Liraglutide Treatment Restores Cardiac Function After Isoprenaline-Induced Myocardial Injury and Prevents Heart Failure in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
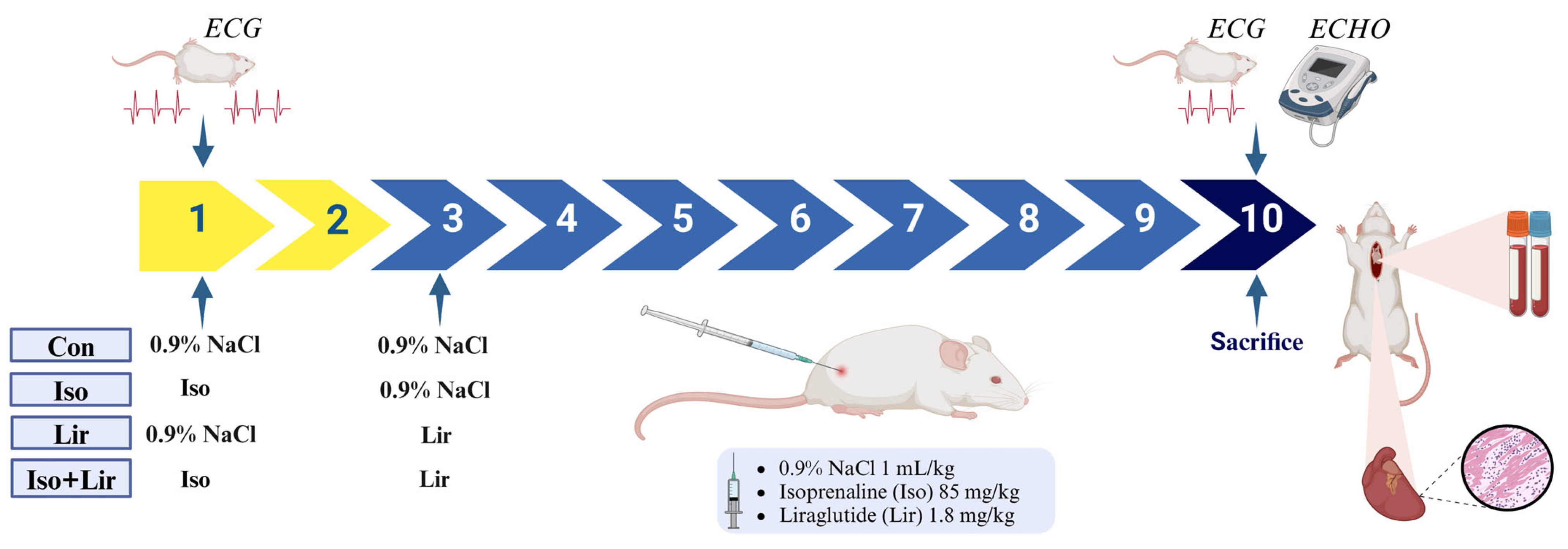
2.2. Experimental Grouping
2.3. Obtaining Blood and Tissue Samples
2.4. Biomarkers of Myocardial Injury and Other Biochemical Parameters
2.5. Prooxidative and Antioxidative Markers
2.6. ECG Recording
2.7. ECHO
2.8. Pathohistological Findings
2.9. Statistical Analysis
3. Results
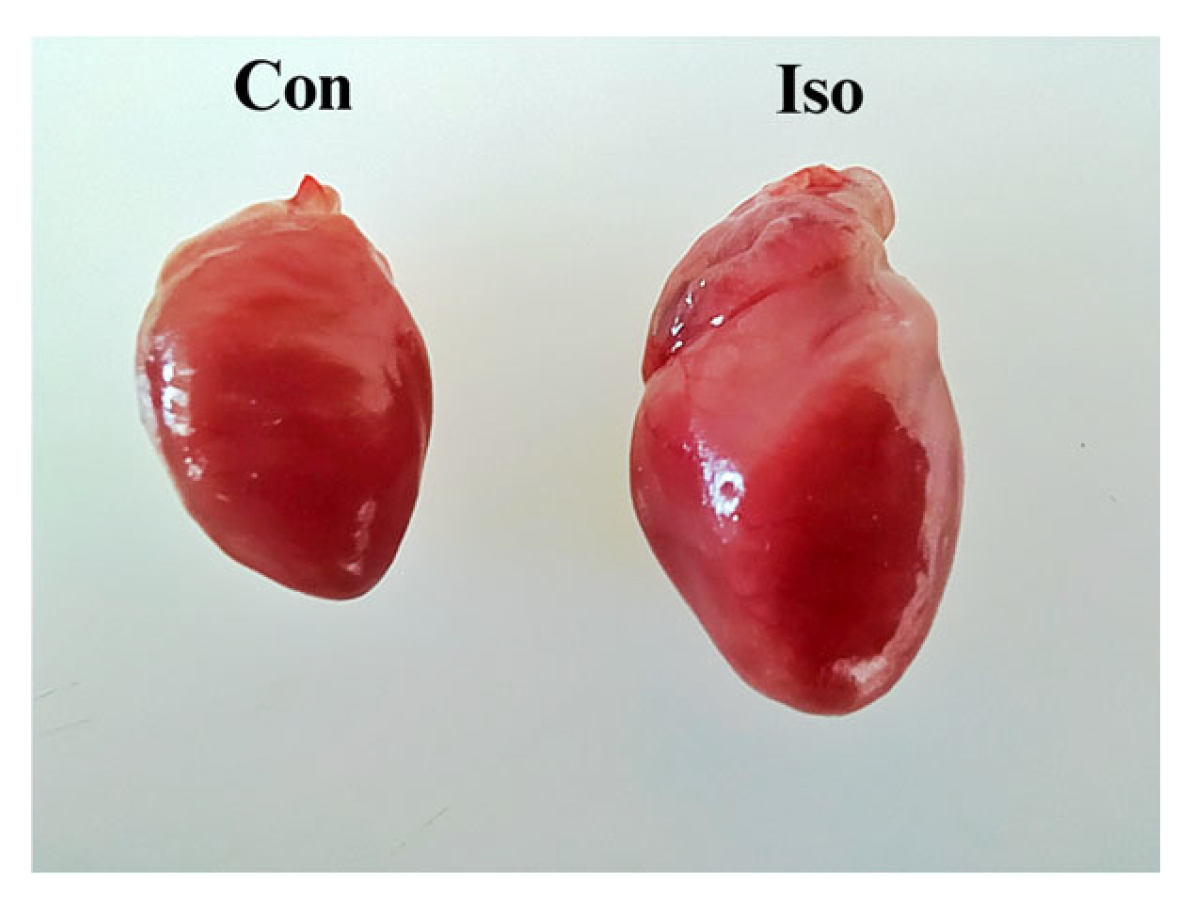
3.1. Effects of Liraglutide Treatment on Body Weight (BW), Heart Weight (HW), and Heart-to-Body Weight (H/BW) Ratio in Rats with Isoprenaline-Induced HF
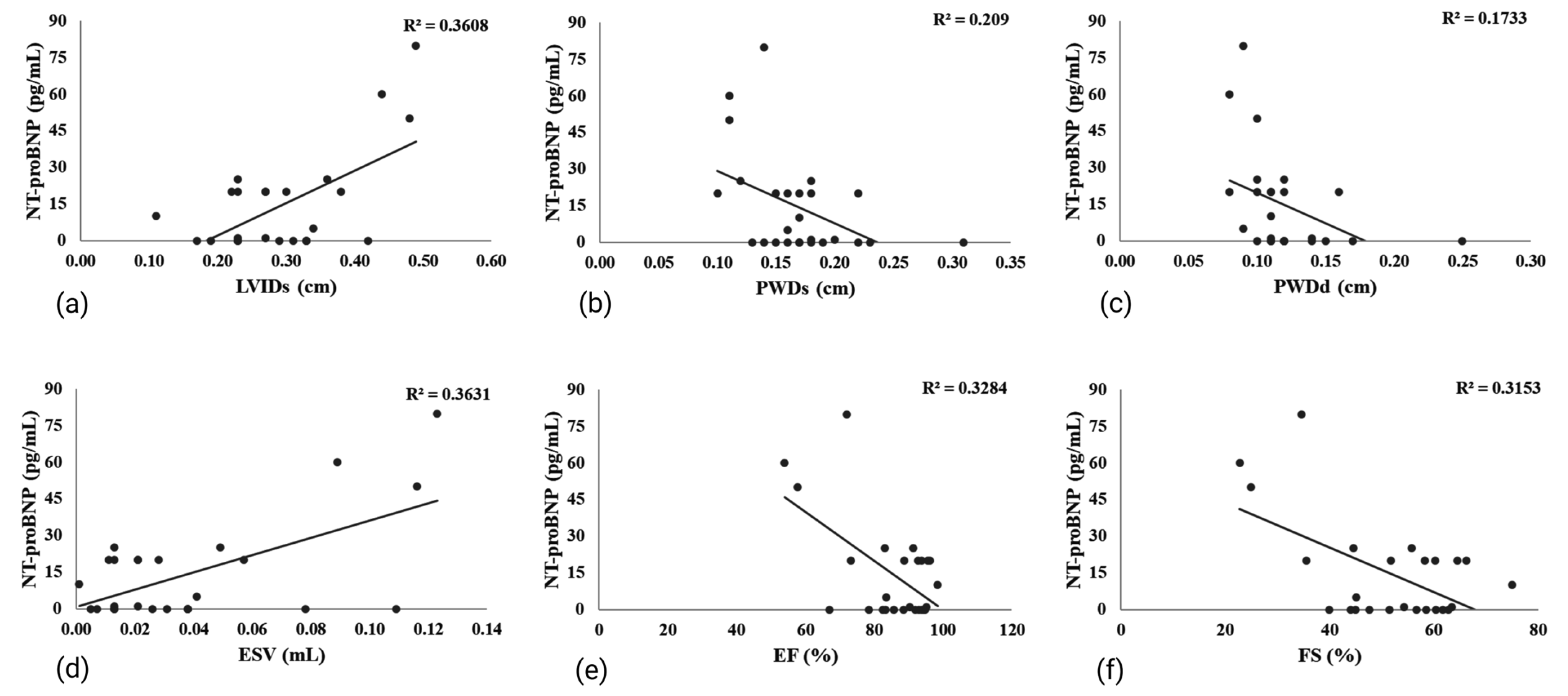
3.2. Effects of Liraglutide Treatment on Biomarkers of MI and Other Biochemical Parameters in Rats with Isoprenaline-Induced HF
3.3. Effects of Liraglutide Treatment on Prooxidative and Antioxidative Markers in Rats with Isoprenaline-Induced HF
3.4. Effects of Liraglutide Treatment on Electrocardiogram (ECG) in Rats with Isoprenaline-Induced HF
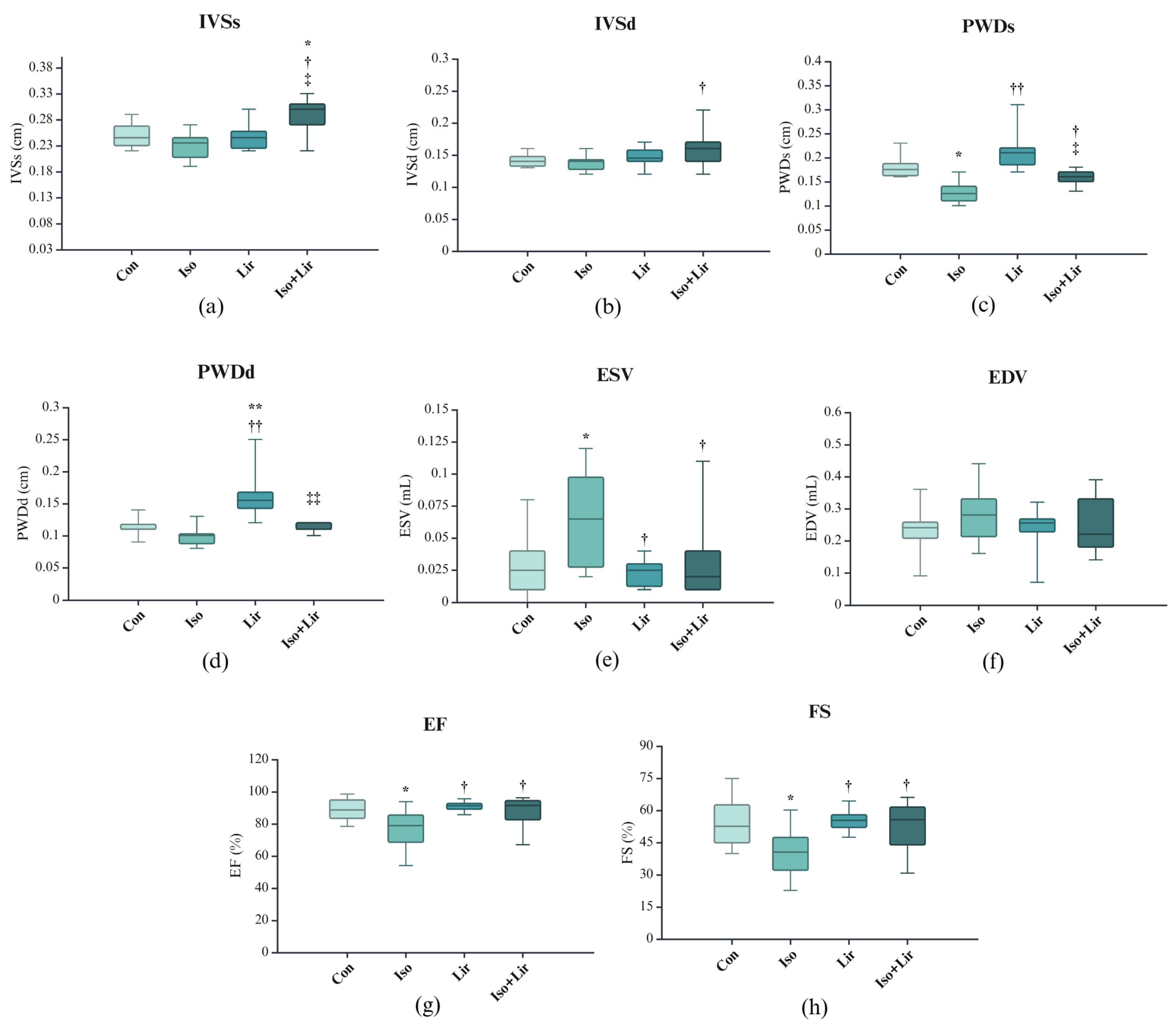
3.5. Effects of Liraglutide Treatment on Echocardiogram (ECHO) in Rats with Isoprenaline-Induced HF
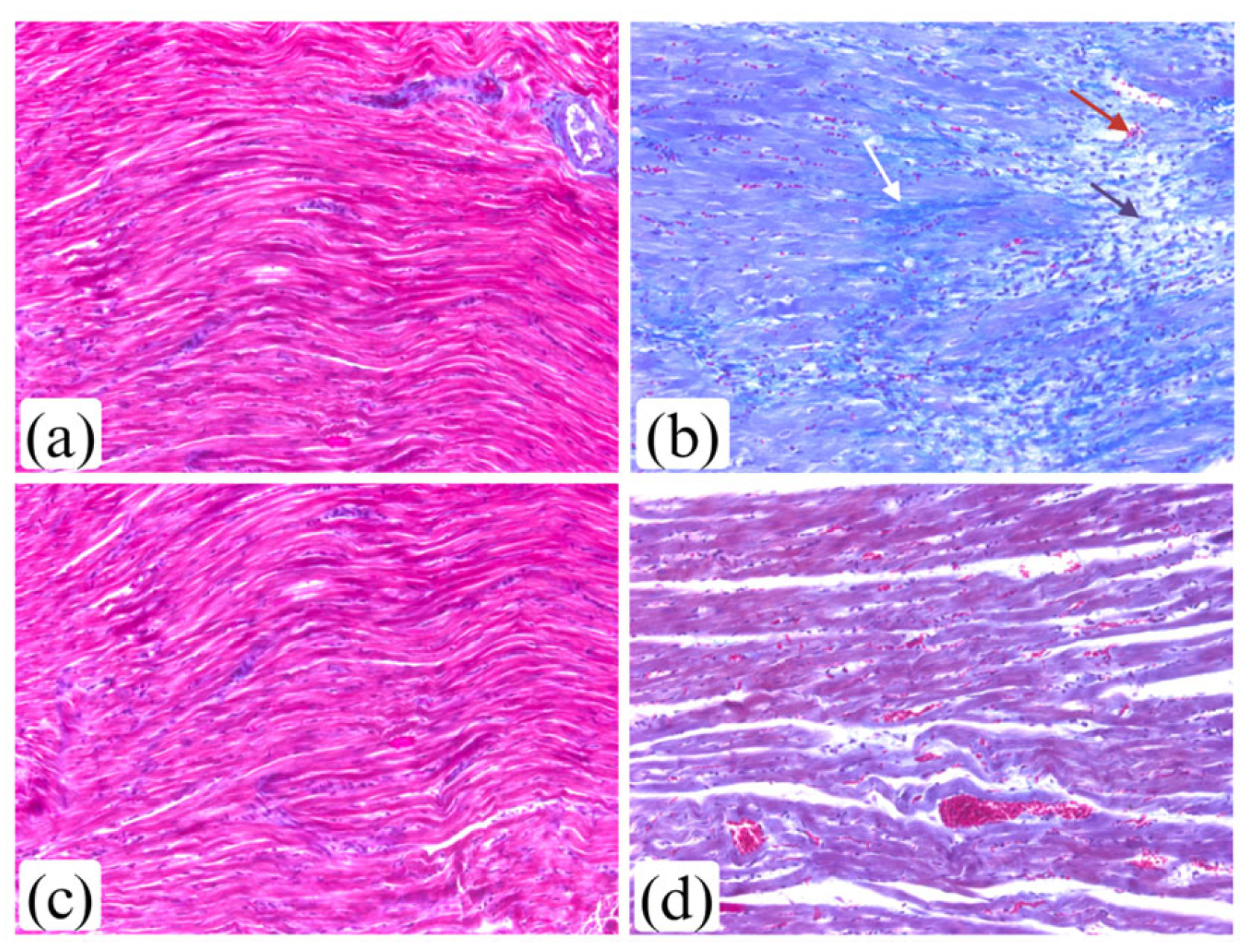
3.6. Effects of Liraglutide Treatment on Myocardium Morphology in Rats with Isoprenaline-Induced HF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCarthy, C.; Murphy, S.; Cohen, J.A.; Rehman, S.; Jones-O’Connor, M.; Olshan, D.S.; Singh, A.; Vaduganathan, M.; Januzzi, J.L.; Wasfy, J.H. Misclassification of Myocardial Injury as Myocardial Infarction: Implications for Assessing Outcomes in Value-Based Programs. JAMA Cardiol. 2019, 4, 460–464. [Google Scholar] [CrossRef]
- Taggart, C.; Wereski, R.; Mills, N.L.; Chapman, A.R. Diagnosis, investigation and management of patients with acute and chronic myocardial injury. J. Clin. Med. 2021, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Onuma, S.; Hao, K.; Godo, S.; Shiroto, T.; Yasuda, S. Pathophysiology and diagnostic pathway of myocardial infarction with non-obstructive coronary arteries. J. Cardiol. 2024, 83, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Adamson, P.D.; Mills, N.L. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart 2017, 103, 10–18. [Google Scholar] [CrossRef]
