Perioperative Considerations of Novel Antidiabetic Agents in Heart Failure Patients Undergoing Cardiac Surgery
Abstract
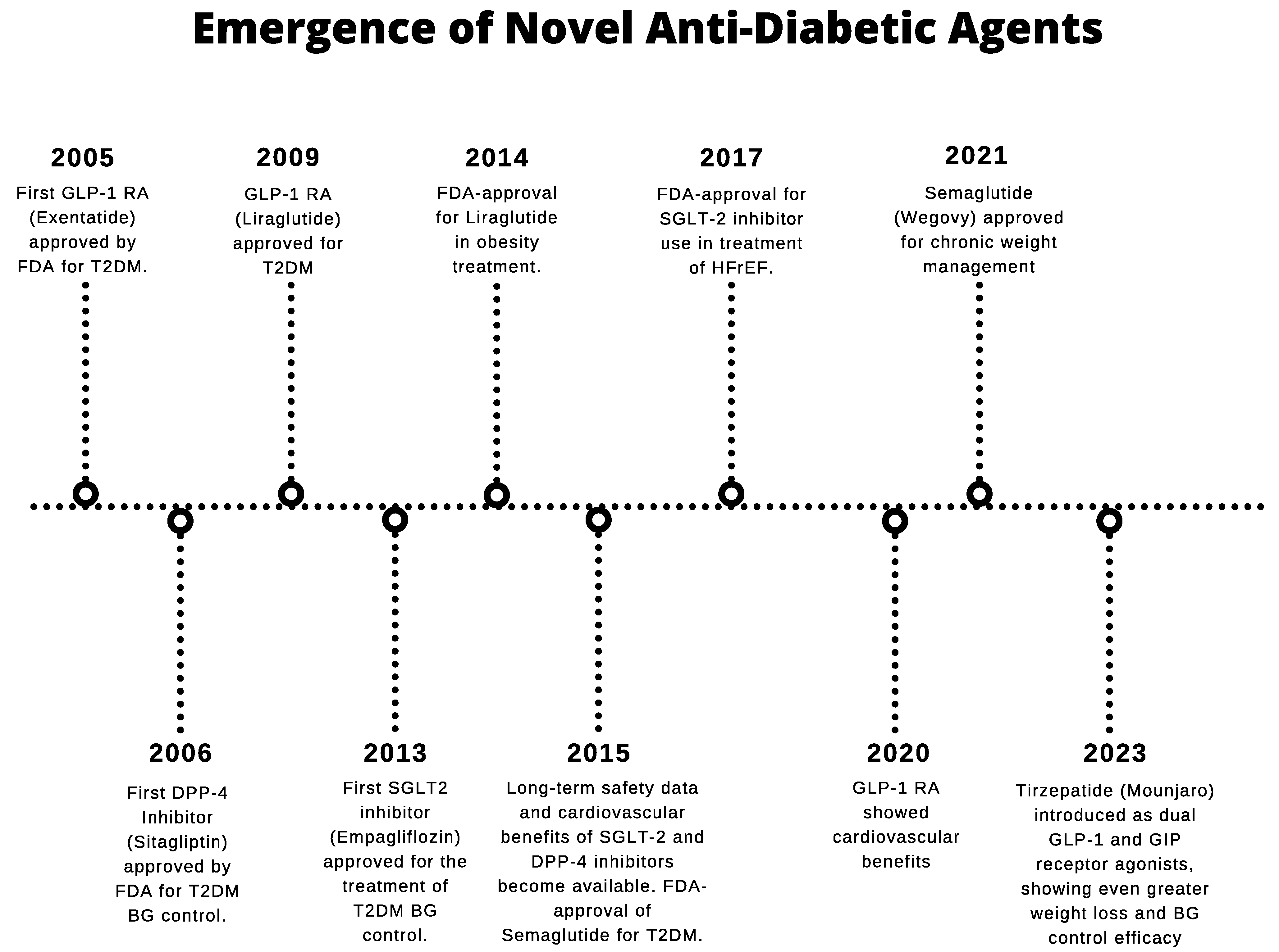
:1. Introduction
2. Overview of Novel Antidiabetic Agents
2.1. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs)
2.2. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
2.3. Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2is)
3. Novel Antidiabetic Agents in HF Management
3.1. GLP-1RAs in HF Management
3.2. DPP-4 Inhibitors in HF Management
3.3. SGLT2is in HF Management
4. Perioperative Considerations of Novel Antidiabetic Use in Cardiac Surgeries
4.1. GLP-1RAs in Cardiac Surgeries
4.2. DPP-4 Inhibitors in Cardiac Surgeries
4.3. SGLT2is in Cardiac Surgeries
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yun, J.-S.; Ko, S.-H. Current Trends in Epidemiology of Cardiovascular Disease and Cardiovascular Risk Management in Type 2 Diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement from the American Heart Association and the Heart Failure Society of America. Circulation 2019, 140, E294–E324. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.A.; Sheeba, F.; Kumar, A.; Ahmad, S.; Blank, N.; Kumari, R.; Kumari, K.; Salame, T.; Khalid, A.; Varrassi, G.; et al. Novel Therapies in Diabetes: A Comprehensive Narrative Review of GLP-1 Receptor Agonists, SGLT2 Inhibitors, and Beyond. Cureus 2023, 15, e51151. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Ferket, B.S.; D’Alessandro, D.A.; Shi, W.; Horvath, K.A.; Rosen, A.; Welsh, S.; Bagiella, E.; Neill, A.E.; Williams, D.L.; et al. Diabetes and the Association of Postoperative Hyperglycemia with Clinical and Economic Outcomes in Cardiac Surgery. Diabetes Care 2016, 39, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; McDonnell, M.; Chipkin, S.R.; Furnary, A.P.; Engelman, R.M.; Sadhu, A.R.; Bridges, C.R.; Haan, C.K.; Svedjeholm, R.; Taegtmeyer, H.; et al. The Society of Thoracic Surgeons Practice Guideline Series: Blood Glucose Management During Adult Cardiac Surgery. The Annals of Thoracic Surgery 2009, 87, 663–669. [Google Scholar] [CrossRef]
