Comprehensive Overview of Cytokine Interplay in Vitiligo: A Decade of Meta-Analyses Systematically Reviewed
Abstract
1. Introduction
2. Materials and Methods
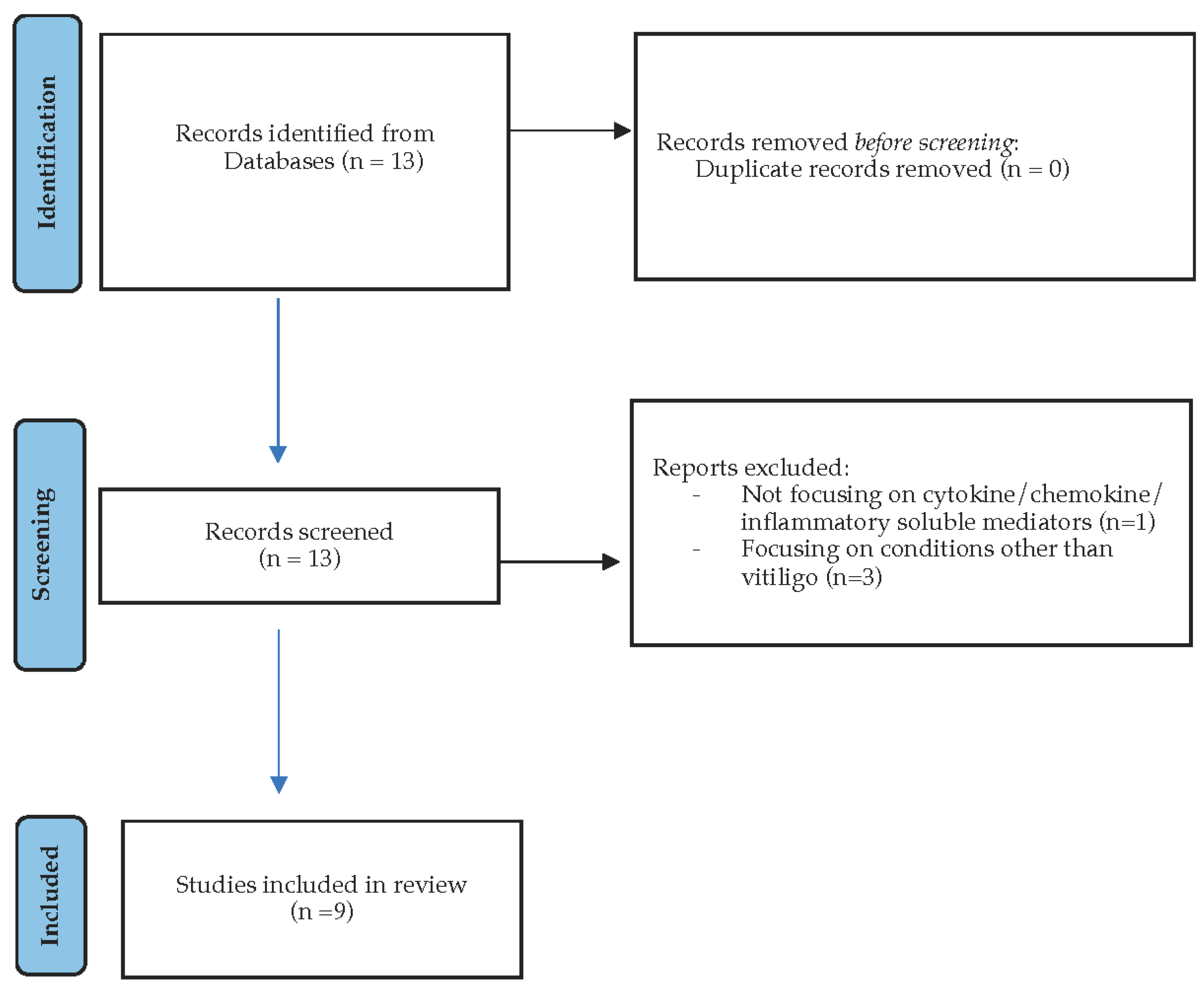
2.1. Search Strategy
2.2. Study Population—Selection
2.3. Data Extraction
3. Results and Discussion
3.1. IFN-γ: A Key Player in Melanocyte Destruction
3.2. Chemokines
3.3. IL-17: A Controversial Player in Vitiligo Pathogenesis and Treatment
3.4. Tumor Necrosis Factor Alpha (TNF-α) and Its Role in Vitiligo
3.5. Immune-Tolerance Disruption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primer 2015, 1, 15011. [Google Scholar] [CrossRef]
- Alikhan, A.; Felsten, L.M.; Daly, M.; Petronic-Rosic, V. Vitiligo: A Comprehensive Overview Part I. Introduction, Epidemiology, Quality of Life, Diagnosis, Differential Diagnosis, Associations, Histopathology, Etiology, and Work-Up. J. Am. Acad. Dermatol. 2011, 65, 473–491. [Google Scholar] [CrossRef]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet Lond. Engl. 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Vedamurthy, M.; Kumar, M.; Boda, S. Exploring the Spectrum of Vitiligo: Clinical and Demographic Perspectives—A Cross-Sectional Study. Cosmoderma 2024, 4, 40. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of Vitiligo and Associated Autoimmune Diseases in Caucasian Probands and Their Families. Pigment. Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef]
- Kussainova, A.; Kassym, L.; Akhmetova, A.; Glushkova, N.; Sabirov, U.; Adilgozhina, S.; Tuleutayeva, R.; Semenova, Y. Vitiligo and Anxiety: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0241445. [Google Scholar] [CrossRef] [PubMed]
- Spritz, R.A.; Santorico, S.A. The Genetic Basis of Vitiligo. J. Investig. Dermatol. 2021, 141, 265–273. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, D.; Yang, H.; Liu, W. The Prevalence and Odds of Depression in Patients with Vitiligo: A Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- D’Arino, A.; Picardo, M.; Truglio, M.; Pacifico, A.; Iacovelli, P. Metabolic Comorbidities in Vitiligo: A Brief Review and Report of New Data from a Single-Center Experience. Int. J. Mol. Sci. 2021, 22, 8820. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, H.J.; Seo, J.M.; Almurayshid, A.; Kim, G.M.; Ezzedine, K.; Bae, J.M. Comorbidities in Patients with Vitiligo: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2023, 143, 777–789.e6. [Google Scholar] [CrossRef]
- Dahir, A.M.; Thomsen, S.F. Comorbidities in Vitiligo: Comprehensive Review. Int. J. Dermatol. 2018, 57, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Esse, I.; Rodriguez, K.H.; Kassels, A.; Shiu, J.; Kraus, C.N. Vulvar Lichen Sclerosus and Vitiligo: Overlap and Clinical Features. J. Am. Acad. Dermatol. 2023, 89, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.; Chang, H. Association between vitiligo and metabolic syndrome: A systematic review and meta-analysis. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 218–221. [Google Scholar] [CrossRef]