- Duan, D.; Li, H.; Chai, S.; Zhang, L.; Fan, T.; Hu, Z.; Feng, Y. The relationship between cardiac oxidative stress, inflammatory cytokine response, cardiac pump function, and prognosis post-myocardial infarction. Sci. Rep. 2024, 14, 8985. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, T.; Cheng, Z. Mechanism of heart failure after myocardial infarction. J. Int. Med. Res. 2023, 51, 03000605231202573. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Sobot, T.; Bajic, Z.; Skrbic, R.; Uletilovic, S.; Mandic-Kovacevic, N.; Cvjetkovic, T.; Malicevic, U.; Djukanovic, D.; Bojic, M.G.; Jovicic, S.; et al. Effect of folic acid on isoprenaline-induced myocardial injury in rats. Physiol. Int. 2024, 111, 80–96. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Cardioprotective potential of garlic oil and its active constituent, diallyl disulphide, in presence of carvedilol during chronic isoprenaline injection-mediated myocardial necrosis in rats. Molecules 2021, 26, 5137. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Zhang, Z.; Cheng, J.; Xu, Z.; Zhu, K.; Ren, Y. Cardioprotective effects of Schisantherin A against isoproterenol-induced acute myocardial infarction through amelioration of oxidative stress and inflammation via modulation of PI3K-AKT/Nrf2/ARE and TLR4/MAPK/NF-κB pathways in rats. BMC Complement. Med. Ther. 2023, 23, 277. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.H.; Fabricius, K.; Barkholt, P.; Niehoff, M.L.; Morley, J.E.; Jelsing, J.; Pyke, C.; Knudsen, L.B.; Farr, S.A.; Vrang, N. The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 46, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Petković-Dabić, J.; Binić, I.; Carić, B.; Umičević-Šipka, S.; Bednarčuk, N.; Dabić, S.; Šitum, M.; Popović-Pejičić, S.; Stojiljković, M.P.; Škrbić, R. Effects of Semaglutide Treatment on Psoriatic Lesions in Obese Patients with Type 2 Diabetes Mellitus: An Open-Label, Randomized Clinical Trial. Biomolecules 2025, 15, 46. [Google Scholar] [CrossRef]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.E.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 2022, 145, 575–585. [Google Scholar] [CrossRef]
- Savchenko, L.G.; Digtiar, N.I.; Selikhova, L.G.; Kaidasheva, E.I.; Shlykova, O.A.; Vesnina, L.E.; Kaidashev, I.P. Liraglutide exerts an anti-inflammatory action in obese patients with type 2 diabetes. Rom. J. Intern. Med. 2019, 57, 233–240. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Li, J.; Mao, Q.; He, J. Effects of liraglutide combined with insulin on oxidative stress and serum MCP-1 and NF-B levels in type 2 diabetes. J. Coll. Physicians Surg. Pak. 2019, 29, 218–221. [Google Scholar] [CrossRef]