- Vervoort, D.; Meuris, B.; Meyns, B.; Verbrugghe, P. Global cardiac surgery: Access to cardiac surgical care around the world. J. Thorac. Cardiovasc. Surg. 2020, 159, 987–996.e6. [Google Scholar] [CrossRef]
- van Straten, A.H.; Soliman Hamad, M.A.; van Zundert, A.A.; Martens, E.J.; Schönberger, J.P.; ter Woorst, J.F.; de Wolf, A.M. Diabetes and Survival after Coronary Artery Bypass Grafting: Comparison with an Age- and Sex-Matched Population. Eur. J. Cardiothorac. Surg. 2010, 37, 1068–1074. [Google Scholar] [CrossRef]
- Leavitt, B.J. Effect of Diabetes and Associated Conditions on Long-Term Survival after Coronary Artery Bypass Graft Surgery. Circulation 2004, 110 (Suppl. S1), II-41. [Google Scholar] [CrossRef]
- Barsness, G.W.; Peterson, E.D.; Ohman, E.M.; Nelson, C.L.; DeLong, E.R.; Reves, J.G.; Smith, P.K.; Anderson, R.D.; Jones, R.H.; Mark, D.B.; et al. Relationship between Diabetes Mellitus and Long-Term Survival after Coronary Bypass and Angioplasty. Circulation 1997, 96, 2551–2556. [Google Scholar] [CrossRef]
- Santos, K.A.Q.; Berto, B.; Sousa, A.G.; Costa, F.A.A.D. Prognosis and Complications of Diabetic Patients Undergoing Isolated Coronary Artery Bypass Surgery. Braz. J. Cardiovasc. Surg. 2015, 31, 7–14. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Baudinaud, P.; Brusset, A.; Nicot, F.; Pechmajou, L.; Salem, J.E.; Estagnasie, P.; Squara, P. Heart failure with preserved ejection fraction as an independent risk factor of mortality after cardiothoracic surgery. J. Thorac. Cardiovasc. Surg. 2018, 156, 188–193.e2. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Tu, J.V.; Eddeen, A.B.; Liu, P.P. Prevalence and Long-Term Survival after Coronary Artery Bypass Grafting in Women and Men with Heart Failure and Preserved versus Reduced Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e008902. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51 (Suppl. S3), S434–S442. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-like Peptide-1: New Regulator in Lipid Metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Costello, R.A. Glucagon-like Peptide-1 Receptor Agonists. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551568/ (accessed on 16 November 2024).
