The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome
Abstract
:1. Introduction
2. Methods
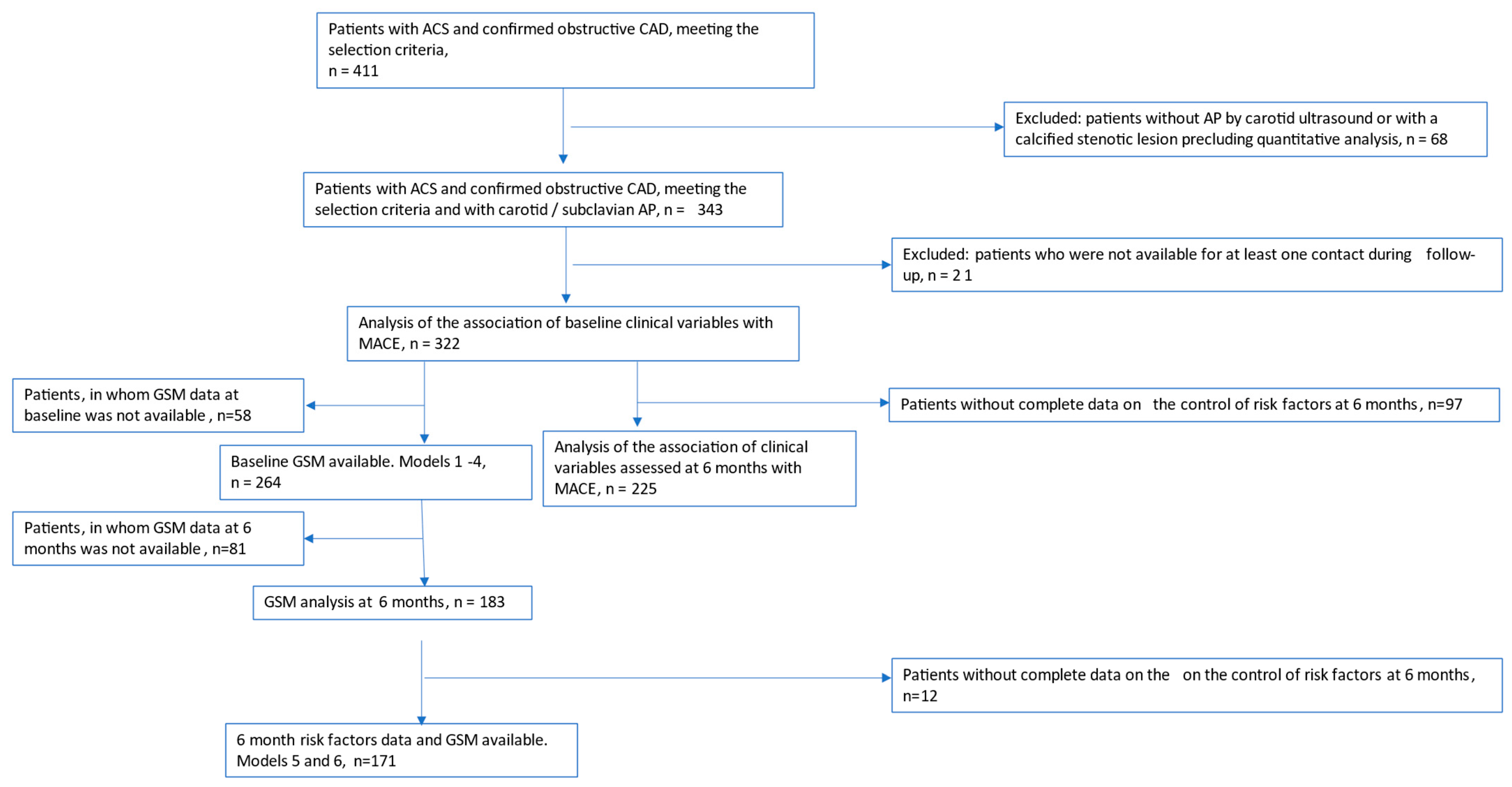
2.1. Selection Criteria
- ACS of any type; obstructive CAD confirmed by coronary angiography; primary invasive strategy (PCI).
- Evidence of atherosclerotic plaque at carotid/subclavian ultrasound study.
- Death or clinically significant bleeding at index hospitalization.