- Bajic, Z.; Sobot, T.; Uletilovic, S.; Mandic-Kovacevic, N.; Cvjetkovic, T.; Malicevic, U.; Djukanovic, D.; Duran, M.; Vesic, N.; Avram, S.; et al. Cardioprotective effects of liraglutide pretreatment on isoprenaline-induced myocardial injury in rats. Can. J. Physiol. Pharmacol. 2023, 101, 258–267. [Google Scholar] [CrossRef]
- Mandić-Kovačević, N.; Kukrić, Z.; Latinović, S.; Cvjetković, T.; Šobot, T.; Bajić, Z.; Maličević, U.; Marinković, S.; Đukanović, Đ.; Uletilović, S.; et al. Antioxidative Potential of Pomegranate Peel Extract: In Vitro and In Vivo Studies. Scr. Medica 2023, 54, 9–18. [Google Scholar] [CrossRef]
- Redfors, B.; Shao, Y.; Omerovic, E. Influence of anesthetic agent, depth of anesthesia and body temperature on cardiovascular functional parameters in the rat. Lab. Anim. 2014, 48, 6–14. [Google Scholar] [CrossRef]
- Taşkıran, E.; Erdoğan, M.A.; Yiğittürk, G.; Erbaş, O. Therapeutic Effects of Liraglutide, Oxytocin and Granulocyte Colony-Stimulating Factor in Doxorubicin-Induced Cardiomyopathy Model: An Experimental Animal Study. Cardiovasc. Toxicol. 2019, 19, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Fenning, A.; Chan, V.; Loch, D.; Wilson, K.; Anderson, B.; Burstow, D. Echocardiographic Assessment of Cardiac Structure and Function in Rats. Heart Lung Circ. 2002, 11, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hubesch, G.; Hanthazi, A.; Acheampong, A.; Chomette, L.; Lasolle, H.; Hupkens, E.; Jespers, P.; Vegh, G.; Wembonyama, C.W.M.; Verhoeven, C.; et al. A Preclinical Rat Model of Heart Failure With Preserved Ejection Fraction With Multiple Comorbidities. Front. Cardiovasc. Med. 2022, 8, 809885. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K. Echocardiographic assessment of cardiac structure and function in chronic renal disease. J. Echocardiogr. 2019, 17, 115–122. [Google Scholar] [CrossRef]
- Hamed, A.B.; Mantawy, E.M.; El-Bakly, W.M.; Abdel-Mottaleb, Y.; Azab, S.S. Putative anti-inflammatory, antioxidant, and anti-apoptotic roles of the natural tissue guardian methyl palmitate against isoproterenol-induced myocardial injury in rats. Futur. J. Pharm. Sci. 2020, 6, 31. [Google Scholar] [CrossRef]
- Qi, C.; Shao, Y.; Liu, X.; Wang, D.; Li, X. The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: Involvement of reactive oxygen species and the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 74, 105733. [Google Scholar] [CrossRef]