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42 (Suppl. S1), S90–S102. [Google Scholar] [CrossRef]
- Parab, P.; Chaudhary, P.; Mukhtar, S.; Moradi, A.; Kodali, A.; Okoye, C.; Klein, D.; Mohamoud, I.; Olanisa, O.O.; Hamid, P. Role of Glucagon-like Peptide-1 (GLP-1) Receptor Agonists in Cardiovascular Risk Management in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Cureus 2023, 15, e45487. [Google Scholar] [CrossRef]
- Rivera, F.B.; Cruz, L.L.A.; Magalong, J.V.; Ruyeras, J.M.M.J.; Aparece, J.P.; Bantayan, N.R.B.; Lara-Breitinger, K.; Gulati, M. Cardiovascular and Renal Outcomes of Glucagon-like Peptide 1 Receptor Agonists among Patients with and without Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Placebo-Controlled Trials. Am. J. Prev. Cardiol. 2024, 18, 100679. [Google Scholar] [CrossRef]
- Khan, M.S.; Fonarow, G.C.; McGuire, D.K.; Hernandez, A.F.; Vaduganathan, M.; Rosenstock, J.; Handelsman, Y.; Verma, S.; Anker, S.D.; McMurray, J.J.V.; et al. Glucagon-like Peptide 1 Receptor Agonists and Heart Failure. Circulation 2020, 142, 1205–1218. [Google Scholar] [CrossRef]
- Joshi, G.; Abdelmalak, B.; Weigel, W.; Soriano, S.; Harbell, M.; Kuo, C.; Stricker, P.; Domino, K. American Society of Anesthesiologists Consensus-Based Guidance on Preoperative Management of Patients (Adults and Children) on Glucagon-like Peptide-1 (GLP-1) Receptor Agonists; American Society of Anesthesiologists (ASA); Volume 2023, Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative (accessed on 20 November 2024).
- Most Patients Can Continue Diabetes, Weight Loss GLP-1 Drugs Before Surgery, Those at Highest Risk for GI Problems Should Follow Liquid Diet Before Procedure; American Society of Anesthesiologists (ASA), 2024; Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2024/10/new-multi-society-glp-1-guidance (accessed on 30 November 2024).
- Kindel, T.L.; Wang, A.Y.; Wadhwa, A.; Schulman, A.R.; Sharaiha, R.Z.; Kroh, M.; Ghanem, O.M.; Levy, S.; Joshi, G.P.; LaMasters, T.L.; et al. Multisociety clinical practice guidance for the safe use of glucagon-like peptide-1 receptor agonists in the perioperative period. Surg. Obes. Relat. Dis. 2024, 20, 1183–1186. [Google Scholar] [CrossRef]
- Robinson, M.; Davidson, A. Aspiration under Anaesthesia: Risk Assessment and Decision-Making. Contin. Educ. Anaesth. Crit. Care Pain 2014, 14, 171–175. [Google Scholar] [CrossRef]
- Ahrén, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Nistala, R.; Savin, V. Diabetes, Hypertension, and Chronic Kidney Disease Progression: Role of DPP-4. Am. J. Physiol. Ren. Physiol. 2017, 312, F661–F670. [Google Scholar] [CrossRef] [PubMed]
- Godinho, R.; Mega, C.; Teixeira-de-Lemos, E.; Carvalho, E.; Teixeira, F.; Fernandes, R.; Reis, F. The Place of Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes Therapeutics: A “Me Too” or “the Special One” Antidiabetic Class? J. Diabetes Res. 2015, 2015, 806979. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hong, J.; Chi, J.; Gu, W.; Ning, G.; Wang, W. Head-to-Head Comparison of Dipeptidyl Peptidase-IV Inhibitors and Sulfonylureas—A Meta-Analysis from Randomized Clinical Trials. Diabetes Metab. Res. Rev. 2014, 30, 241–256. [Google Scholar] [CrossRef]
- Crowley, K.; Scanaill, P.; Hermanides, J.; Buggy, D.J. Current Practice in the Perioperative Management of Patients with Diabetes Mellitus: A Narrative Review. Br. J. Anaesth. 2023, 131, 242–252. [Google Scholar] [CrossRef]
- Kirk, J.K.; Gonzales, C.F. Preoperative Considerations for Patients with Diabetes. Expert Rev. Endocrinol. Metab. 2023, 18, 503–512. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- Boutsikos, I.; Beltsios, E.; Schmack, B.; Pantazopoulos, I.; Chatzis, D.G. Sodium glucose Co-Transporter 2 inhibitors and the cardiovascular System: Current knowledge and future expectations. Heart Int. 2023, 17, 12. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes/Metab. Res. Rev. 2004, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.H.; Chan, M.C.Y. SGLT2 inhibitors: The next blockbuster multifaceted drug? Medicina 2023, 59, 388. [Google Scholar] [CrossRef] [PubMed]
