Artificial Intelligence in the Surgery-First Approach: Harnessing Deep Learning for Enhanced Condylar Reshaping Analysis: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
- Cone-beam computed tomography (CBCT) (T0) and 12-month postoperative (T1) imaging;
- Dentoskeletal deformities of Class II or Class III requiring orthognathic surgery;
- No previous orthognathic surgery or craniofacial trauma;
- Full availability of clinical data.
- Included incomplete CBCT imaging;
- Follow-up of less than 12 months;
- Patient with temporomandibular joint disorders.
2.2. Imaging Protocol and Data Acquisition
- Slice thickness: 0.5 mm.
- Voxel resolution: isotropic at 0.3 mm.
- Field of view adjusted to capture the maxillofacial complex, including the TMJ and condyles.
2.3. Surgical Accuracy
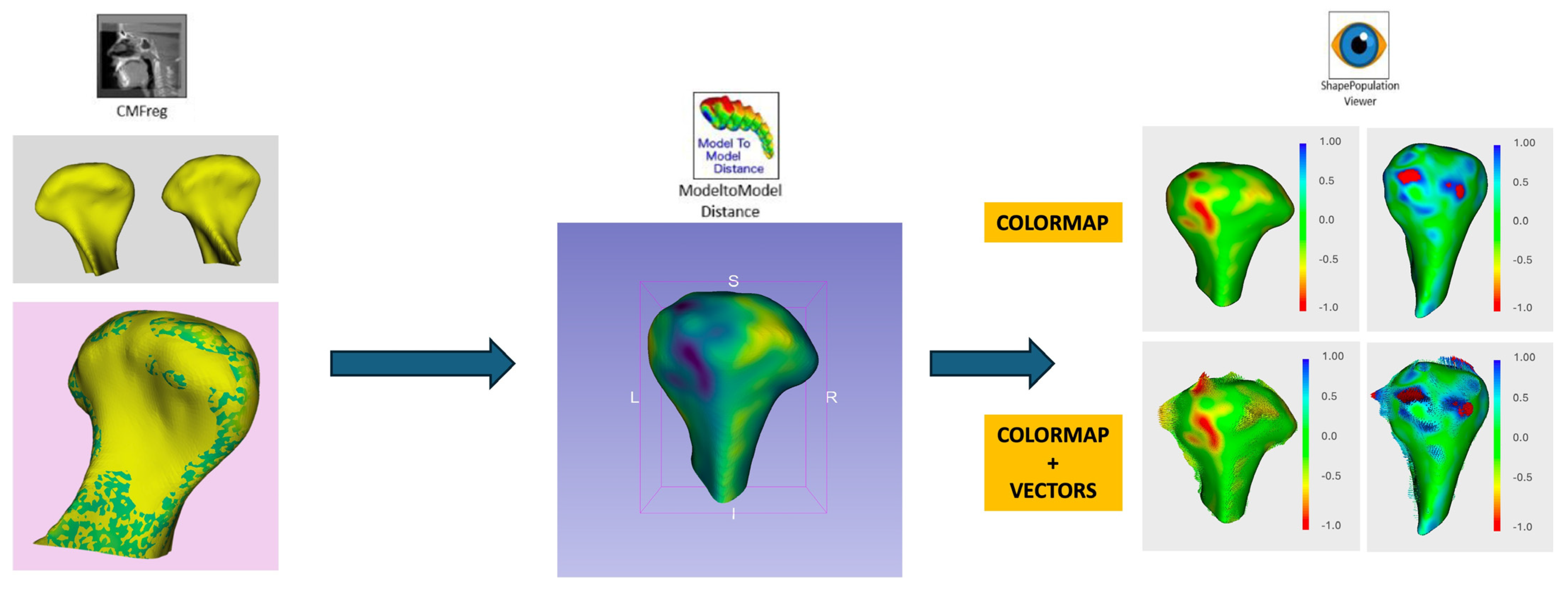
2.4. Digital Workflow and Morphological Analysis
- Registration and Alignment:
- 2.