- Left ventricular ejection fraction (EF) <30% and/or heart failure NYHA class IV at hospital discharge.
- Planned coronary artery bypass surgery (CABG).
- Statin intolerance.
- Severe comorbidity with a life expectancy of less than 1 year.
- Stenotic calcific carotid lesion precluding quantitative atherosclerotic plaque analysis.
2.2. Goals of Revascularization and Medical Therapy
2.3. Ultrasound Study Protocol
2.4. Follow-Up and Registration of MACE
2.5. Statistical Analysis
3. Results
3.1. Variables Assessed at Baseline and 6 Months After Index ACS and the Risk of MACEs
3.2. Calculators Based on the Identified Predictors
3.3. Equations for Calculating Individual Risk
3.3.1. Calculator 1
3.3.2. Calculator 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Health Statistics. Deaths and Mortality. Available online: https://www.cdc.gov/nchs/fastats/deaths.htm (accessed on 18 January 2025).
- Mensah, G.; Fuster, V.; Murray, C.; Roth, G.; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Itzahki Ben Zadok, O.; Ben-Gal, T.; Abelow, A.; Shechter, A.; Zusman, O.; Iakobishvili, Z.; Cohen, T.; Shlomo, N.; Kornowski, R.; Eisen, A. Temporal Trends in the Characteristics, Management and Outcomes of Patients with Acute Coronary Syndrome According to Their Killip Class. Am. J. Cardiol. 2019, 124, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Baldetti, L.; Pagnesi, M.; Latib, A.; Colombo, A.; Libby, P.; Giannini, F. Medical Therapy for Long-Term Prevention of Atherothrombosis Following an Acute Coronary Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72 Pt. A, 2886–2903. [Google Scholar] [CrossRef] [PubMed]
- Evbayekha, E.O.; Okobi, O.E.; Okobi, T.; Ibeson, E.C.; Nwafor, J.N.; Ozobokeme, O.E.; Olawoye, A.; Ngoladi, I.A.; Boms, M.G.; Habib, F.A.; et al. The Evolution of Hypertension Guidelines Over the Last 20+ Years: A Comprehensive Review. Cureus 2022, 14, e31437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schüpke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 16, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Boden, W.E. Why optimal medical therapy should be a universal standard of care. J. Am. Coll. Cardiol. 2015, 66, 774–776. [Google Scholar] [CrossRef]
- Kuo, F.; Gardener, H.; Dong, C.; Cabral, D.; Della-Morte, D.; Blanton, S.H.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke 2012, 43, 1755–1760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spence, J.D. Measurement of carotid plaque burden. Curr. Opin. Lipidol. 2020, 31, 291–298. [Google Scholar] [CrossRef]
- Björnsdóttir, G. Longitudinal Changes in Size and Composition of Carotid Artery Plaques Using Ultrasound: Adaptation and Validation of Methods (Inter- and Intraobserver Variability). J. Vasc. Ultrasound 2018, 38, 198–208. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Page, C.B.; Berman, D.S.; Chaitman, B.R.; Picard, M.H.; Kwong, R.Y.; O’Brien, S.M.; Huang, Z.; et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144, 1024–1038. [Google Scholar] [CrossRef]
- Bytyçi, I.; Shenouda, R.; Wester, P.; Henein, M.Y. Carotid Atherosclerosis in Predicting Coronary Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e224–e237. [Google Scholar] [CrossRef] [PubMed]
