Metabolic and Microcirculatory Changes in Severe Renal Ischemia–Reperfusion and Ischemic Preconditioning in the Rat: Are They Detectable in the First Hour of Reperfusion?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Operative Protocol
2.2. Laboratory Techniques
2.3. Blood Flow and Microcirculatory Investigations
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Blood Gases, pH, Electrolytes, and Metabolites
3.2. Hematological Parameters
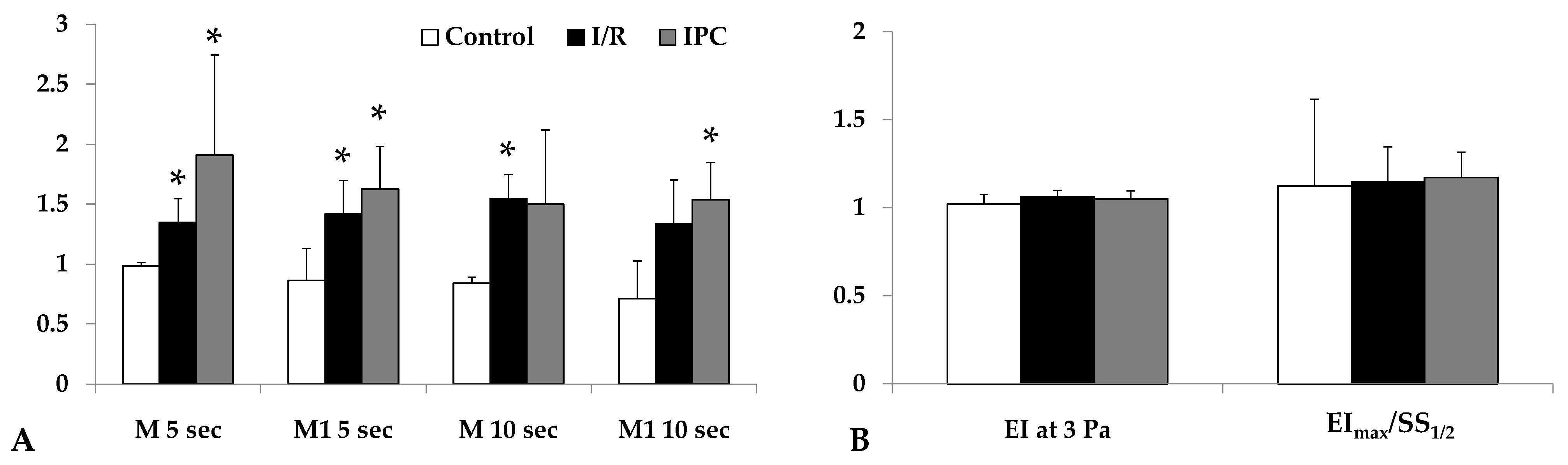
3.3. Micro-Rheological Parameters
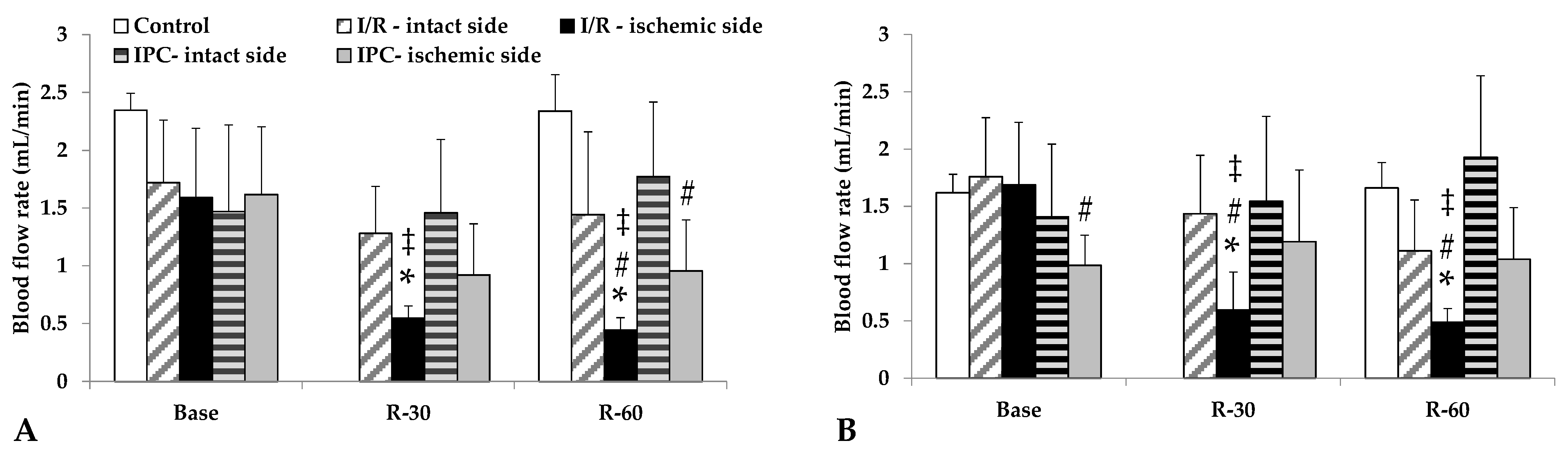
3.4. Blood Flow Rates
3.5. Microcirculatory Parameters
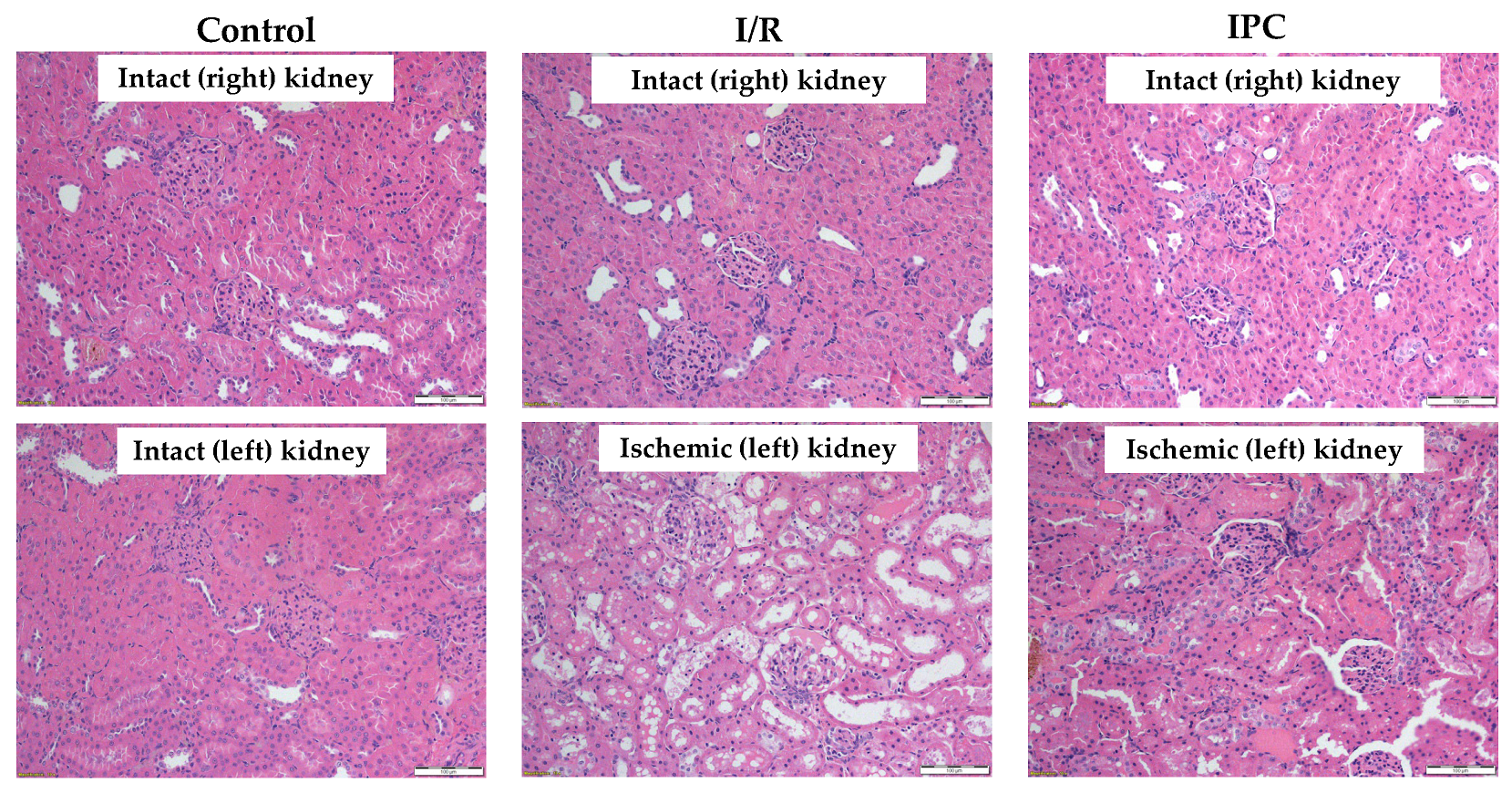
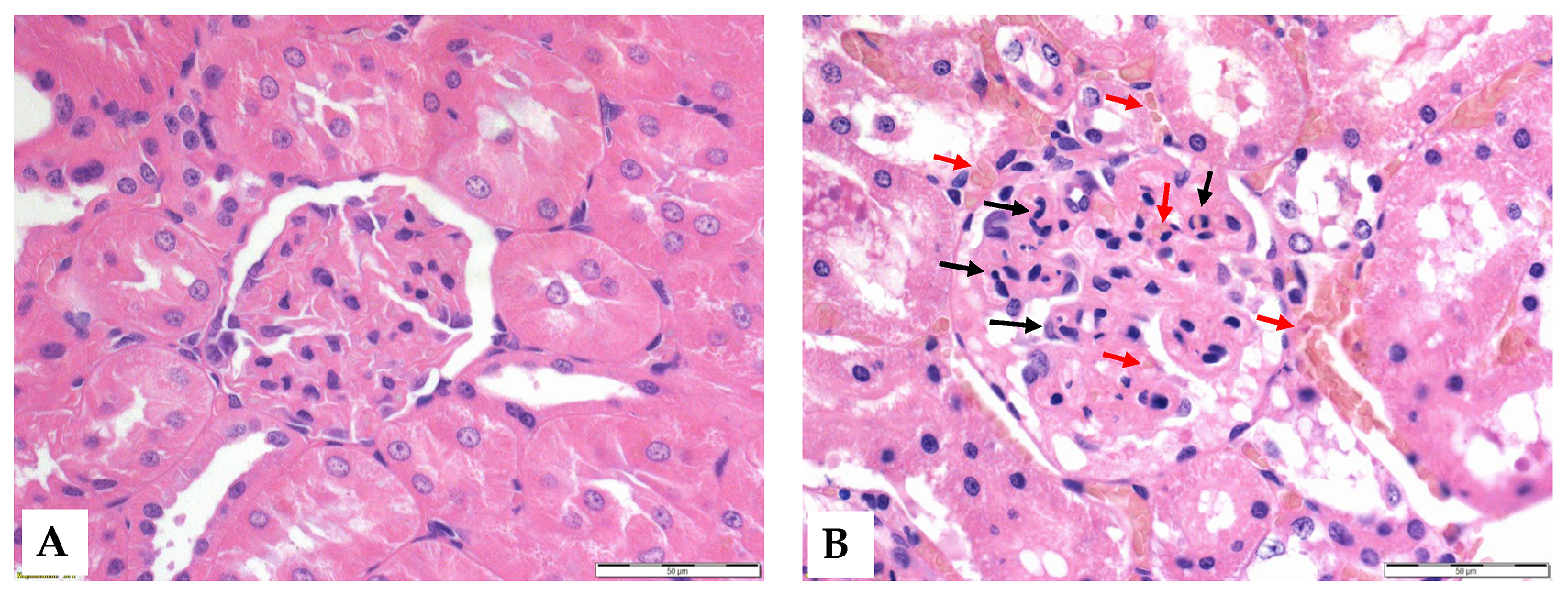
3.6. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos Dias, F.; Metelo Coimbra, C.; Roncon-Albuquerque, R., Jr. Renal warm ischemia in organ donors after circulatory death. Exp. Clin. Transplant. 2021, 19, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.E.; Endre, Z.H. Definitions, phenotypes, and subphenotypes in acute kidney injury—Moving towards precision medicine. Nephrology 2023, 28, 83–96. [Google Scholar] [CrossRef]
- Raynaud, M.; Al-Awadhi, S.; Louis, K.; Zhang, H.; Su, X.; Goutaudier, V.; Wang, J.; Demir, Z.; Wei, Y.; Truchot, A.; et al. Prognostic biomarkers in kidney transplantation: A systematic review and critical appraisal. J. Am. Soc. Nephrol. 2024, 35, 177–188. [Google Scholar] [CrossRef]
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Bashir, K. Acute Kidney Injury. 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Bahde, R.; Vowinkel, T.; Unser, J.; Anthoni, C.; Hölzen, J.P.; Suwelack, B.; Senninger, N.; Wolters, H.H. Prognostic factors for kidney allograft survival in the Eurotransplant Senior Program. Ann. Transplant. 2014, 19, 201–209. [Google Scholar] [CrossRef]