- Morphological Analysis:
- Surface deviation: Bone remodeling was measured in millimeters, categorizing changes as resorption (<0 mm deviation) or formation (>0 mm deviation).
- Mean surface change: Calculated across all condylar regions for both SFA and SLA groups.
- 3.
- Linear and Rotational Displacement Analysis:
- Linear displacement: ≤2 mm;
- Rotational deviation: ≤2°.
- 4.
- Statistical Modeling of Condylar Behavior:
2.5. Statistical Analysis
- Descriptive Statistics:
- ○
- Mean, standard deviation, and range for continuous variables.
- ○
- Frequencies and percentages for categorical data.
- Comparative Analysis:
- ○
- Independent t-tests for normally distributed data (e.g., mean surface deviations).
- ○
- Mann–Whitney U tests for non-normally distributed data.
- ○
- Chi-square tests for categorical variables (e.g., rates of bone resorption vs. formation).
- Multivariate Regression Models:
- 4.
- Machine Learning Analysis for Morphological Prediction:
- ○
- CNN-based features from the Dental Segmentator were integrated into support vector machine (SVM) and decision tree (DT) models to identify key predictors of condylar changes.
- ○
- Model performance was evaluated using 10-fold cross-validation.
- 5.
- Significance Testing and Adjustments:
- ○
- Statistical significance was set at p < 0.05.
- ○
- False discovery rate (FDR) adjustments were applied for multiple comparisons.
- 6.
- Effect Sizes and Correlation Coefficients:
2.6. Outcome Measures
- Linear displacement (mm).
- Rotational displacement (degrees).
- Mean surface deviation (mm).
- Bone resorption rates stratified by the ROI.
- Impact of dentoskeletal classification on condylar adaptation.
3. Results
3.1. Patient Demographics and Sample Characteristics
3.2. Surgical Accuracy
- Linear deviations (mm):
- ○
- SFA: 0.70 ± 0.10 mm.
- ○
- SLA: 0.62 ± 0.08 mm.
- ○
- No significant difference between groups (p = 0.076).
- Rotational deviations (°):
- ○
- SFA: 1.18° ± 0.12.
- ○
- SLA: 1.11° ± 0.09.
- ○
- No significant difference (p = 0.065).
3.3. Morphological Changes in Condylar Surface
- Bone resorption:
- ○
- Observed in 48.9% of condyles for the SFA and 42.6% for the SLA.
- ○
- Resorption primarily affected anterolateral (63.2%) and lateral (56.8%) regions in both groups.
- ○
- Mean resorption:
- ▪
- SFA: 0.39 ± 0.09 mm.
- ▪
- SLA: 0.33 ± 0.07 mm.
- ▪
- The difference was not statistically significant (p = 0.084).
- Bone formation:
- ○
- Noted in 41.2% of condyles for the SFA and 36.5% for the SLA.
- ○
- Formation was more prevalent in the anteromedial (61.3%) and superior (52.7%) regions.
- ○
- Mean formation:
- ▪
- SFA: 0.21 ± 0.15 mm.
- ▪
- SLA: 0.13 ± 0.11 mm.
- ▪
- The difference approached significance (p = 0.049).
- Mean absolute surface deviation:
- ○
- SFA: 0.43 ± 0.06 mm.
- ○
- SLA: 0.35 ± 0.07 mm.
- ○
- The SFA exhibited slightly greater deviations that were statistically significant (p < 0.05).