- Cismaru, G.; Serban, T.; Tirpe, A. Ultrasound Methods in the Evaluation of Atherosclerosis: From Pathophysiology to Clinic. Biomedicines 2021, 9, 418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguirre, A.D.; Arbab-Zadeh, A.; Soeda, T.; Fuster, V.; Jang, I.K. Optical Coherence Tomography of Plaque Vulnerability and Rupture: JACC Focus Seminar Part 1/3. J. Am. Coll. Cardiol. 2021, 78, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunner, G.; Virani, S.S.; Sun, W.; Liu, L.; Dodge, R.C.; Nambi, V.; Coresh, J.; Mosley, T.H.; Sharrett, A.R.; Boerwinkle, E.; et al. Associations Between Carotid Artery Plaque Burden, Plaque Characteristics, and Cardiovascular Events: The ARIC Carotid Magnetic Resonance Imaging Study. JAMA Cardiol. 2021, 6, 79–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuster, V.; García-Álvarez, A.; Devesa, A.; Mass, V.; Owen, R.; Quesada, A.; Fuster, J.J.; García-Lunar, I.; Pocock, S.; Sánchez-González, J.; et al. Influence of Subclinical Atherosclerosis Burden and Progression on Mortality. J. Am. Coll. Cardiol. 2024, 84, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367, Erratum in Eur. Heart J. 2021, 42, 1908; Erratum in Eur. Heart J. 2021, 42, 1925; Erratum in Eur. Heart J. 2021, 42, 2298. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, T.S.; von Lueder, T.G.; Zannad, F.; Rossignol, P.; Duarte, K.; Chouihed, T.; Dickstein, K.; Atar, D.; Agewall, S.; Girerd, N.; et al. Relationship between left ventricular ejection fraction and mortality after myocardial infarction complicated by heart failure or left ventricular dysfunction. Int. J. Cardiol. 2018, 272, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.; Jimenez, E. Re-evaluating the Role of CABG in Acute Coronary Syndromes. Curr. Cardiol. Rep. 2020, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Bershtein, L.L.; Zbyshevskaya, E.V.; Katamadze, N.O.; Kuzmina-Krutetskaya, A.M.; Volkov, A.V.; Andreeva, A.E.; Gumerova, V.E.; Bitakova, F.I.; Sayganov, S.A. ISCHEMIA—The Largest Ever Randomized Study in Stable Coronary Artery Disease. Baseline Characteristics of Enrolled Patients in One Russian Site. Kardiologiia 2017, 57, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1046–e1081, Erratum in Circulation 2019, 139, e1178–e1181. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020, 41, 111–188, Erratum in Eur Heart J. 2020, 41, 4255. PMID: 31504418. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.V.; Sergienko, I.V.; Aronov, D.M.; Arabidze, G.G.; Akhmedzhanov, N.M.; Bazhan, S.S.; Balakhonova, T.V.; Barbarash, O.L.; Boytsov, S.A.; Bubnova, M.G.; et al. Diagnostics and correction of lipid metabolism disorders in order to prevent and treat atherosclerosis. Russian recommendations VI revision. J. Atheroscler. Dyslipidemias 2017, 3, 5–22. [Google Scholar]
- von Reutern, G.M.; Goertler, M.W.; Bornstein, N.M.; Del Sette, M.; Evans, D.H.; Hetzel, A.; Kaps, M.; Perren, F.; Razumovky, A.; von Reutern, M.; et al. Grading carotid stenosis using ultrasonic methods. Stroke 2012, 43, 916–921, Erratum in Stroke 2012, 43, e54. [Google Scholar] [CrossRef] [PubMed]