- Nemeth, N.; Toth, E.; Nemes, B. Agents targeting ischemia-reperfusion injury. In Current Immunosuppressive Therapy in Organ Transplantation; Huifang, C., Shiguang, Q., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 487–533. [Google Scholar]
- Jiang, S.H.; Kennard, A.L.; Walters, G.D. Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol. 2018, 19, 344. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Recent advances in acute kidney injury and its consequences and impact on chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 397–405. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-reperfusion injury reduces long term renal graft survival: Mechanism and beyond. EBioMedicine. 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Kataria, A.; Athreya, A.; Gupta, G. Biomarkers in kidney transplantation. Adv. Kidney Dis. Health. 2024, 31, 427–435. [Google Scholar] [CrossRef]
- Westphal, S.G.; Mannon, R.B. Biomarkers of rejection in kidney transplantation. Am. J. Kidney Dis. 2024, 85, 364–374. [Google Scholar] [CrossRef]
- Eltzschig, H.; Eckle, T. Ischemia and reperfusion–from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and reperfusion injury in kidney transplantation: Relevant mechanisms in injury and repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Zhang, H.; Chen, H.; Hao, J.; Liu, H.; Li, X. Mitochondrial metabolism and targeted treatment strategies in ischemic-induced acute kidney injury. Cell Death Discov. 2024, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, C.; Xiang, Z.; Lin, T.; Ling, J.; Hu, H. Role of mitochondria in renal ischemia-reperfusion injury. FEBS J. 2024, 291, 5365–5378. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Cucchiari, D.; Podestà, M.A.; Ponticelli, C. Pathophysiology of rejection in kidney transplantation. Expert Rev. Clin. Immunol. 2024, 20, 1471–1481. [Google Scholar] [CrossRef]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. The “no-reflow” phenomenon: Basic science and clinical correlates. Heart 2002, 87, 162–168. [Google Scholar] [CrossRef]