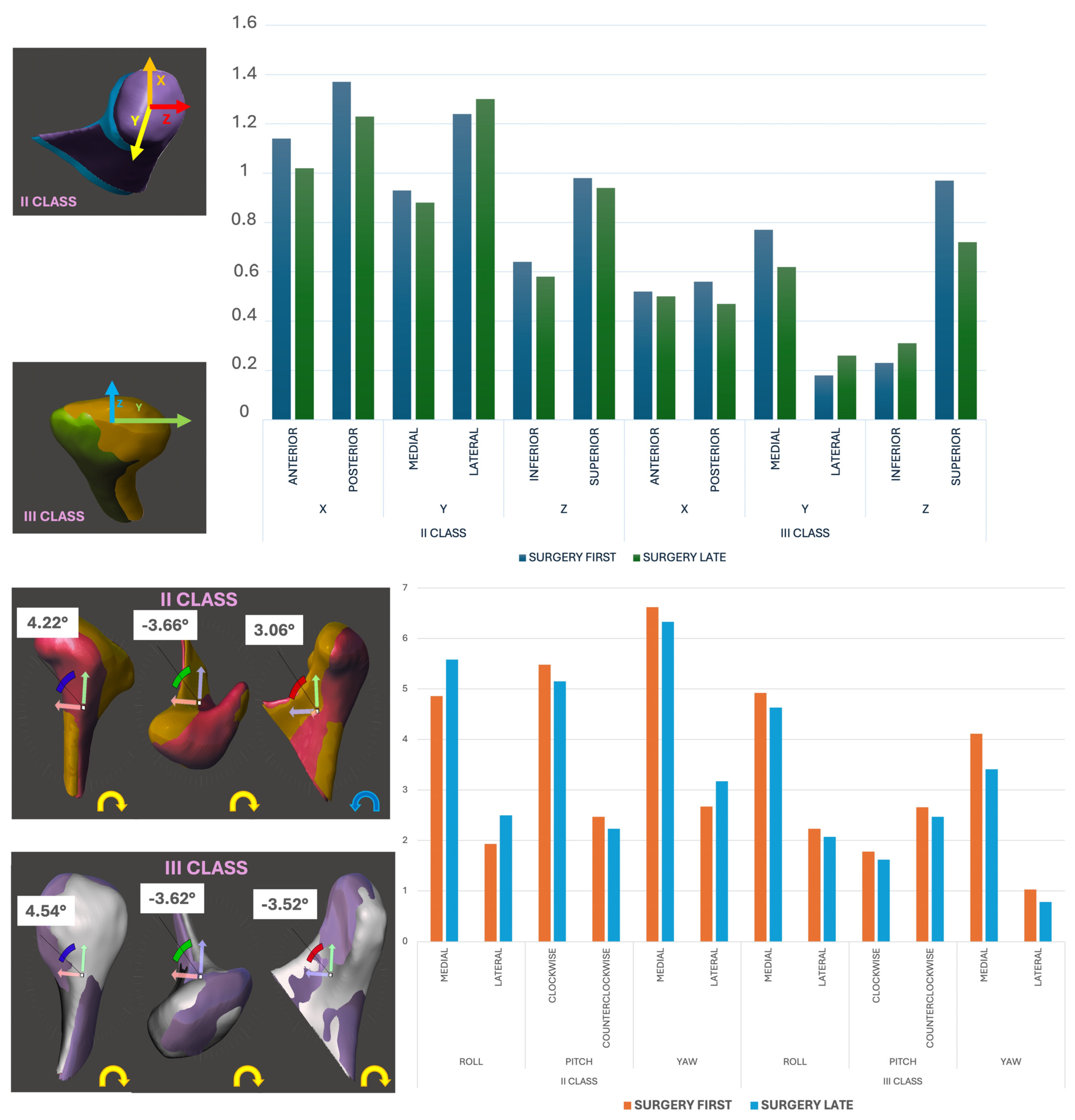
3.4. Linear and Rotational Displacement Analysis
- Linear displacement (mm):
- ○
- SFA: Lateral displacement was higher in Class II patients (mean: 0.88 ± 0.12 mm, p = 0.032).
- ○
- SLA: Comparable across axes (mean: 0.78 ± 0.10 mm).
- Rotational displacement (°):
- ○
- SFA Class III patients exhibited pronounced medial roll (89.3%), medial yaw (92.8%), and counterclockwise pitch (57.1%).
- ○
- The SLA showed reduced but similar trends (roll: 83.6%; yaw: 85.4%; pitch: 52.3%).
3.5. Influence of Dentoskeletal Classification
- Class II patients:
- ○
- Greater resorption in superior regions (73.5%) and posterior displacement (53.4%).
- ○
- More frequent formation in anterior regions (62.8%).