- Bershtein, L.L.; Boldueva, S.A.; Kochanov, I.N.; Lunina, M.D.; Naiden, T.V.; Evdokimov, D.S.; Tandelov, B.M.; Podmetin, P.S.; Zbyshevskaya, E.V.; Gumerova, V.E.; et al. Variability of Measurement and Feasibility of Assessing Changes in Brachiocepahlic Atherosclerotic Plaque After Acute Coronary Syndrome. Kardiologiia 2023, 63, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Okkonen, M.; Havulinna, A.S.; Ukkola, O.; Huikuri, H.; Pietila, A.; Koukkunen, H.; Lehto, S.; Mustonen, J.; Ketonen, M.; Airaksinen, J.; et al. Risk factors for major adverse cardiovascular events after the first acute coronary syndrome. Ann. Med. 2021, 53, 817–823. [Google Scholar] [CrossRef]
- Bilgin, M.; Akkaya, E.; Dokuyucu, R. The Role of Triglyceride/HDL Ratio, Triglyceride-Glucose Index, and Pan-Immune-Inflammation Value in the Differential Diagnosis of Acute Coronary Syndrome and Predicting Mortality. J. Clin. Med. 2024, 13, 4832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fox, K.A.; Fitzgerald, G.; Puymirat, E.; Huang, W.; Carruthers, K.; Simon, T.; Coste, P.; Monsegu, J.; Gabriel Steg, P.; Danchin, N.; et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014, 4, e004425. [Google Scholar] [CrossRef]
- Spadafora, L.; Betti, M.; D’Ascenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Post-Discharge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Quadri, G.; et al. Machine learning- based prediction of adverse events following an acute coronary syndrome (PRAISE): A modelling study of pooled datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef]
- Bauer, D.; Toušek, P. Risk Stratification of Patients with Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 4574. [Google Scholar] [CrossRef] [PubMed]
- Yahud, E.; Tzuman, O.; Fink, N.; Goldenberg, I.; Goldkorn, R.; Peled, Y.; Lev, E.; Asher, E. Trends in long-term prognosis according to left ventricular ejection fraction after acute coronary syndrome. J. Cardiol. 2020, 76, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Stampouloglou, P.K.; Anastasiou, A.; Bletsa, E.; Lygkoni, S.; Chouzouri, F.; Xenou, M.; Katsarou, O.; Theofilis, P.; Zisimos, K.; Tousoulis, D.; et al. Diabetes Mellitus in Acute Coronary Syndrome. Life 2023, 13, 2226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, K.; Niida, T.; Yuki, H.; Kinoshita, D.; Fujimoto, D.; Lee, H.; McNulty, I.; Takano, M.; Nakamura, S.; Kakuta, T.; et al. Coronary Plaque Characteristics and Underlying Mechanism of Acute Coronary Syndromes in Different Age Groups of Patients with Diabetes. J. Am. Heart Assoc. 2023, 12, e031474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johner, N.; Gencer, B.; Roffi, M. Routine beta-blocker therapy after acute coronary syndromes: The end of an era? Eur. J. Clin. Investig. 2024, 54, e14309. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bliden, K.; Tantry, U.S.; Gurbel, P.A.; Kanjwal, M.Y.; Lundgren, S.W. Role of Beta Blockers After Acute Coronary Syndrome With Preserved Ejection Fraction. Am. J. Cardiol. 2025, 235, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Barbarash, O.L.; Kashtalap, V.V. Diagnosis of latent forms of non-coronary atherosclerosis in acute coronary syndrome patients. Is there any practical sense? Complex Issues Cardiovasc. Dis. 2012, 1, 12–16. [Google Scholar] [CrossRef]