- Chasis, H.; Jolliffe, N.; Smith, H.W. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine and urea by man. J. Clin. Investig. 1933, 12, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Smith, D.; Shulman, G.I.; Papachristou, D.; DeFronzo, R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Investig. 1987, 79, 1510–1515. [Google Scholar] [CrossRef]
- Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes; National Institute of Diabetes and Digestive and Kidney Diseases: 2020. Available online: https://www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes#:~:text=The%20first%20SGLT2%20inhibitor%20to,Jardiance%C2%AE)%20in%20August%202014 (accessed on 15 November 2024).
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S125–S143. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacodynamics, efficacy and safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) inhibitors for the treatment of Type 2 diabetes mellitus. Drugs 2014, 75, 33–59. [Google Scholar] [CrossRef]
- Madhok, J.; Vanneman, M.W. SGLT-2 inhibitors: Proliferating indications and perioperative pitfalls. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1815–1819. [Google Scholar] [CrossRef]
- Handelsman, Y.; Henry, R.R.; Bloomgarden, Z.T.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Ferrannini, E.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on the Association of SGLT-2 Inhibitors and Diabetic Ketoacidosis. Endocr. Pract. 2016, 22, 753–762. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Fiemotonghan, B.-E.; Okoye, O.K.; Agu-Ben, C.M.; Onyekweli, S.O.; Amapu, D.A.; Ikpegbu, R.; Asekhauno, M.; et al. A comprehensive review of heart failure: Unraveling the etiology, decoding pathophysiological mechanisms, navigating diagnostic modalities, exploring pharmacological interventions, advocating lifestyle modifications, and charting the horizon of emerging therapies in the complex landscape of chronic cardiac dysfunction. Medicine 2024, 103, e36895. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Gao, Z.; Barth, A.S.; DiSilvestre, D.; Akar, F.G.; Tian, Y.; Tanskanen, A.; Kass, D.A.; Winslow, R.L.; Tomaselli, G.F. Key pathways associated with heart failure development revealed by gene networks correlated with cardiac remodeling. Physiol. Genom. 2008, 35, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Baman, J.R.; Ahmad, F.S. Heart failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in human heart failure: Major mediators and therapeutic targets. Front. Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Saraiva, F.; Sharma, A.; Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Leite, A.R.; Borges-Canha, M.; Carvalho, D.; Packer, M.; Zannad, F.; et al. Glucagon-like Peptide 1 Receptor Agonists in Patients with Type 2 Diabetes with and without Chronic Heart Failure: A Meta-Analysis of Randomized Placebo-Controlled Outcome Trials. Diabetes Obes. Metab. 2023, 25, 1495–1502. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of Liraglutide, a Glucagon-like Peptide-1 Analogue, on Left Ventricular Function in Stable Chronic Heart Failure Patients with and without Diabetes ( LIVE )—A Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Eur. J. Heart Fail. 2016, 19, 69–77. [Google Scholar] [CrossRef]