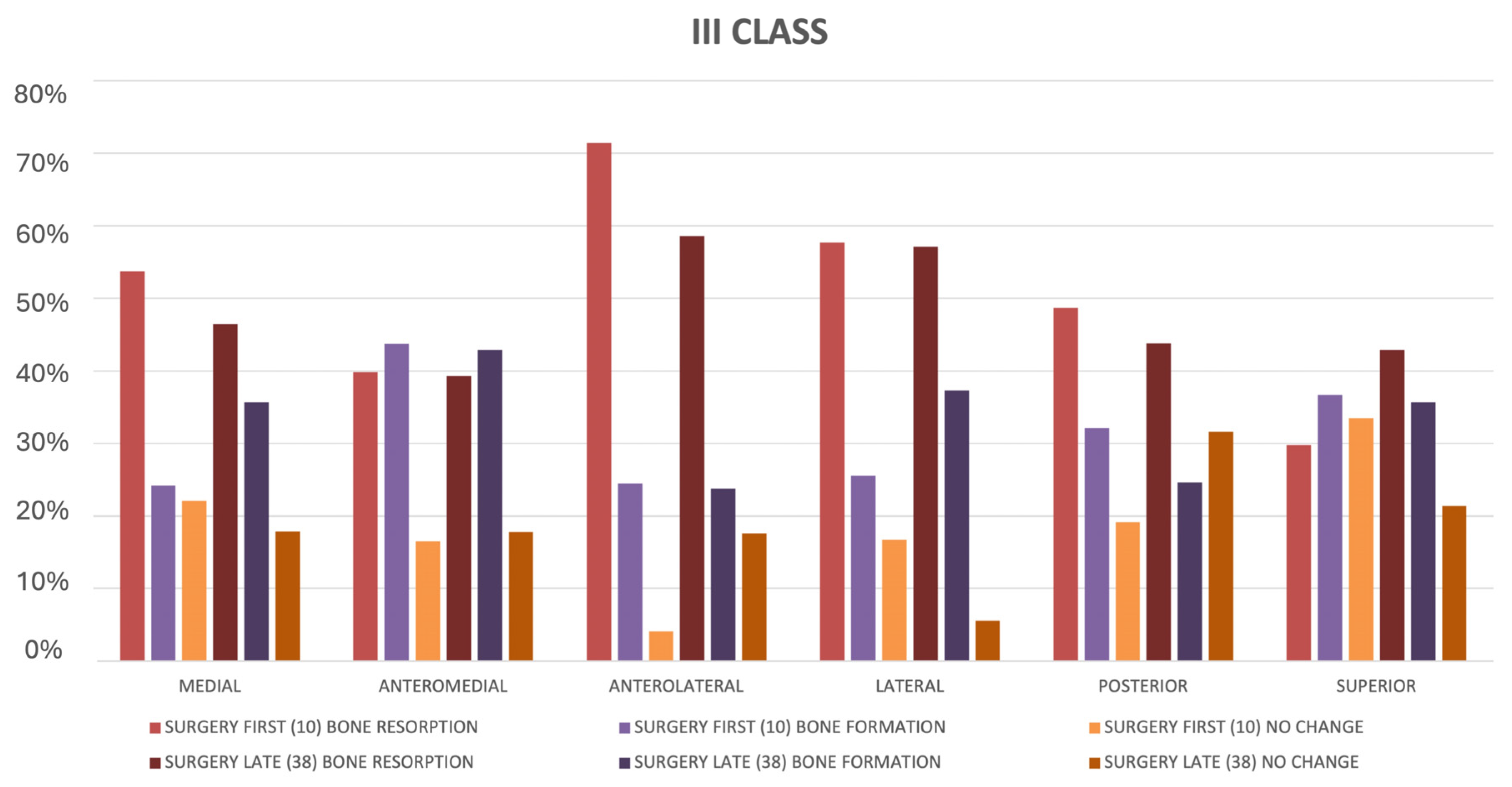
- Class III patients:
- ○
- Significant medial displacement (58.1%) and superior resorption (76.2%).
- ○
- Resorption tended to cluster in posteromedial regions (63.7%).