- Bershtein, L.L.; Lunina, M.D.; Evdokimov, D.S.; Nayden, T.V.; Gumerova, V.E.; Kochanov, I.N.; Ivanov, A.A.; Boldueva, S.A.; Resnyanskaya, E.D.; Zbyshevskaya, E.V.; et al. Associations of the severity of carotid and subclavian atherosclerosis with the major risk factors, clinical and angiographic variables in patients with acute coronary syndrome. Cardiovasc. Ther. Prev. 2024, 23, 51–62. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T.; Luo, Y.; Jiao, L. Identification Markers of Carotid Vulnerable Plaques: An Update. Biomolecules 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Futamata, M.; Matsuoka, S.; Shimizu, T.; Yoshizaki, T.; Obata, J.E.; Nakamura, T.; Fujioka, D.; Watanabe, Y.; Nakamura, K.; Watanabe, K.; et al. Echolucency of the carotid artery is associated with short-term plaque progression and positive remodeling in the culprit coronary artery in AMI survivors. J. Cardiol. 2017, 70, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, B.; Coggi, D.; Bonomi, A.; Amato, M.; Capra, N.; Colombo, G.I.; Sansaro, D.; Ravani, A.; Savonen, K.; Giral, P.; et al. Determinants of Carotid Wall Echolucency in a Cohort of European High Cardiovascular Risk Subjects: A Cross-Sectional Analysis of IMPROVE Baseline Data. Biomedicines 2024, 12, 737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bos, D.; Arshi, B.; van den Bouwhuijsen, Q.J.A.; Ikram, M.K.; Selwaness, M.; Vernooij, M.W.; Kavousi, M.; van der Lugt, A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J. Am. Coll. Cardiol. 2021, 77, 1426–1435. [Google Scholar] [CrossRef] [PubMed]

| Total | Without MACE | With MACE | |
|---|---|---|---|
| History and clinical characteristics | |||
| Male | |||
| No | 98 (30.4%) | 86 (30.3%) | 12 (31.6%) |
| Yes | 224 (69.6%) | 198 (69.7%) | 26 (68.4%) |
| Age (years) | 65 [59; 73] | 66 [59; 73] | 62 [59; 72] |
| Body mass index | 28 [26; 31] | 28 [26; 31] | 29 [26; 31] |
| Smoking | |||
| Never | 171 (53.1%) | 155 (54.6%) | 16 (42.1%) |
| Smokes now/previously | 151 (46.9%) | 129 (45.4%) | 22 (57.9%) |
| Arterial hypertension | |||
| No | 129 (40.2%) | 114 (40.3%) | 15 (39.5%) |
| Yes | 192 (59.8%) | 169 (59.7%) | 23 (60.5%) |
| Diabetes | |||
| No/on diet | 255 (79.2%) | 234 (82.4%) | 21 (55.3%) |
| Oral medication/insulin | 67 (20.8%) | 50 (17.6%) | 17 (44.7%) |
| Peripheral arterial disease | |||
| No | 296 (91.9%) | 261 (91.9%) | 35 (92.1%) |
| Yes | 26 (8.1%) | 23 (8.1%) | 3 (7.9%) |
| History of myocardial infarction | |||
| No | 232 (72.0%) | 210 (73.9%) | 22 (57.9%) |
| Yes | 90 (28.0%) | 74 (26.1%) | 16 (42.1%) |
| History of stroke | |||
| No | 297 (92.2%) | 265 (93.3%) | 32 (84.2%) |
| Yes | 25 (7.8%) | 19 (6.7%) | 6 (15.8%) |
| Moderate-intensity physical activity (min/week) | 200 [40; 420] | 205 [50; 415] | 165 [30; 420] |
| Vigorous physical activity (min/week) | 20 [0; 60] | 20 [5; 60] | 20 [0; 50] |
| Physical activity as per Guidelines | |||
| No | 121 (37.6%) | 105 (37.0%) | 16 (42.1%) |
| Yes | 201 (62.4%) | 179 (63.0%) | 22 (57.9%) |
| Clinical variables related to ACS | |||
| Time from onset of pain to PCI (min) | 340 [180; 630] | 300 [180; 612] | 430 [200; 720] |