- Baskurt, O.K. Mechanisms of blood rheology alterations. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 170–190. [Google Scholar]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” phenomenon: A contemporary review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef]
- Freitas, F.; Attwell, D. Pericyte-mediated constriction of renal capillaries evokes no-reflow and kidney injury following ischaemia. eLife 2022, 11, e74211. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Kierulf-Lassen, C.; Nieuwenhuijs-Moeke, G.J.; Krogstrup, N.V.; Oltean, M.; Jespersen, B.; Dor, F.J. Molecular mechanisms of renal ischemic conditioning strategies. Eur. Surg. Res. 2015, 55, 151–183. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S.; Jia, P.; Xu, X.; Song, N.; Zhang, T.; Chen, R.; Ding, X. Protection of remote ischemic preconditioning against acute kidney injury: A systematic review and meta-analysis. Crit. Care. 2016, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Veighey, K.; MacAllister, R. Ischemic conditioning in kidney transplantation. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 330–336. [Google Scholar] [CrossRef]
- Varga, G.; Ghanem, S.; Szabo, B.; Nagy, K.; Pal, N.; Tanczos, B.; Somogyi, V.; Barath, B.; Deak, A.; Peto, K.; et al. Renal ischemia-reperfusion-induced metabolic and micro-rheological alterations and their modulation by remote organ ischemic preconditioning protocols in the rat. Clin. Hemorheol. Microcirc. 2019, 71, 225–236. [Google Scholar] [CrossRef]
- Varga, G.; Ghanem, S.; Szabo, B.; Nagy, K.; Pal, N.; Tanczos, B.; Somogyi, V.; Barath, B.; Deak, A.; Matolay, O.; et al. Which remote ischemic preconditioning protocol is favorable in renal ischemia-reperfusion injury in the rat? Clin. Hemorheol. Microcirc. 2020, 76, 439–451. [Google Scholar] [CrossRef]