3.6. Performance of Deep Learning Segmentation
- Segmentation accuracy:
- ○
- Mean deviation: 0.95 ± 0.23 mm compared to manual segmentation (intraclass correlation coefficient: 0.94).
- Processing time:
- ○
- Reduced from ~7 h (manual) to ~5 min per scan.
4. Discussion
4.1. Introduction to the Surgery-First Approach
4.2. Adaptive Remodeling and Clinical Implications
4.3. Influence of Dentoskeletal Class
5. Conclusions
5.1. Limitations of the Study
5.1.1. Retrospective Design
5.1.2. Sample Size
5.1.3. Short Follow-Up Duration
5.1.4. Dentoskeletal Class
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.S.; Hsu, S.S.; Chen, Y.R. Systematic review of the surgery-first approach in orthognathic surgery. Biomed. J. 2014, 37, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Valls-Ontañón, A.; Triginer-Roig, S.; Trujillo, I.; Brabyn, P.J.; Giralt-Hernando, M.; Hernández-Alfaro, F. Three-dimensional evaluation of postoperative stability: A comparative study between surgery-first and surgery-late protocols. Int. J. Oral Maxillofac. Surg. 2023, 52, 353–360. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K. 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support. Intraoperative Imaging Image-Guided Therapy; Ferenc, A.J., Ed.; Springer: New York, NY, USA, 2014; Volume 3, pp. 277–289. ISBN 978-1-4614-7656-6/978-1-4614-7657-3. [Google Scholar]
- Dot, G.; Chaurasia, A.; Dubois, G.; Savoldelli, C.; Haghighat, S.; Azimian, S.; Rahbar Taramsari, A.; Sivaramakrishnan, G.; Issa, J.; Dubey, A.; et al. DentalSegmentator: Robust deep learning-based CBCT image segmentation. medRxiv 2024. [Google Scholar] [CrossRef]
- Beccuti, M.L.; Cozzani, M.; Antonini, S.; Doldo, T.; Raffaini, M. “Surgery First” vs. “Traditional Sequence” Surgery: A Qualitative Study of Health Experiences in 46 Bimaxillary Orthognathic Patients. J. Maxillofac. Oral Surg. 2022, 21, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Kosowski, T.R.; McCarthy, C.; Reavey, P.L.; Scott, M.A.; Wilkins, E.G.; Cano, S.J.; Klassen, A.F.; Carr, N.; Cordeiro, P.G.; Pusic, A.L. A systematic review of patient-reported outcome measures after facial cosmetic surgery and/or nonsurgical facial rejuvenation. Plast. Reconstr. Surg. 2009, 123, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Consorti, G.; Monarchi, G.; Betti, E.; Balercia, P. The Impact of Professional Oral Hygiene in Orthognathic Surgery. J. Craniofac. Surg. 2023, 34, e646–e648. [Google Scholar] [CrossRef]
- Wolford, L.M.; Reiche-Fischel, O.; Mehra, P. Changes in temporomandibular joint dysfunction after orthognathic surgery. J. Oral Maxillofac. Surg. 2003, 61, 655–660. [Google Scholar] [CrossRef]
- Petronis, Z.; Janovskiene, A.; Rokicki, J.P.; Razukevicius, D. Three-Dimensional Mandibular Condyle Remodeling Post-Orthognathic Surgery: A Systematic Review. Medicina 2024, 60, 1683. [Google Scholar] [CrossRef]
- Li, J.; Ryu, S.-Y.; Park, H.-J.; Kook, M.-S.; Jung, S.; Han, J.J.; Oh, H.-K. Changes in condylar position after BSSRO with and without Le Fort I osteotomy via surgery-first approach in mandibular prognathism with facial asymmetry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Yang, Y.M.; Kim, Y.I.; Cho, B.H.; Jung, Y.H.; Hwang, D.S. Effect of bimaxillary surgery on adaptive condylar head remodeling: Metric analysis and image interpretation using cone-beam computed tomography volume superimposition. J. Oral Maxillofac. Surg. 2012, 70, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Epker, B.N.; Fish, L. Surgical-orthodontic correction of open-bite deformity. Am. J. Orthod. 