- Fudim, M.; White, J.; Pagidipati, N.J.; Lokhnygina, Y.; Wainstein, J.; Murin, J.; Iqbal, N.; Öhman, P.; Lopes, R.D.; Reicher, B.; et al. Effect of Once-Weekly Exenatide in Patients with Type 2 Diabetes Mellitus with and without Heart Failure and Heart Failure–Related Outcomes. Circulation 2019, 140, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Packer, M.; Ferreira, J.P. Increased Risk of Heart Failure Hospitalization with GLP-1 Receptor Agonists in Patients with Reduced Ejection Fraction: A Meta-Analysis of the EXSCEL and FIGHT Trials. J. Card. Failure 2023. [Google Scholar] [CrossRef]
- Mannucci, E.; Nreu, B.; Montereggi, C.; Ragghianti, B.; Gallo, M.; Giaccari, A.; Monami, M. Cardiovascular Events and All-Cause Mortality in Patients with Type 2 Diabetes Treated with Dipeptidyl Peptidase-4 Inhibitors: An Extensive Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2745–2755. [Google Scholar] [CrossRef]
- Packer, M. Do DPP-4 Inhibitors Cause Heart Failure Events by Promoting Adrenergically Mediated Cardiotoxicity? Clues from Laboratory Models and Clinical Trials. Circ. Res. 2018, 122, 928–932. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Deng, K.; Liu, J.; Vandvik, P.O.; Zhao, P.; Zhang, L.; Shen, J.; Bala, M.M.; Sohani, Z.N.; et al. Dipeptidyl Peptidase-4 Inhibitors and Risk of Heart Failure in Type 2 Diabetes: Systematic Review and Meta-analysis of Randomised and Observational Studies. BMJ 2016, 352, i610. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Obley, A.J.; Shamliyan, T.; Hicks, L.A.; Harrod, C.S.; Crandall, C.J. Newer Pharmacologic Treatments in Adults with Type 2 Diabetes: A Clinical Guideline from the American College of Physicians. Ann. Intern. Med. 2024, 177, 658–666. [Google Scholar] [CrossRef]
- Enzan, N.; Matsushima, S.; Kaku, H.; Tohyama, T.; Nagata, T.; Ide, T.; Tsutsui, H. Beneficial Effects of Dipeptidyl Peptidase-4 Inhibitors on Heart Failure with Preserved Ejection Fraction and Diabetes. JACC Asia 2023, 3, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2018, 380, 347–357. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2020, 384, 117–128. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; De Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Butler, J.; Farmakis, D.; Zannad, F.; Ofstad, A.P.; Ferreira, J.P.; Green, J.B.; Rosenstock, J.; Schnaidt, S.; Brueckmann, M.; et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation 2022, 146, 676–686. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction. Circulation 2020, 143, 326–336. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Angermann, C.E.; Collins, S.P.; Teerlink, J.R.; Ponikowski, P.; Biegus, J.; Comin-Colet, J.; Ferreira, J.P.; Mentz, R.J.; Nassif, M.E.; et al. Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: Results from the EMPULSE trial. Circulation 2022, 146, 279–288. [Google Scholar] [CrossRef]
- Sindhvananda, W.; Poopuangpairoj, W.; Jaiprasat, T.; Ongcharit, P. Comparison of Glucose Control by Added Liraglutide to Only Insulin Infusion in Diabetic Patient Undergoing Cardiac Surgery: A Preliminary Randomized-Controlled Trial. Ann. Card. Anaesth. 2023, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hulst, A.H.; Visscher, M.J.; Godfried, M.B.; Thiel, B.; Gerritse, B.M.; Scohy, T.V.; Bouwman, R.A.; Willemsen, M.G.A.; Hollmann, M.W.; Preckel, B.; et al. Liraglutide for Perioperative Management of Hyperglycaemia in Cardiac Surgery Patients: A Multicentre Randomized Superiority Trial. Diabetes Obes. Metab. 2019, 22, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Tanaka, A.; Asakura, K.; Koezuka, R.; Tochiya, M.; Ohata, Y.; Tamanaha, T.; Son, C.; Shimabara, Y.; Fujita, T.; et al. Addition of Low-Dose Liraglutide to Insulin Therapy Is Useful for Glycaemic Control during the Peri-Operative Period: Effect of Glucagon-like Peptide-1 Receptor Agonist Therapy on Glycaemic Control in Patients Undergoing Cardiac Surgery (GLOLIA Study). Diabet. Med. 2019, 36, 1621–1628. [Google Scholar] [CrossRef]