- Nemeth, N.; Peto, K.; Magyar, Z.; Klarik, Z.; Varga, G.; Oltean, M.; Mantas, A.; Czigany, Z.; Tolba, R.H. Hemorheological and microcirculatory factors in liver ischemia-reperfusion injury—An update on pathophysiology, molecular mechanisms and protective strategies. Int. J. Mol. Sci. 2021, 22, 1864. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Klarik, Z.; Toth, E.; Mester, A.; Furka, I.; Miko, I. Simultaneous investigation of hemodynamic, microcirculatory and arterio-venous micro-rheological parameters in infrarenal or suprarenal aortic cross-clamping model in the rat. Clin. Hemorheol. Microcirc. 2014, 57, 339–353. [Google Scholar] [CrossRef]
- Peto, K.; Nemeth, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Somogyi, V.; Tanczos, B.; Deak, A.; Bidiga, L.; Frecska, E.; et al. Hemorheological and metabolic consequences of renal ischemia-reperfusion and their modulation by N,N-dimethyl-tryptamine on a rat model. Clin. Hemorheol. Microcirc. 2018, 70, 107–117. [Google Scholar] [CrossRef]
- Nemeth, N.; Deak, A.; Szentkereszty, Z.; Peto, K. Effects and influencing factors on hemorheological variables taken into consideration in surgical pathophysiology research. Clin. Hemorheol. Microcirc. 2018, 69, 133–140. [Google Scholar] [CrossRef]
- Ghanem, S.; Lesznyak, T.; Fazekas, L.; Tanczos, B.; Barath, B.; Nasser, M.; Horvath, L.; Bidiga, L.; Szabo, B.; Deak, A.; et al. Microrheology, microcirculation and structural compensatory mechanisms of a chronic kidney disease rat model. A preliminary study. Clin. Hemorheol. Microcirc. 2020, 75, 47–56. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, S.R. Pathophysiology of red blood cell trapping in ischemic acute kidney injury. Compr. Physiol. 2023, 14, 5325–5343. [Google Scholar] [CrossRef] [PubMed]
- Peto, K.; Nemeth, N.; Brath, E.; Takacs, I.E.; Baskurt, O.K.; Meiselman, H.J.; Furka, I.; Miko, I. The effects of renal ischemia-reperfusion on hemorheological factors: Preventive role of allopurinol. Clin. Hemorheol. Microcirc. 2007, 37, 347–358. [Google Scholar] [PubMed]