- Elshaer, A.; El-Awady, W.S.; Masoud, M.M.; Elmenshawy, M.D.; Wageeh, S.; Shehata, I.E. Comparative Study Among Three Different Models for Induction of Acute Myocardial Infarction in Rats. Eur. Heart J. Suppl. 2021, 23, D1–D7. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, W.; Zhang, J.; Dong, Y.; Wu, J.; Zhang, Y.; Dai, C.; Zhang, T.; Yang, G.; Zhang, Y.; et al. Bellidifolin ameliorates isoprenaline-induced cardiac hypertrophy by the Nox4/ROS signalling pathway through inhibiting BRD4. Cell Death Discov. 2023, 9, 279. [Google Scholar] [CrossRef]
- Li, Y.; He, B.; Zhang, C.; Xia, T.; Zeng, C. Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway. Nutrients 2023, 15, 1340. [Google Scholar] [CrossRef]
- Yoon, J.J.; Tai, A.L.; Kim, H.Y.; Han, B.H.; Shin, S.; Lee, H.S.; Kang, D.G. TongGuanWan Alleviates Doxorubicin- and Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Modulating Apoptotic and Fibrotic Pathways. Int. J. Mol. Sci. 2024, 25, 10573. [Google Scholar] [CrossRef]
- Baynes, K.C.R.; Nicholas, M.D.; Shojaee-Moradie, F.; Umpleby, A.M.; Giannoulis, M.G. Acute regulation of plasma leptin by isoprenaline in lean and obese fasted subjects. Diabetes Obes. Metab. 2006, 8, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Gasoyan, H.; Pfoh, E.R.; Schulte, R.; Le, P.; Butsch, W.S.; Rothberg, M.B. One-Year Weight Reduction With Semaglutide or Liraglutide in Clinical Practice. JAMA Netw. Open 2024, 7, e2433326. [Google Scholar] [CrossRef] [PubMed]
- Anamalley, R.; Rajassageran, L.; Apparoo, Y.; Jauri, M.H.; Kamisah, Y.; Yunos, N.M.; Zainalabidin, S. Repeated Administration of Low Dose Isoprenaline on the Rat’s Cardiovascular System. Sains Malays. 2022, 51, 2147–2157. [Google Scholar] [CrossRef]
- Li, L.; Fang, H.; Yu, Y.H.; Liu, S.X.; Yang, Z.Q. Liquiritigenin attenuates isoprenaline-induced myocardial fibrosis in mice through the tgf-β1/smad2 and akt/erk signaling pathways. Mol. Med. Rep. 2021, 24, 686. [Google Scholar] [CrossRef]
- Pham, V.A.; Tran, H.T.; Mai, T.P.; Nguyen, L.H.; Nguyen, V.H.; Nguyen, T.H.; Bui, S.S.; Van Vu, A.; Do, H.T.; Trinh, Q.V. Myocardial infarction model induced by isoproterenol in rats and potential cardiovascular protective effect of a nattokinase-containing hard capsule. Phytomed. Plus 2023, 3, 100472. [Google Scholar] [CrossRef]