- Oosterom-Eijmael, M.J.P.; Hermanides, J.; van Raalte, D.H.; Kouw, I.W.K.; DeVries, J.H.; Hulst, A.H. Continuous Glucose Monitoring and the Effect of Liraglutide in Cardiac Surgery Patients: A Sub-Study of the Randomized Controlled GLOBE Trial. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1965–1971. [Google Scholar] [CrossRef]
- Hulst, A.H.; Visscher, M.J.; Cherpanath, T.G.V.; van de Wouw, L.; Godfried, M.B.; Thiel, B.; Gerritse, B.M.; Scohy, T.V.; Bouwman, R.A.; Willemsen, M.G.A.; et al. Effects of Liraglutide on Myocardial Function after Cardiac Surgery: A Secondary Analysis of the Randomised Controlled GLOBE Trial. J. Clin. Med. 2020, 9, 673. [Google Scholar] [CrossRef]
- Cardona, S.; Tsegka, K.; Pasquel, F.J.; Jacobs, S.; Halkos, M.; Keeling, W.B.; Davis, G.M.; Fayfman, M.; Albury, B.; Urrutia, M.A.; et al. Sitagliptin for the Prevention and Treatment of Perioperative Hyperglycemia in Patients with Type 2 Diabetes Undergoing Cardiac Surgery: A Randomized Controlled Trial. Diabetes Obes. Metab. 2021, 23, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Massetti, M.; Testa, N.; Di Martino, L.; Castellano, G.; Turriziani, F.; Sasso, F.C.; Torella, M.; De Feo, M.; Santulli, G.; et al. Effects of Sodium-Glucose Transporter 2 Inhibitors (SGLT2-I) in Patients with Ischemic Heart Disease (IHD) Treated by Coronary Artery Bypass Grafting via MiECC: Inflammatory Burden, and Clinical Outcomes at 5 Years of Follow-Up. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Al Namat, R.; Duceac, L.D.; Chelaru, L.; Dabija, M.G.; Guțu, C.; Marcu, C.; Popa, M.V.; Popa, F.; Goroftei, E.R.B.; Țarcă, E. Post-Coronary Artery Bypass Grafting Outcomes of Patients with/without Type-2 Diabetes Mellitus and Chronic Kidney Disease Treated with SGLT2 Inhibitor Dapagliflozin: A Single-Center Experience Analysis. Diagnostics 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Cagliostro, M.; Hundal, P.; Ting, P.; Patel, S.; Sudarshan, S.; Thomas, J.; Morris, K.; Mancini, D.M.; Moss, N.; Lala, A.; et al. Safety and effects of SGLT-2 inhibitor use among LVAD patients with type 2 diabetes mellitus. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 18, 100154. [Google Scholar] [CrossRef]
- Fardman, A.; Kodesh, A.; Siegel, A.J.; Segev, A.; Regev, E.; Maor, E.; Berkovitch, A.; Kuperstein, R.; Morgan, A.; Nahum, E.; et al. The safety of sodium glucose transporter 2 inhibitors and trends in clinical and hemodynamic parameters in patients with left ventricular assist devices. Artif. Organs 2024, 48, 902–911. [Google Scholar] [CrossRef]
| Clinical Trial | SGLT2i | Subjects | T2DM | HF | MACE | Target Population | Outcome |
|---|---|---|---|---|---|---|---|
| DECLARE-TIMI 58 (2018) | Dapagliflozin | 17,160 | + | ↓ | ND | Patients with T2DM and established atherosclerotic CV disease or multiple risk factors for CV disease | * SGLT2is did not result in a higher or lower rate of MACE, but did result in a lower rate of CV death or hospitalization for HF |
| DAPA-HF (2019) | Dapagliflozin | 4744 | − | ↓ | ↓ | NYHA II/III/IV HF and EF ≤40% | Lower risk of worsening HF or CV deaths, regardless of DM status |
| SOLOIST-WHF (2020) | Sotagliflozin | 1222 | + | ↓ | ↓ | T2DM patients recently hospitalized for worsening HF | Lower worsening HF and lower CV deaths |
| EMPEROR-Reduced (2020) | Empagliflozin | 3730 | − | ↓ | NA | NYHA II/III/IV HF and EF ≤ 40% | Lower risk and total number of inpatient and outpatient worsening HF at 12 days after starting SGLT2is |
| EMPULSE (2022) | Empagliflozin | 530 | − | NA | ↓ | Acute HF (both de novo and decompensated chronic HF with reduced or preserved EF, and with or without T2DM) | Improved symptoms, lesser physical limitations, and better quality of life as early as 15 days after starting SGLT2is and maintained through 90 days |
| DELIVER (2022) | Dapagliflozin | 6263 | − | ↓ | ↓ | Chronic HF and LVEF > 40%, i.e., preserved or mildly reduced | Lower combined risk of worsening HF or CV deaths |