- Green, C.J.; Knight, J.; Precious, S.; Simpkin, S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: A 10-year experience. Lab. Anim. 1981, 15, 163–170. [Google Scholar] [CrossRef]
- Flecknell, P. Laboratory Animal Anaesthesia, 4th ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2015; pp. 163–165. [Google Scholar]
- Owji, S.M.; Nikeghbal, E.; Moosavi, S.M. Comparison of ischaemia-reperfusion-induced acute kidney injury by clamping renal arteries, veins or pedicles in anaesthetized rats. Exp. Physiol. 2018, 103, 1390–1402. [Google Scholar] [CrossRef]
- Hardeman, M.; Goedhart, P.; Shin, S. Methods in hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 242–266. [Google Scholar]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index—Shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef]
- Hutchings, S.; Watts, S.; Kirkman, E. The Cytocam video microscope. A new method for visualising the microcirculation using Incident Dark Field technology. Clin. Hemorheol. Microcirc. 2016, 62, 261–271. [Google Scholar] [CrossRef]
- Hutchings, S.D.; Naumann, D.N.; Watts, S.; Wilson, C.; Burton, C.; Wendon, J.; Kirkman, E. Microcirculatory perfusion shows wide inter-individual variation and is important in determining shock reversal during resuscitation in a porcine experimental model of complex traumatic hemorrhagic shock. Intensive Care Med. Exp. 2016, 4, 17. [Google Scholar] [CrossRef]
- Banga, N.R.; Homer-Vanniasinkam, S.; Graham, A.; Al-Mukhtar, A.; White, S.A.; Prasad, K.R. Ischaemic preconditioning in transplantation and major resection of the liver. Br. J. Surg. 2005, 92, 528–538. [Google Scholar] [CrossRef]
- Bruzzese, L.; Lumet, G.; Vairo, D.; Guiol, C.; Guieu, R.; Faure, A. Hypoxic preconditioning in renal ischaemia-reperfusion injury: A review in pre-clinical models. Clin. Sci. 2021, 135, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y. Ischemic preconditioning of the kidney. Bull. Exp. Biol. Med. 2021, 171, 567–571. [Google Scholar] [CrossRef]