- Keihanian, F.; Moohebati, M.; Saeidinia, A.; Mohajeri, S.A.; Madaeni, S. Therapeutic effects of medicinal plants on isoproterenol-induced heart failure in rats. Biomed. Pharmacother. 2021, 134, 111101. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Juhl, C.R.; Janus, C.; Lundgren, J.R.; Martinussen, C.; Wiingaard, C.; Knudsen, C.; Frikke-Schmidt, R.; Stallknecht, B.M.; Holst, J.J.; et al. Weight loss maintenance with exercise and liraglutide improves glucose tolerance, glucagon response, and beta cell function. Obesity 2023, 31, 977–989. [Google Scholar] [CrossRef]
- Fiserova, I.; Trinh, M.D.; Elkalaf, M.; Vacek, L.; Heide, M.; Martinkova, S.; Bechynska, K.; Kosek, V.; Hajslova, J.; Fiser, O.; et al. Isoprenaline modified the lipidomic profile and reduced β-oxidation in HL-1 cardiomyocytes: In vitro model of takotsubo syndrome. Front. Cardiovasc. Med. 2022, 9, 917989. [Google Scholar] [CrossRef]
- Chang, G.R.; Chen, W.K.; Hou, P.H.; Mao, F.C. Isoproterenol exacerbates hyperglycemia and modulates chromium distribution in mice fed with a high fat diet. J. Trace Elem. Med. Biol. 2017, 44, 315–321. [Google Scholar] [CrossRef]
- Tawfik, M.K.; Ameen, A.M. Cardioprotective effect of ranolazine in nondiabetic and diabetic male rats subjected to isoprenaline-induced acute myocardial infarction involves modulation of AMPK and inhibition of apoptosis. Can. J. Physiol. Pharmacol. 2019, 97, 661–674. [Google Scholar] [CrossRef]
- Hosseini, A.; Rajabian, A.; Sobhanifar, M.A.; Alavi, M.S.; Taghipour, Z.; Hasanpour, M.; Iranshahi, M.; Boroumand-Noughabi, S.; Banach, M.; Sahebkar, A. Attenuation of isoprenaline-induced myocardial infarction by Rheum turkestanicum. Biomed. Pharmacother. 2022, 148, 112775. [Google Scholar] [CrossRef]
- Marinković, S.T.; Đukanović, Đ.; Duran, M.; Bajic, Z.; Sobot, T.; Uletilović, S.; Mandić-Kovacević, N.; Cvjetković, T.; Maksimović, Ž.M.Ž.M.; Maličević, U.; et al. Pomegranate peel extract attenuates isoprenaline-induced Takotsubo-like myocardial injury in rats. Pharmaceutics 2023, 15, 1697. [Google Scholar] [CrossRef]
- Abdelrahaman, D.; Habotta, O.A.; Taher, E.S.; El-Ashry, E.S.; Ibrahim, I.; Abdeen, A.; Ibrahim, A.M.; Ibrahim, R.M.; Anwer, H.; Mihaela, O.; et al. Suppression of NLRP3 inflammasome orchestrates the protective efficacy of tiron against isoprenaline-induced myocardial injury. Front. Pharmacol. 2024, 15, 1379908. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Ahmad, T.; Ikram, M.; Khan, T.; Shah, A.J.; Mahnashi, M.H.; Alhasaniah, A.H.; Al Awadh, A.A.; Almazni, I.A.; Alshahrani, M.M. Cardioprotective Potential of Aqueous Extract of Fumaria indica on Isoproterenol-Induced Myocardial Infarction in SD Rats. Oxid. Med. Cell. Longev. 