1977, 71, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Weissheimer, A.; Menezes, L.M.; Koerich, L.; Pham, J.; Cevidanes, L.H.S. Fast three-dimensional superimposition of cone beam computed tomography for orthopaedics and orthognathic surgery evaluation. Int. J. Oral Maxillofac. Surg. 2015, 44, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Oh, J.H.; Kim, S.G. Virtual surgical plan with custom surgical guide for orthognathic surgery: Systematic review and meta-analysis. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 39. [Google Scholar] [CrossRef] [PubMed]
- Sillmann, Y.M.; Monteiro, J.L.G.C.; Eber, P.; Baggio, A.M.P.; Peacock, Z.S.; Guastaldi, F.P.S. Empowering surgeons: Will artificial intelligence change oral and maxillofacial surgery? Int. J. Oral Maxillofac. Surg. 2024, 54, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Committeri, U.; Barone, S.; Arena, A.; Fusco, R.; Troise, S.; Maffia, F.; Tramontano, S.; Bonavolontà, P.; Abbate, V.; Granata, V.; et al. New perspectives in the differential diagnosis of jaw lesions: Machine learning and inflammatory biomarkers. J. Stomatol. Oral Maxillofac. Surg. 2024, 125 (Suppl. S4), 101912. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Ma, C.-Y.; Ko, E.W.-C. Prediction of surgery-first approach orthognathic surgery using deep learning models, International. J. Oral Maxillofac. Surg. 2024, 53, 942–949. [Google Scholar] [CrossRef]
- Hernández-Alfaro, F.; Guijarro-Martínez, R.; Molina-Coral, A.; Badía-Escriche, C. “Surgery first” in bimaxillary orthognathic surgery. J. Oral Maxillofac. Surg. 2011, 69, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Farhad; Naini, B.; Witherow, H.; Daljit; Gill, S. Point-counterpoint: Conventional jaw surgery versus the surgery first approach. Semin. Orthod. 2022, 28, 4. [Google Scholar] [CrossRef]
- Badiali, G.; Costabile, E.; Lovero, E.; Pironi, M.; Rucci, P.; Marchetti, C.; Bianchi, A. Virtual Orthodontic Surgical Planning to Improve the Accuracy of the Surgery-First Approach: A Prospective Evaluation. J. Oral Maxillofac. Surg. 2019, 77, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Trevisiol, L.; Bersani, M.; Martinez Garza, A.; Alvarado, E.; Arnett, G.W.; D’Agostino, A. Accuracy of virtual surgical planning in bimaxillary orthognathic surgery with mandible first sequence: A retrospective study. J. Craniomaxillofac. Surg. 2023, 51, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Nicolielo, L.F.P.; Van Dessel, J.; Shaheen, E.; Letelier, C.; Codari, M.; Politis, C.; Lambrichts, I.; Jacobs, R. Validation of a novel imaging approach using multi-slice CT and cone-beam CT to follow-up on condylar remodeling after bimaxillary surgery. Int. J. Oral Sci. 2017, 9, 139–144. [Google Scholar] [CrossRef]
- Nunes de Lima, V.; Faverani, L.P.; Santiago, J.F.; Palmieri, C.; Magro Filho, O.; Pellizzer, E.P. Evaluation of condylar resorption rates after orthognathic surgery in class II and III dentofacial deformities: A systematic review. J. Cranio-Maxillofac. Surg. 2018, 46, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Abbate, V.; Audino, G.; Dell’Aversana Orabona, G.; Friscia, M.; Bonavolontà, P.; Lo Faro, C.; Committeri, U.; Cuéllar, C.N.; Iaconetta, G.; Califano, L. Condylar Reshape in Orthognathic Surgery: Morphovolumetric and Densitometric Analysis Based on 3D Imaging and Digital Workflow. J. Maxillofac. Oral Surg. 2022, 21, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Politis, C.; Van De Vyvere, G.; Agbaje, J.O. Condylar resorption after orthognathic surgery. J. Craniofac. Surg. 2019, 30, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Barghan, S.; Tetradis, S.; Mallya, S. Application of cone beam computed tomography for assessment of the temporomandibular joints. Aust. Dent. J. 2012, 57, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ravelo, V.; Olate, G.; de Moraes, M.; Huentequeo, C.; Sacco, R.; Olate, S. Condylar Positional Changes in Skeletal Class II and Class III Malocclusions after Bimaxillary Orthognathic Surgery. J. Pers. Med. 2023, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Vandeput, A.S.; Verhelst, P.J.; Jacobs, R.; Shaheen, E.; Swennen, G.; Politis, C. Condylar changes after orthognathic surgery for class III dentofacial deformity: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 193–202. [Google Scholar] [CrossRef]
- Perillo, L.; Auconi, P.; d’Apuzzo, F.; Grassia, V.; Scazzocchio, M.; Nucci, L.; McNamara, J.A.; Franchi, L. Machine learning in the prognostic appraisal of Class III growth. Semin. Orthod. 2021, 27, 96–108. [Google Scholar] [CrossRef]
- Giovacchini, F.; Gilli, M.; Mitro, V.; Bensi, C.; Tullio, A. Rapid prototyping: Applications in oral and maxillofacial surgery. J. Oral Med. Oral Surg. 2021, 27, 2020050. [Google Scholar] [CrossRef]
- Gruber, L.J.; Egger, J.; Bönsch, A.; Kraeima, J.; Ulbrich, M.; van den Bosch, V.; Motmaen, I.; Wilpert, C.; Ooms, M.; Isfort, P.; et al. Accuracy and Precision of Mandible Segmentation and Its Clinical Implications: Virtual Reality, Desktop Screen and Artificial Intelligence. Expert Syst. Appl. 2024, 239, 122275. [Google Scholar] [CrossRef]
- Patcas, R.; Bernini, D.A.J.; Volokitin, A.; Agustsson, E.; Rothe, R.; Timofte, R. Applying artificial intelligence to assess the impact of orthognathic treatment on facial attractiveness and estimated age. Int. J. Oral Maxillofac. Surg. 2019, 48, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Raith, S.; Wolff, S.; Steiner, T.; Modabber, A.; Weber, M.; Hölzle, F.; Fischer, H. Planning of mandibular reconstructions based on statistical shape models. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 99–112. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Committeri, U.; Monarchi, G.; Gilli, M.; Caso, A.R.; Sacchi, F.; Abbate, V.; Troise, S.; Consorti, G.; Giovacchini, F.; Mitro, V.; et al. Artificial Intelligence in the Surgery-First Approach: Harnessing Deep Learning for Enhanced Condylar Reshaping Analysis: A Retrospective Study. Life 2025, 15, 134. https://doi.org/10.3390/life15020134
Committeri U, Monarchi G, Gilli M, Caso AR, Sacchi F, Abbate V, Troise S, Consorti G, Giovacchini F, Mitro V, et al. Artificial Intelligence in the Surgery-First Approach: Harnessing Deep Learning for Enhanced Condylar Reshaping Analysis: A Retrospective Study. Life. 2025; 15(2):134. https://doi.org/10.3390/life15020134
Chicago/Turabian StyleCommitteri, Umberto, Gabriele Monarchi, Massimiliano Gilli, Angela Rosa Caso, Federica Sacchi, Vincenzo Abbate, Stefania Troise, Giuseppe Consorti, Francesco Giovacchini, Valeria Mitro, and et al. 2025. "Artificial Intelligence in the Surgery-First Approach: Harnessing Deep Learning for Enhanced Condylar Reshaping Analysis: A Retrospective Study" Life 15, no. 2: 134. https://doi.org/10.3390/life15020134
APA StyleCommitteri, U., Monarchi, G., Gilli, M., Caso, A. R., Sacchi, F., Abbate, V., Troise, S., Consorti, G., Giovacchini, F., Mitro, V., Balercia, P., & Tullio, A. (2025). Artificial Intelligence in the Surgery-First Approach: Harnessing Deep Learning for Enhanced Condylar Reshaping Analysis: A Retrospective Study. Life, 15(2), 134. https://doi.org/10.3390/life15020134