- Ortega-Trejo, J.A.; Bobadilla, N.A. Is renal ischemic preconditioning an alternative to ameliorate the short- and long-term consequences of acute kidney injury? Int. J. Mol. Sci. 2023, 24, 8345. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Hobson, R.W., 2nd; Miyasaka, M.; Granger, D.N.; Durán, W.N. Inhibition of white blood cell adhesion at reperfusion decreases tissue damage in postischemic striated muscle. J. Vasc. Surg. 1996, 24, 187–193. [Google Scholar] [CrossRef]
- Adar, R.; Franklin, A.; Salzman, E.W. Letter: Hemoconcentration in acute nonocclusive mesenteric ischemia. JAMA 1974, 228, 27. [Google Scholar] [PubMed]
- Tejchman, K.; Sierocka, A.; Kotowski, M.; Zair, L.; Pilichowska, E.; Ostrowski, M.; Sieńko, J. Acid-base balance disorders during kidney preservation in cold ischemia. Transplant. Proc. 2020, 52, 2036–2042. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Dong, Z. Rodent models of AKI-CKD transition. Am. J. Physiol. Renal Physiol. 2018, 315, F1098–F1106. [Google Scholar] [CrossRef]
- Shiva, N.; Sharma, N.; Kulkarni, Y.A.; Mulay, S.R.; Gaikwad, A.B. Renal ischemia/reperfusion injury: An insight on in vitro and in vivo models. Life Sci. 2020, 256, 117860. [Google Scholar] [CrossRef]
- Lerink, L.J.S.; de Kok, M.J.C.; Mulvey, J.F.; Le Dévédec, S.E.; Markovski, A.A.; Wüst, R.C.I.; Alwayn, I.P.J.; Ploeg, R.J.; Schaapherder, A.F.M.; Bakker, J.A.; et al. Preclinical models versus clinical renal ischemia reperfusion injury: A systematic review based on metabolic signatures. Am. J. Transplant. 2022, 22, 344–370. [Google Scholar] [CrossRef]

| Variable | Sample | Control | I/R | IPC |
|---|---|---|---|---|
| pH | base | 7.41 ± 0.06 | 7.45 ± 0.06 | 7.41 ± 0.07 |
| R-60 | 7.36 ± 0.01 | 7.04 ± 0.09 *# | 7.1 ± 0.08 *# | |
| pO2 [mmHg] | base | 64.68 ± 6.13 | 67.2 ± 7.95 | 62.77 ± 16.55 |
| R-60 | 65.63 ± 1.33 | 40.51 ± 13.28 *# | 39.67 ± 6.93 *# | |