2022, 2022, 2112956. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Amjad, W.; Inayat, F.; Nadeem, M.; Weissman, S.; Malik, M.I.; Jajja, A.A.; Khan, A.; Tabibian, J.H. The effects of liraglutide on liver enzymes and metabolic factors in patients with nonalcoholic steatohepatitis: A meta-analysis of randomized controlled trials. Gastroenterol. Rev. 2023, 18, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.; Rajkumar, D. Effect of glutathione in lipid profile in isoprenaline induced myocardial infarction in male albino rats. MedPulse Int. J. Physiol. 2019, 11, 45–48. [Google Scholar] [CrossRef]
- Galal, O.; Mostafa, A.; Mohamed, H.; Ahmed, A.R.H.; Hashim, M.S.; Mohamed, N. Cardioprotective Effects of Nano-Vitamin D on Isoprenaline-Induced Myocardial Infarction Rat Model. SVU-Int. J. Med. Sci. 2022, 5, 136–151. [Google Scholar] [CrossRef]
- Lan, T.; Zeng, Q.; Jiang, W.; Liu, T.; Xu, W.; Yao, P.; Lu, W. Metabolism disorder promotes isoproterenol-induced myocardial injury in mice with high temperature and high humidity and high-fat diet. BMC Cardiovasc. Disord. 2022, 22, 133. [Google Scholar] [CrossRef]
- Ahsan, F.; Mahmood, T.; Wani, T.A.; Zargar, S.; Siddiqui, M.H.; Usmani, S.; Shamim, A.; Wahajuddin, M. Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats. Life 2022, 12, 1063. [Google Scholar] [CrossRef]
- Nowrouzi-Sohrabi, P.; Soroush, N.; Tabrizi, R.; Shabani-Borujeni, M.; Rezaei, S.; Jafari, F.; Hosseini-Bensenjan, M.; Stricker, B.H.; van Hoek, M.; Ahmadizar, F. Effect of Liraglutide on Cardiometabolic Risk Profile in People with Coronary Artery Disease with or without Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 618208. [Google Scholar] [CrossRef]
- Aoki, K.; Kamiyama, H.; Takihata, M.; Taguri, M.; Shibata, E.; Shinoda, K.; Yoshii, T.; Nakajima, S.; Terauchi, Y. Effect of liraglutide on lipids in patients with type 2 diabetes: A pilot study. Endocr. J. 2020, 67, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, M.; Draginic, N.; Jeremic, J.; Samanovic, A.M.; Stojkov, S.; Mitrovic, S.; Jeremic, N.; Radonjic, T.; Srejovic, I.; Bolevich, S.; et al. Protective Role of Vitamin B1 in Doxorubicin-Induced Cardiotoxicity in Rats: Focus on Hemodynamic, Redox, and Apoptotic Markers in Heart. Front. Physiol. 2021, 12, 690619. [Google Scholar] [CrossRef] [PubMed]