- Kang, P.; Zhang, W.; Ji, Z.; Shao, Z.; Li, C. Association between vitiligo and relevant components of metabolic syndrome: A systematic review and meta-analysis. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 629–641. [Google Scholar] [CrossRef]
- Liang, X.; Guo, F.; Zhang, M.; Wang, C.; Lin, N.; Liu, L.; Chen, Y.; Liu, F.; Du, Y.; Li, L.; et al. Risk Factors for Cardiovascular Diseases in Patients with Vitiligo: An Analysis of Current Evidence. Ann. Med. 2024, 56, 2326297. [Google Scholar] [CrossRef]
- Azzazi, Y.; Mostafa, W.Z.; Sayed, K.S.; Alhelf, M.; Safwat, M.; Mahrous, A.; El Lawindi, M.; Ragab, N. Support for Increased Cardiovascular Risk in Non-Segmental Vitiligo among Egyptians: A Hospital-Based, Case-Control Study. Pigment. Cell Melanoma Res. 2021, 34, 598–604. [Google Scholar] [CrossRef]
- Bellei, B.; Picardo, M. Premature Cell Senescence in Human Skin: Dual Face in Chronic Acquired Pigmentary Disorders. Ageing Res. Rev. 2020, 57, 100981. [Google Scholar] [CrossRef]
- Papaccio, F.; Ottaviani, M.; Truglio, M.; D’Arino, A.; Caputo, S.; Pacifico, A.; Iacovelli, P.; Di Nardo, A.; Picardo, M.; Bellei, B. Markers of Metabolic Abnormalities in Vitiligo Patients. Int. J. Mol. Sci. 2024, 25, 10201. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Ko, C.-H. The Role of Oxidative Stress in Vitiligo: An Update on Its Pathogenesis and Therapeutic Implications. Cells 2023, 12, 936. [Google Scholar] [CrossRef]
- Dell’anna, M.L.; Picardo, M. A Review and a New Hypothesis for Non-Immunological Pathogenetic Mechanisms in Vitiligo. Pigment. Cell Res. 2006, 19, 406–411. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M. Interleukin-17 Level in Patients with Vitiligo: A Systematic Review and Meta-Analysis. Australas. J. Dermatol. 2020, 61, e208–e212. [Google Scholar] [CrossRef]
- Giri, P.S.; Mistry, J.; Dwivedi, M. Meta-Analysis of Alterations in Regulatory T Cells’ Frequency and Suppressive Capacity in Patients with Vitiligo. J. Immunol. Res. 2022, 2022, 6952299. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shi, D.; Zhu, X. The Association Between Tumor Necrosis Factor-α-308 G/A Polymorphism and Risk for Vitiligo: A Meta-Analysis. Int. J. Dermatol. 2015, 54, 1045–1053. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Associations between TNF-α Polymorphisms and Susceptibility to Rheumatoid Arthritis and Vitiligo: A Meta-Analysis. Genet. Mol. Res. GMR 2015, 14, 5548–5559. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Qi, J.H.; Huang, C.W.; Yang, T.; Shi, N.; Chen, Y.J. Meta-Analysis of the TNF-α-308G/A Polymorphism and Vitiligo Risk. Genet. Mol. Res. GMR 2015, 14, 17296–17304. [Google Scholar] [CrossRef]