| pCO2 [mmHg] | base | 39.7 ± 4.08 | 33.05 ± 6.9 | 37.51 ± 8.65 |
| R-60 | 45.2 ± 1.83 | 70.6 ± 13.68 *# | 55.87 ± 13.04 *# | |
| K+ [mmol/L] | base | 3.63 ± 0.64 | 3.8 ± 0.44 | 3.41 ± 0.68 |
| R-60 | 3.76 ± 0.51 | 4.54 ± 1.07 | 4.91 ± 0.05 *# | |
| lactate [mmol/L] | base | 1.81 ± 0.64 | 1.91 ± 0.74 | 1.96 ± 1.06 |
| R-60 | 1.73 ± 0.74 | 3.86 ± 1.76 *# | 3.41 ± 1.78 * | |
| creatinine [μmol/L] | base | 0.35 ± 0.03 | <0.3 | <0.3 |
| R-60 | 0.41 ± 0.06 | 0.71 ± 0.28 | 0.76 ± 0.29 |
| Variable | Sample | Control | I/R | IPC |
|---|---|---|---|---|
| WBC [109/L] | base | 8.92 ± 1.64 | 7.31 ± 2.67 | 9.8 ± 5.84 |
| R-60 | 7.2 ± 0.62 | 5.51 ± 1.37 # | 6.17 ± 2.18 | |
| RBC [1012/L] | base | 6.58 ± 0.35 | 6.95 ± 0.87 | 6.35 ± 0.9 |
| R-60 | 7.39 ± 0.24 | 8.47 ± 0.61 # | 8.07 ± 1.75 | |
| Hct [%] | base | 40.83 ± 4.82 | 39.99 ± 10.69 | 38.95 ± 4.33 |
| R-60 | 43.82 ± 0.52 | 55.61 ± 3.13 *# | 52.62 ± 9.8 * | |
| Plt [109/L] | base | 750.8 ± 96.8 | 849.4 ± 345.9 | 803.4 ± 197.4 |
| R-60 | 426 ± 76.5 | 1014 ± 99.1 # | 989.5 ± 186 # |
| Variable | Sample | Control | I/R | IPC |
|---|---|---|---|---|
| blood flow rate [mL/min] | base | 4.26 ± 0.5 | 3.94 ± 0.62 | 4.66 ± 1.05 |
| R-60 | 4.04 ± 0.39 | 3.81 ± 0.6 | 4.09 ± 1.01 |
| Variable | Sample | Control Group | I/R Group | IPC Group | ||
|---|---|---|---|---|---|---|
| Intact Side | Ischemic Side | Intact Side | Ischemic Side | |||
| PVD [mm/mm2] | base | 4.77 ± 0.27 | 4.38 ± 2.05 | 4.99 ± 1.79 | 4.26 ± 1.2 | 3.36 ± 2.99 |
| R-60 | 4.18 ± 4.11 | 4.84 ± 1.78 | 2.98 ± 0.74 | 3.96 ± 1.53 | ||
| PPV [%] | base | 27.29 ± 2.78 | 25.58 ± 9.71 | 25.56 ± 12.64 | 26.48 ± 12.22 | 22.45 ± 19.08 |
| R-60 | 22.15 ± 16.47 | 29.53 ± 11.68 | 19.03 ± 6.76 | 28.08 ± 13.47 | ||
| MFI [au] | base | 2.85 ± 0.36 | 2.83 ± 0.41 | 2.83 ± 0.4 | 2.8 ± 0.42 | 2.9 ± 0.41 |
| R-60 | 2.72 ± 0.46 | 2.54 ± 0.52 | 2.7 ± 0.48 | 2.63 ± 0.51 | ||
| Ratio of Cells/View | Lateralization | Control | I/R | IPC |