- Bajic, Z.; Sobot, T.; Amidzic, L.; Vojinovic, N.; Jovicic, S.; Gajic Bojic, M.; Djuric, D.M.; Stojiljkovic, M.P.; Bolevich, S.; Skrbic, R. Liraglutide Protects Cardiomyocytes against Isoprenaline-Induced Apoptosis in Experimental Takotsubo Syndrome. Biomedicines 2024, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- Fathiazad, F.; Tamarzadeh, N.; Alsos, D.; Garjani, A.; Vaez, H. The effect of astragaloside IV on isoproterenol-induced myocardial infarction in rats. Pharm. Sci. 2019, 25, 100–110. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Zhu, Q.; Li, N.; Zhou, H. Liraglutide, a glucagon-like peptide-1 analog, inhibits high glucose-induced oxidative stress and apoptosis in neonatal rat cardiomyocytes. Exp. Ther. Med. 2019, 17, 3734–3740. [Google Scholar] [CrossRef]
- El-Shafey, M.; El-Agawy, M.S.E.-d.; Eldosoky, M.; Ebrahim, H.A.; Elsherbini, D.M.A.; El-Sherbiny, M.; Asseri, S.M.; Elsherbiny, N.M. Role of Dapagliflozin and Liraglutide on Diabetes-Induced Cardiomyopathy in Rats: Implication of Oxidative Stress, Inflammation, and Apoptosis. Front. Endocrinol. 2022, 13, 862394. [Google Scholar] [CrossRef]
- Konopelski, P.; Ufnal, M. Electrocardiography in rats: A comparison to human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef]
- Forte, E.; Panahi, M.; Baxan, N.; Ng, F.S.; Boyle, J.J.; Branca, J.; Bedard, O.; Hasham, M.G.; Benson, L.; Harding, S.E.; et al. Type 2 MI induced by a single high dose of isoproterenol in C57BL/6J mice triggers a persistent adaptive immune response against the heart. J. Cell. Mol. Med. 2021, 25, 229–243. [Google Scholar] [CrossRef]




| Groups (Mean ± SE) | ||||
|---|---|---|---|---|
| Parameters | Con | Iso | Lir | Iso + Lir |
| BW (g) | 261.33 ± 10.78 | 228.33 ± 6.31 | 216.33 ± 8.16 * | 199.56 ± 8.86 ** † |
| HW (g) | 0.80 ± 0.02 | 0.82 ± 0.02 | 0.71 ± 0.03 † | 0.68 ± 0.03 * † |
| H/BW ratio | 3.05 ± 0.07 | 3.62 ± 0.09 * | 3.22 ± 0.06 † | 3.40 ± 0.09 * |
| Groups (Mean ± SE) | ||||
|---|---|---|---|---|
| Parameters | Con | Iso | Lir | Iso + Lir |
| AST (U/L) | 250.58 ± 39.95 | 363.78 ± 41.80 | 284.50 ± 36.84 | 350.67 ± 49.68 |
| ALT (U/L) | 108.67 ± 32.53 | 130.11 ± 7.94 * | 80.17 ± 12.46 † | 123.83 ± 18.64 |
| LDH (U/L) | 1202.67 ± 355.89 | 1968.67 ± 299.43 | 1491.00 ± 264.07 | 1916.67 ± 399.24 |
| Glucose (mmol/L) | 24.58 ± 3.02 | 17.07 ± 2.47 * | 22.38 ± 1.62 | 21.30 ± 1.01 |
| Groups (Mean ± SE) | ||||
|---|---|---|---|---|
| Parameters | Con | Iso | Lir | Iso + Lir |