| EMPEROR-Preserved (2022) | Empagliflozin | 5988 | +/− | ↓ | ↓ | Symptomatic HF and EF > 40%, elevated natriuretic peptide levels, and evidence of cardiac changes or previous HF hospitalization | Improvement in worsening HF outcomes; also slowed decline in kidney function—regardless of T2DM status |
| Study: Author (Year), Type | Antidiabetic Agent | Subjects (n) | T2DM (n) | HF (n) | Others | Target Population and Cardiac Surgery Type | Outcomes |
|---|---|---|---|---|---|---|---|
| Sindhvananda et al. (2023) [70] RCT | GLP-1RA (Liraglutide) | 56 | Yes (56) | No | T2DM patients undergoing CABG | Significantly lower BG levels in patients on Liraglutide and insulin before, during, and after CABG compared to insulin-only controls. | |
| Hulst et al. (2020) RCT [71] | GLP-1RA (Liraglutide) | 278 | Yes (43) | No | Patients with or without T2DM undergoing elective cardiac surgery | Significantly lower total intraoperative insulin doses and a significantly lower number of insulin administrations, as well as better postoperative BG control in the Liraglutide treatment group compared to the placebo control group. | |
| Makino et al. (2019) [72] RCT | GLP-1RA (Liraglutide) | 70 | Yes (70) | No | T2DM patients undergoing CABG | Significantly lower M values (proximity index of target glucose level) in the Liraglutide treatment group compared to the insulin-only control group, suggestive of better glycemic control. | |
| Oosterom-Eijmael et al. (2024) [73] Prospective cohort | GLP-1RA (Liraglutide) | 25 | Yes (3) | No | Patients with or without T2DM undergoing elective cardiac surgery | The Liraglutide treatment group had increased glycemic time in range compared to the placebo control group. | |
| Hulst et al. (2020) [74] Secondary analysis | GLP-1RA (Liraglutide) | 261 | Yes (42) | No | Postoperative patients with or without T2DM after elective cardiac surgery | The Liraglutide group had a lower ICU admission rate and higher rates of normal LV systolic function postoperatively compared to the control group. | |
| Cardona et al. (2021) [75] RCT | DPP-4 inhibitor (Sitagliptin) | 182 | Yes (182) | No | T2DM patients undergoing CABG | No differences in hypoglycemia rate, mean daily glucose, hospital stay, or readmissions after discharge in the DPP-4 inhibitor group compared to placebo. Lower insulin requirement in the DPP-4 inhibitor group upon transfer from ICU to wards. | |
| Sardu et al. (2021) [76] Prospective cohort | SGLT2i | 648 | Yes (188) | No | Yes, IHD (188) | IHD patients undergoing CABG via MiECC | Patients without T2DM had lower levels of inflammatory markers postoperatively. Among T2DM patients, those treated with SGLT2is had lower levels of inflammatory markers compared to the non-SGLT2i group after surgery. |
| Al Namat et al. (2023) [77] Prospective cohort | SGLT2i (Dapagliflozin) | 120 | Yes (65) | Yes (87) | Yes, CKD (35) | Age ≥ 40 with clinical indication for CABG surgery | All patients underwent post-CABG rehabilitation and SGLT2i treatment. Regardless of cardiac statuses, rehabilitation and SGLT2i treatment led to improved mean EF, glycemic status, and renal function in patients with or without T2DM and with or without CKD. |
| Cagliostro et al. (2022) [78] Retrospective cohort | SGLT2i | 34 | Yes (34) | Yes (17) | Yes, CKD (16) | T2DM patients undergoing LVAD implantation | Lower BUN levels at 180-day follow-up with SGLT2i treatment but no significant change in BMI, HbA1c, or diuretic dose. |
| Fardman et al. (2023) [79] Retrospective cohort | SGTL2i | 29 | Yes (23) | Yes (29) | Patients undergoing LVAD implantation initiated on SGLT2i postoperatively | SGLT2i initiation after LVAD placement associated with decreased daily furosemide dose, weight, and sPAP but possibly higher RV dysfunction rate. No change in LVAD parameters. |