|---|---|---|---|---|
| Intact cells [%] | intact side | 67.91 ± 4.76 | 79.76 ± 14.82 | 69.30 ± 5.11 |
| ischemic side | n.a. | 27.54 ± 13.16 *# | 17.36 ± 4.42 *# | |
| Pyknotic cells [%] | intact side | 23.34 ± 3.67 | 10.86 ± 5.94 # | 16.86 ± 4.41 # |
| ischemic side | n.a. | 20.96 ± 8.07 | 26.36 ± 12.22 | |
| Hydropic cells [%] | intact side | 11.04 ± 4.53 | 9.37 ± 9.52 | 20.68 ± 4.99 # |
| ischemic side | n.a. | 51.48 ± 11.12 *# | 56.27 ± 9.38 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorjan, D.M.; Fazekas, L.A.; Varga, A.; Matrai, A.A.; Bidiga, L.; Lesznyak, T.; Deak, A.; Peto, K.; Nemeth, N. Metabolic and Microcirculatory Changes in Severe Renal Ischemia–Reperfusion and Ischemic Preconditioning in the Rat: Are They Detectable in the First Hour of Reperfusion? Life 2025, 15, 592. https://doi.org/10.3390/life15040592
Adorjan DM, Fazekas LA, Varga A, Matrai AA, Bidiga L, Lesznyak T, Deak A, Peto K, Nemeth N. Metabolic and Microcirculatory Changes in Severe Renal Ischemia–Reperfusion and Ischemic Preconditioning in the Rat: Are They Detectable in the First Hour of Reperfusion? Life. 2025; 15(4):592. https://doi.org/10.3390/life15040592
Chicago/Turabian StyleAdorjan, David Martin, Laszlo Adam Fazekas, Adam Varga, Adam Attila Matrai, Laszlo Bidiga, Tamas Lesznyak, Adam Deak, Katalin Peto, and Norbert Nemeth. 2025. "Metabolic and Microcirculatory Changes in Severe Renal Ischemia–Reperfusion and Ischemic Preconditioning in the Rat: Are They Detectable in the First Hour of Reperfusion?" Life 15, no. 4: 592. https://doi.org/10.3390/life15040592
APA StyleAdorjan, D. M., Fazekas, L. A., Varga, A., Matrai, A. A., Bidiga, L., Lesznyak, T., Deak, A., Peto, K., & Nemeth, N. (2025). Metabolic and Microcirculatory Changes in Severe Renal Ischemia–Reperfusion and Ischemic Preconditioning in the Rat: Are They Detectable in the First Hour of Reperfusion? Life, 15(4), 592. https://doi.org/10.3390/life15040592








