] [CrossRef]
- Aramini, B.; Masciale, V.; Radaelli, L.F.Z.; Sgarzani, R.; Dominici, M.; Stella, F. The sternum reconstruction: Present and future perspectives. Front. Oncol. 2022, 12, 975603. [Google Scholar] [CrossRef]
- Iarussi, T.; Pardolesi, A.; Camplese, P.; Sacco, R. Composite chest wall reconstruction using titanium plates and mesh preserves chest wall function. J. Thorac. Cardiovasc. Surg. 2010, 140, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, X.; Zhao, J.; Chen, C.; Chen, C.; Chen, J.; Chen, K.-N.; Cao, T.; Chen, M.-W.; Duan, H.; et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl. Lung Cancer Res. 2021, 10, 4057–4083. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.A.; Hameed, P.; Whenish, R.; Elsen, R.S.; G, A.; Jaiswal, A.K.; Prashanth, K.G.; Manivasagam, G. A Review on Development of Bio-Inspired Implants Using 3D Printing. Biomimetics 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next generation surgical meshes for drug delivery and tissue engineering applications: Materials, design and emerging manufacturing technologies. BioDesign Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2020, 6, 666–683. [Google Scholar] [CrossRef]
- Herrera, H.R.; Mijangos, J.; Weiner, R.S.; Ginsburg, M.E. Mediastinal cutaneous fistulae arising as a complication of major cardiac surgery: The value of sinography and injection of methylene blue in their radical excision and immediate repair. Br. J. Plast. Surg. 1983, 36, 421–424. [Google Scholar] [CrossRef]
- Gur, E.; Stern, D.; Weiss, J.; Herman, O.; Wertheym, E.; Cohen, M.; Shafir, R. Clinical-radiological evaluation of poststernotomy wound infection. Plast. Reconstr. Surg. 1998, 101, 348–355. [Google Scholar] [CrossRef]
- Hota, P.; Dass, C.; Erkmen, C.; Donuru, A.; Kumaran, M. Poststernotomy Complications: A Multimodal Review of Normal and Abnormal Postoperative Imaging Findings. AJR Am. J. Roentgenol. 2018, 211, 1194–1205. [Google Scholar] [CrossRef]
- Friebe, B.; Apostolova, I.; Ricke, J. Radiological Diagnostics of Postoperative Sternal Osteomyelitis. In Deep Sternal Wound Infections; Horch, R.E., Willy, C., Kutschka, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 21–27. [Google Scholar]
- van de Belt, H.; Neut, D.; Uges, D.R.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Bertazzoni Minelli, E.; Benini, A.; Magnan, B.; Bartolozzi, P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J. Antimicrob. Chemother. 2004, 53, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.A.; Duncan, C.P.; Beauchamp, C.P. Long-term elution of antibiotics from bone-cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J. Arthroplast. 1998, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Chang, Y.H.; Chen, S.H.; Ueng, S.W.; Shih, C.H. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: A study of 46 patients at an average follow-up of 107 days. J. Orthop. Res. 2006, 24, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.; Regitz, T.; Schmitt, E.; Jung, W.; Anagnostakos, K. In vivo and in vitro studies of antibiotic release from and bacterial growth inhibition by antibiotic-impregnated polymethylmethacrylate hip spacers. Antimicrob. Agents Chemother. 2006, 50, 332–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neut, D.; de Groot, E.P.; Kowalski, R.S.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Gentamicin-loaded bone cement with clindamycin or fusidic acid added: Biofilm formation and antibiotic release. J. Biomed. Mater. Res. A 2005, 73, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Duguid, I.G.; Evans, E.; Brown, M.R.; Gilbert, P. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis; evidence for cell-cycle dependency. J. Antimicrob. Chemother. 1992, 30, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Salvati, E.A.; Callaghan, J.J.; Brause, B.D.; Klein, R.F.; Small, R.D. Reimplantation in infection: Elution of gentamicin from cement and beads. Clin. Orthop. Relat. Res. 1986, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Huang, K.C.; Tai, C.L. Liquid gentamicin in bone cement spacers: In vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J. Trauma 2009, 66, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.; Syed, F.; Raman, G.; Bhalla, A.; Muldoon, E.; Hadley, S.; Smith, E.; Rao, M. Two-stage arthroplasty for prosthetic joint infection: A systematic review of acute kidney injury, systemic toxicity and infection control. J. Arthroplast. 2013, 28, 1490–1498.e1. [Google Scholar] [CrossRef] [PubMed]
- Timofte, D.; Tanasescu, M.D.; Balcangiu-Stroescu, A.E.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, A.; Constantin, P.C.; Cosconel, C.I.; et al. Dyselectrolytemia-management and implications in hemodialysis. Exp. Ther. Med. 2021, 21, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verras, G.I.; Mulita, F. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: A prospective single-center study. Front. Surg. 2024, 11, 1379410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coghill, M.T.; Wallis, G.A.; Kirshbom, P.M.; Maxey, T.S. Successful Salvage of an Extracardiac Fontan in the Setting of Purulent Mediastinitis using Antibiotic-Impregnated Beads. J. Pediatr. Intensive Care 2018, 7, 163–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Genovese, E.A.; Avgerinos, E.D.; Baril, D.T.; Makaroun, M.S.; Chaer, R.A. Bio-absorbable antibiotic impregnated beads for the treatment of prosthetic vascular graft infections. Vascular 2016, 24, 590–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stone, P.A.; Mousa, A.Y.; Hass, S.M.; Dearing, D.D.; Campbell, J.R., 2nd; Parker, A.; Thompson, S.; AbuRahma, A.F. Antibiotic-loaded polymethylmethacrylate beads for the treatment of extracavitary vascular surgical site infections. J. Vasc. Surg. 2012, 55, 1706–1711. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robu, M.; Margarint, I.M.; Draganita, A.; Guzu, M.; Iliescu, V.A. Antibiotic-Loaded PMMA Beads for Recurrent Sternocutaneous Fistula: Expanding the Surgical Armamentarium in Post-Sternotomy Osteomyelitis: Case Report and Literature Review. Life 2025, 15, 1547. https://doi.org/10.3390/life15101547
Robu M, Margarint IM, Draganita A, Guzu M, Iliescu VA. Antibiotic-Loaded PMMA Beads for Recurrent Sternocutaneous Fistula: Expanding the Surgical Armamentarium in Post-Sternotomy Osteomyelitis: Case Report and Literature Review. Life. 2025; 15(10):1547. https://doi.org/10.3390/life15101547
Chicago/Turabian StyleRobu, Mircea, Irina Maria Margarint, Andrei Draganita, Miruna Guzu, and Vlad Anton Iliescu. 2025. "Antibiotic-Loaded PMMA Beads for Recurrent Sternocutaneous Fistula: Expanding the Surgical Armamentarium in Post-Sternotomy Osteomyelitis: Case Report and Literature Review" Life 15, no. 10: 1547. https://doi.org/10.3390/life15101547
APA StyleRobu, M., Margarint, I. M., Draganita, A., Guzu, M., & Iliescu, V. A. (2025). Antibiotic-Loaded PMMA Beads for Recurrent Sternocutaneous Fistula: Expanding the Surgical Armamentarium in Post-Sternotomy Osteomyelitis: Case Report and Literature Review. Life, 15(10), 1547. https://doi.org/10.3390/life15101547