| Heart rate on admission (in min) | 72 [65; 80] | 72 [65; 80] | 72 [67; 80] |
| Heart rate at discharge (in min) | 66 [61; 71] | 65 [61; 71] | 66 [62; 73] |
| SBP (mmHg) | 140 [130; 150] | 140 [130; 150] | 140 [125; 155] |
| Hemoglobin (g/L) | 141 [130; 151] | 142 [130; 151] | 136 [129; 146] |
| Creatinine (µmol/L) | 92 [80; 108] | 94 [81; 108] | 84 [73; 102] |
| eGFR * (mL/min/1.73 m2) | 69 [56; 82] | 69 [55; 82] | 74 [58; 89] |
| LDL-C (mmol/L) | 3 [2; 4] | 3 [2; 4] | 3 [2; 4] |
| LDL-C/HDL-C | 3 [2; 4] | 3 [2; 4] | 3 [2; 4] |
| ACS type | |||
| STEMI | 137 (42.7%) | 124 (43.8%) | 13 (34.2%) |
| NSTEMI | 94 (29.3%) | 78 (27.6%) | 16 (42.1%) |
| UA | 90 (28.0%) | 81 (28.6%) | 9 (23.7%) |
| Killip class | |||
| 1 | 254 (89.4%) | 34 (89.5%) | 288 (89.4%) |
| ≥2 | 30 (10.6%) | 4 (10.5%) | 34 (10.6%) |
| Number of ECG leads with ST elevation/depression | 3 [0; 5] | 3 [0; 5] | 3 [0; 4] |
| Area of LV dyssynergy by echocardiography (%) | 12 [0; 25] | 12 [0; 25] | 18 [6; 25] |
| Number of segments with WMA | 2 [0; 4] | 2 [0; 4] | 3 [1; 4] |
| WMSI | 1 [0; 2] | 1 [0; 2] | 1 [1; 2] |
| LV ejection fraction (%) | 61 [54; 66] | 62 [55; 66] | 59 [52; 64] |
| Presence of LM stenosis | |||
| No | 285 (88.5%) | 252 (88.7%) | 33 (86.8%) |
| Yes | 37 (11.5%) | 32 (11.3%) | 5 (13.2%) |
| Three-vessel coronary artery disease | |||
| No | 229 (71.3%) | 207 (73.1%) | 22 (57.9%) |
| Yes | 92 (28.7%) | 76 (26.9%) | 16 (42.1%) |
| SYNTAX score | 14 (8–22) | 14 (8–21) | 14 (10–26) |
| Troponin (max, pg/mL) | 647 [104; 3700] | 647 [103; 3947] | 604 [109; 2619] |
| Charlson comorbidity index | 4 [2; 5] | 4 [2; 5] | 4 [3; 6] |
| PRECISE-DAPT score | 18 [12; 27] | 18 [12; 27] | 14 [10; 21] |
| Characteristics of the carotid/subclavian AP | |||
| Hsum (mm) | 7 [4; 11] | 8 [4; 12] | 7 [4; 10] |
| TPA (mm2) | 50 [27; 89] | 51 [28; 90] | 44 [22; 73] |
| Standardized GSM | 94 [72; 122] | 95 [73; 124] | 83 [58; 108] |
| Standardized GSM < 81 | |||
| No | 178 (66.9%) | 162 (69.2%) | 16 (50.0%) |
| Yes | 88 (33.1%) | 72 (30.8%) | 16 (50.0%) |
| Treatment at hospital discharge | |||
| Complete revascularization | |||
| No | 186 (57.8%) | 159 (56.0%) | 27 (71.1%) |
| Yes | 136 (42.2%) | 125 (44.0%) | 11 (28.9%) |
| High-intensity statins | |||
| No | 15 (4.7%) | 14 (4.9%) | 1 (2.6%) |
| Yes | 307 (95.3%) | 270 (95.1%) | 37 (97.4%) |
| Ezetimibe | |||
| No | 299 (92.9%) | 262 (92.3%) | 37 (97.4%) |
| Yes | 23 (7.1%) | 22 (7.7%) | 1 (2.6%) |
| DATT | |||
| No | 8 (2.5%) | 6 (2.1%) | 2 (5.3%) |
| Yes | 314 (97.5%) | 278 (97.9%) | 36 (94.7%) |
| ACE inhibitors/ARBs | |||
| No | 10 (3.1%) | 10 (3.5%) | 0 (0.0%) |
| Yes | 312 (96.9%) | 274 (96.5%) | 38 (100.0%) |
| Beta-blockers | |||
| No | 25 (7.8%) | 19 (6.7%) | 6 (15.8%) |
| Yes | 297 (2.2%) | 265 (3.3%) | 32 (84.2%) |
| Total | Without MACE | With MACE | |
|---|---|---|---|
| Risk factor control | |||
| SBP (mm Hg) | 125 [120; 130] | 125 [120; 130] | 130 [120; 135] |
| DBP (mm Hg) | 75 [70; 80] | 75 [70; 80] | 80 [70; 80] |
| Target blood pressure | |||
| No | 56 (18.6%) | 48 (18.0%) | 8 (22.9%) |
| Yes | 245 (81.4%) | 218 (82.0%) | 27 (77.1%) |
| LDL-C (mmol/L) | 2 [2; 2] | 2 [2; 2] | 2 [2; 2] |