| TC (mmol/L) | 1.00 ± 0.08 | 1.80 ± 0.23 * | 0.75 ± 0.02 * †† | 1.13 ± 0.17 † ‡ |
| HDL (mmol/L) | 0.37 ± 0.03 | 0.69 ± 0.07 * | 0.28 ± 0.02 †† | 0.46 ± 0.07 † |
| LDL (mmol/L) | 0.16 ± 0.02 | 0.19 ± 0.03 | 0.10 ± 0.00 † | 0.14 ± 0.02 |
| TG (mmol/L) | 0.75 ± 0.15 | 1.03 ± 0.45 | 0.66 ± 0.41 | 0.88 ± 0.16 |
| Groups (Mean ± SE) | ||||
|---|---|---|---|---|
| Parameters | Con | Iso | Lir | Iso + Lir |
| HR 1 (bpm) | 243.22 ± 13.09 | 436.51 ± 7.94 ** | 245.67 ± 10.75 †† | 439.28 ± 14.87 ** ‡‡ |
| HR 2 (bpm) | 240.38 ± 4.30 | 220.88 ± 6.28 | 237.64 ± 6.05 | 226.13 ± 11.90 |
| QT 1 (s) | 0.173 ± 0.007 | 0.089 ± 0.005 ** | 0.167 ± 0.004 †† | 0.094 ± 0.004 ** ‡‡ |
| QT 2 (s) | 0.170 ± 0.010 | 0.200 ± 0.017 | 0.173 ± 0.008 | 0.188 ± 0.010 |
| QRS 1 (mV) | 0.280 ± 0.018 | 0.405 ± 0.045 * | 0.268 ± 0.009 † | 0.411 ± 0.042 * ‡ |
| QRS 2 (mV) | 0.302 ± 0.033 | 0.204 ± 0.024 * | 0.290 ± 0.019 † | 0.210 ± 0.027 * ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajic, Z.; Sobot, T.; Smitran, A.; Uletilovic, S.; Mandić-Kovačević, N.; Cvjetkovic, T.; Malicevic, U.; Stanetic, B.; Đukanović, Đ.; Maticic, M.; et al. Liraglutide Treatment Restores Cardiac Function After Isoprenaline-Induced Myocardial Injury and Prevents Heart Failure in Rats. Life 2025, 15, 443. https://doi.org/10.3390/life15030443
Bajic Z, Sobot T, Smitran A, Uletilovic S, Mandić-Kovačević N, Cvjetkovic T, Malicevic U, Stanetic B, Đukanović Đ, Maticic M, et al. Liraglutide Treatment Restores Cardiac Function After Isoprenaline-Induced Myocardial Injury and Prevents Heart Failure in Rats. Life. 2025; 15(3):443. https://doi.org/10.3390/life15030443
Chicago/Turabian StyleBajic, Zorislava, Tanja Sobot, Aleksandra Smitran, Snezana Uletilovic, Nebojša Mandić-Kovačević, Tanja Cvjetkovic, Ugljesa Malicevic, Bojan Stanetic, Đorđe Đukanović, Milka Maticic, and et al. 2025. "Liraglutide Treatment Restores Cardiac Function After Isoprenaline-Induced Myocardial Injury and Prevents Heart Failure in Rats" Life 15, no. 3: 443. https://doi.org/10.3390/life15030443
APA StyleBajic, Z., Sobot, T., Smitran, A., Uletilovic, S., Mandić-Kovačević, N., Cvjetkovic, T., Malicevic, U., Stanetic, B., Đukanović, Đ., Maticic, M., Jovicic, S., Djuric, D. M., Stojiljkovic, M. P., & Skrbic, R. (2025). Liraglutide Treatment Restores Cardiac Function After Isoprenaline-Induced Myocardial Injury and Prevents Heart Failure in Rats. Life, 15(3), 443. https://doi.org/10.3390/life15030443