| Antidiabetic Class | |||
|---|---|---|---|
| GLP-1RAs | DPP-4 Inhibitors | SGLT2is | |
| Glycemic control | Significant reduction in HbA1c by ~1–1.2%. | Moderate reduction in HbA1c (0.5–0.8%). | Significant reduction in HbA1c (0.4–1.08%) and lower fasting plasma glucose as monotherapy or combined use with other agents. |
| HF with T2DM | Lower incidence of hospitalization for new-onset HFpEF but not in patients with established HF at baseline. Neutral or harmful in HFrEF. | Lower incidence of hospitalization and composite of cardiovascular death in HFpEF. | Improved outcomes (less worsening HF, hospitalization and/or urgent visits for HF, and quality of life) for HFpEF, HFmrEF, and HFrEF. |
| Cardiac surgery outcomes | Improved perioperative BG control with lower intraoperative insulin requirements. | Limited studies: No differences in frequency of hypoglycemia, mean daily glucose, hospital length of stay, surgical reinterventions, or readmissions after discharge in the DPP-4 inhibitor group compared to placebo. Lower insulin requirement in the DPP-4 inhibitor group upon transfer from ICU to wards. | CABG: Significant decrease in ischemic risk, improved mean EF, glycemic status, and renal function with or without T2DM, with or without CKD in the SGLT2i treatment group compared to the control. Lower-level inflammatory markers and ameliorated clinical outcomes at the 5-year postop point via MiECC. LVAD: Reduction in BUN and sPAP. Questionable reduction in weight/BMI and daily diuretic requirements. Possible higher RV dysfunction prevalence. No change in HbA1c or LVAD parameters. |
| Adverse events | GI upset (nausea/vomiting), delayed gastric emptying, appetite suppression, renal dysfunction, arrhythmias, and worsening of existing ischemic heart disease. | Upper respiratory tract infection, nasopharyngitis, headache, UTI, and arthralgia. | Volume depletion, UTI/yeast infection, hypoglycemia, and euglycemic DKA. |
| Perioperative recommendations | If the risk of delayed gastric emptying/aspiration is low, continue before surgery. If the risk of delayed gastric emptying/aspiration is high, hold GLP-1RAs on the day of surgery if on a daily dosing schedule or the week prior to surgery if on a weekly dosing schedule, as well as a 24 hr preoperative liquid diet Resume GLP-1RAs after surgery if patient is able to safely tolerate oral intake. | Continue DPP-4 inhibitor use or withhold on day of surgery. May resume DPP-4 inhibitor use immediately after surgery. | Hold SGLT2is at least 3 days before surgery. Monitor closely for glucose level and acid/base status after surgery and resume SGLT2is if able to tolerate oral intake and maintain adequate hydration. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Bitzas, S.; Perez, D.; Schwartz, J.; Zaidi, S.; Oster, J.; Bergese, S.D. Perioperative Considerations of Novel Antidiabetic Agents in Heart Failure Patients Undergoing Cardiac Surgery. Life 2025, 15, 427. https://doi.org/10.3390/life15030427
Wang A, Bitzas S, Perez D, Schwartz J, Zaidi S, Oster J, Bergese SD. Perioperative Considerations of Novel Antidiabetic Agents in Heart Failure Patients Undergoing Cardiac Surgery. Life. 2025; 15(3):427. https://doi.org/10.3390/life15030427
Chicago/Turabian StyleWang, Ashley, Savannah Bitzas, Dilsa Perez, Jonathon Schwartz, Saleem Zaidi, Jonathan Oster, and Sergio D. Bergese. 2025. "Perioperative Considerations of Novel Antidiabetic Agents in Heart Failure Patients Undergoing Cardiac Surgery" Life 15, no. 3: 427. https://doi.org/10.3390/life15030427
APA StyleWang, A., Bitzas, S., Perez, D., Schwartz, J., Zaidi, S., Oster, J., & Bergese, S. D. (2025). Perioperative Considerations of Novel Antidiabetic Agents in Heart Failure Patients Undergoing Cardiac Surgery. Life, 15(3), 427. https://doi.org/10.3390/life15030427