| LDL-C/HDL-C | 2 [1; 2] | 2 [1; 2] | 2 [1; 2] |
| Target LDL-C * | |||
| No | 198 (87.6%) | 175 (86.6%) | 23 (95.8%) |
| Yes | 28 (12.4%) | 27 (13.4%) | 1 (4.2%) |
| Current smoker | |||
| No | 213 (73.2%) | 188 (72.9%) | 25 (75.8%) |
| Yes | 78 (26.8%) | 70 (27.1%) | 8 (24.2%) |
| Physical activity as per Guidelines | |||
| No | 109 (37.2%) | 94 (36.4%) | 15 (42.9%) |
| Yes | 184 (62.8%) | 164 (63.6%) | 20 (57.1%) |
| Number of uncontrolled risk factors | |||
| 0–2 | 181 (80.8%) | 167 (83.5%) | 14 (58.3%) |
| 3–4 | 43 (19.2%) | 33 (16.5%) | 10 (41.7%) |
| Characteristics of the carotid/subclavian AP | |||
| Hsum (mm) | 7 [4; 11] | 8 [4; 11] | 6 [4; 9] |
| TPA (mm2) | 51 [27; 88] | 52 [28; 91] | 43 [27; 64] |
| Standardized GSM | 94 [73; 119] | 96 [76; 119] | 80 [58; 109] |
| Standardized GSM < 81 | |||
| No | |||
| Yes | |||
| Hsum compared to baseline | |||
| No change | 166 (75.1%) | 147 (75.0%) | 19 (76.0%) |
| Decreased | 18 (8.1%) | 15 (7.7%) | 3 (12.0%) |
| Increased | 37 (16.7%) | 34 (17.3%) | 3 (12.0%) |
| TPA compared to baseline | |||
| No change | 133 (60.2%) | 118 (60.2%) | 15 (60.0%) |
| Decreased | 23 (10.4%) | 21 (10.7%) | 2 (8.0%) |
| Increased | 65 (29.4%) | 57 (29.1%) | 8 (32.0%) |
| Standardized GSM compared to baseline | |||
| No change | 115 (62.8%) | 102 (63.7%) | 13 (56.5%) |
| Decreased | 9 (4.9%) | 8 (5.0%) | 1 (4.3%) |
| Increased | 59 (32.2%) | 50 (31.2%) | 9 (39.1%) |
| Treatment | |||
| High-intensity statins | |||
| No | 29 (9.8%) | 26 (10.0%) | 3 (8.6%) |
| Yes | 266 (90.2%) | 234 (90.0%) | 32 (91.4%) |
| Ezetimibe | |||
| No | 260 (88.1%) | 228 (87.7%) | 32 (91.4%) |
| Yes | 35 (11.9%) | 32 (12.3%) | 3 (8.6%) |
| DATT | |||
| No | 17 (5.8%) | 15 (5.8%) | 2 (5.7%) |
| Yes | 278 (94.2%) | 245 (94.2%) | 33 (94.3%) |
| ACE inhibitors/ARBs | |||
| No | 15 (5.1%) | 14 (5.4%) | 1 (2.9%) |
| Yes | 280 (94.9%) | 246 (94.6%) | 34 (97.1%) |
| Beta-blockers | |||
| No | 27 (9.2%) | 22 (8.5%) | 5 (14.3%) |
| Yes | 268 (90.8%) | 238 (91.5%) | 30 (85.7%) |
| Baseline Variables | HR | 95% CI | p |
|---|---|---|---|
| Diabetes | |||
| No/diet | |||
| Oral medication/insulin | 3.09 | 1.63–5.87 | 0.001 |
| History of myocardial infarction | |||
| No | |||
| Yes | 2.11 | 1.11–4.03 | 0.023 |
| Charlson comorbidity index | 1.27 | 1.08–1.49 | 0.004 |
| LV ejection fraction (%) | 0.86 | 0.75–0.98 | 0.026 |
| Three-vessel coronary artery disease | |||
| No | |||
| Yes | 2.11 | 1.10–4.02 | 0.024 |
| Standardized GSM < 81 | |||
| No | |||
| Yes | 1.91 | 0.95–3.87 | 0.071 |
| Heart rate at discharge (bpm) | 1.05 | 1.01–1.08 | 0.007 |
| Beta-blockers at discharge | |||
| No | |||
| Yes | 0.41 | 0.17–0.98 | 0.045 |
| Variables assessed after 6 months | |||
| Uncontrolled risk factors | |||
| 0–2 | |||
| 3–4 | 2.91 | 1.28–6.58 | 0.010 |
| Standardized GSM < 81 | |||
| No | |||
| Yes | 2.39 | 1.04–5.48 | 0.040 |
| Predictors | HR | Original Model | After Internal Validation | ||
|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | ||
| Model 1 | |||||
| Diabetes | 2.22 | 1.08–4.57 | 0.030 | 1.04–4.74 | 0.039 |
| Standardized GSM < 81 | 2.06 | 1.02–4.19 | 0.045 | 1.03–4.15 | 0.042 |
| Decrease in LV ejection fraction (by every 5% from 60%) | 1.22 | 1.03–1.46 | 0.023 | 1.02–1.47 | 0.030 |
| Model 2 | |||||
| Diabetes | 2.28 | 1.11–4.68 | 0.025 | 1.04–4.99 | 0.039 |
| Standardized GSM < 81 | 1.98 | 0.98–4.03 | 0.058 | 1.00–3.94 | 0.038 |
| Decrease in LV ejection fraction (by every 5% from 60%) | 1.24 | 1.04–1.48 | 0.015 | 1.05–1.46 | 0.011 |
| No beta-blockers at discharge | 3.24 | 1.32–7.97 | 0.011 | 1.07–9.82 | 0.038 |
| Model 3 | |||||
| Charlson comorbidity index | 1.24 | 1.05–1.48 | 0.014 | 1.03–1.50 | 0.021 |
| Standardized GSM < 81 | 2.01 | 0.99–4.08 | 0.052 | 1.05–3.85 | 0.034 |
| Decrease in LV ejection fraction by every 5% (from 60%) | 1.22 | 1.03–1.44 | 0.022 | 1.00–1.49 | 0.050 |
| Model 4 | |||||
| Charlson comorbidity index | 1.27 | 1.06–1.52 | 0.008 | 1.05–1.54 | 0.013 |
| Standardized GSM < 81 | 1.97 | 0.97–3.99 | 0.061 | 0.90–4.31 | 0.091 |
| Decrease in LV ejection fraction (by every 5% from 60%) | 1.23 | 1.04–1.46 | 0.015 | 1.03–1.48 | 0.025 |
| No beta-blockers at discharge | 3.46 | 1.40–8.55 | 0.007 | 1.27–9.40 | 0.015 |
| Predictors | HR | Original Model | After Internal Validation | ||
|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | ||
| Model 5 | |||||
| Diabetes | 3.80 | 1.27–11.35 | 0.017 | 1.33–10.81 | 0.012 |
| Standardized GSM < 81 at 6 months | 3.77 | 1.43–9.92 | 0.007 | 1.16–12.20 | 0.027 |
| ≥3 uncorrected risk factors at 6 months | 3.11 | 1.17–8.25 | 0.023 | 1.15–8.42 | 0.026 |
| Model 6 | |||||
| Charlson comorbidity index | 1.31 | 1.03–1.68 | 0.030 | 1.01–1.70 | 0.038 |
| Standardized GSM < 81 at 6 months | 4.03 | 1.52–10.65 | 0.005 | 1.29–12.54 | 0.016 |
| ≥3 uncorrected risk factors at 6 months | 2.27 | 0.87–5.89 | 0.092 | 0.79–6.49 | 0.126 |
| Calculator | Baseline Predictor Values | 180 Days | 360 Days | 720 Days |
|---|---|---|---|---|
|
| 2.05% | 3.35% | 11.19% |
|
| 0.93% | 1.52% | 5.29% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bershtein, L.L.; Sumin, A.N.; Kutina, A.V.; Lunina, M.D.; Evdokimov, D.S.; Nayden, T.V.; Gumerova, V.E.; Kochanov, I.N.; Ivanov, A.A.; Boldueva, S.A.; et al. The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome. Life 2025, 15, 431. https://doi.org/10.3390/life15030431
Bershtein LL, Sumin AN, Kutina AV, Lunina MD, Evdokimov DS, Nayden TV, Gumerova VE, Kochanov IN, Ivanov AA, Boldueva SA, et al. The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome. Life. 2025; 15(3):431. https://doi.org/10.3390/life15030431
Chicago/Turabian StyleBershtein, Leonid L., Alexey N. Sumin, Anna V. Kutina, Marina D. Lunina, Dmitrii S. Evdokimov, Tatyana V. Nayden, Viktoriya E. Gumerova, Igor N. Kochanov, Arkadii A. Ivanov, Svetlana A. Boldueva, and et al. 2025. "The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome" Life 15, no. 3: 431. https://doi.org/10.3390/life15030431
APA StyleBershtein, L. L., Sumin, A. N., Kutina, A. V., Lunina, M. D., Evdokimov, D. S., Nayden, T. V., Gumerova, V. E., Kochanov, I. N., Ivanov, A. A., Boldueva, S. A., Evdokimova, E. D., Zbyshevskaya, E. V., Evtushenko, A. E., Piltakyan, V. K., & Sayganov, S. A. (2025). The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome. Life, 15(3), 431. https://doi.org/10.3390/life15030431