- Dutta, T.; Sengupta, S.; Adhya, S.; Saha, A.; Sengupta, D.; Mondal, R.; Naskar, S.; Bhattacharjee, S.; Sengupta, M. Identification of TNF-α as Major Susceptible Risk Locus for Vitiligo: A Systematic Review and Meta-Analysis Study in the Asian Population. Dermatol. Basel Switz. 2024, 240, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.S.; Begum, R.; Dwivedi, M. Meta-Analysis for Association of TNFA-308(G > A) SNP with Vitiligo Susceptibility. Gene 2022, 809, 146027. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.-C. Association between Interferon- γ +874 T/A Polymorphism and Susceptibility to Autoimmune Diseases: A Meta-Analysis. Lupus 2016, 25, 710–718. [Google Scholar] [CrossRef]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.M.; van Geel, N. A Meta-Analysis of Chemokines in Vitiligo: Recruiting Immune Cells Towards Melanocytes. Front. Immunol. 2023, 14, 1112811. [Google Scholar] [CrossRef]
- Lambe, T.; Leung, J.C.H.; Bouriez-Jones, T.; Silver, K.; Makinen, K.; Crockford, T.L.; Ferry, H.; Forrester, J.V.; Cornall, R.J. CD4 T Cell-Dependent Autoimmunity against a Melanocyte Neoantigen Induces Spontaneous Vitiligo and Depends upon Fas-Fas Ligand Interactions. J. Immunol. 2006, 177, 3055–3062. [Google Scholar] [CrossRef]
- De Benedetti, F.; Prencipe, G.; Bracaglia, C.; Marasco, E.; Grom, A.A. Targeting Interferon-γ in Hyperinflammation: Opportunities and Challenges. Nat. Rev. Rheumatol. 2021, 17, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sousa, J.; Echeverria, D.; Fan, X.; Hsueh, Y.-C.; Afshari, K.; MeHugh, N.; Cooper, D.A.; Vangjeli, L.; Monopoli, K.; et al. RNAi-Based Modulation of IFN-γ Signaling in Skin. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 2709–2721. [Google Scholar] [CrossRef]
- Liu, B.; Shen, J.; Li, J.; Tian, B.; Zhou, B.; Gui, J.; Li, Z.; Zhang, Y.; Hu, W.; Li, Q. Candidate Approaches for Predicting Vitiligo Recurrence: An Effective Model and Biomarkers. Front. Immunol. 2025, 16, 1468665. [Google Scholar] [CrossRef]
- Seneschal, J.; Boniface, K.; D’Arino, A.; Picardo, M. An Update on Vitiligo Pathogenesis. Pigment. Cell Melanoma Res. 2021, 34, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, Y.; Sun, Y.; Shi, W.; Yang, J.; Zhu, L.; Li, M. Interferon-Gamma Inhibits Melanogenesis and Induces Apoptosis in Melanocytes: A Pivotal Role of CD8+ Cytotoxic T Lymphocytes in Vitiligo. Acta Derm. Venereol. 2015, 95, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Le, Q.; Tong, J.; Wang, H. The IFN-γ-CXCL9/CXCL10-CXCR3 Axis in Vitiligo: Pathological Mechanism and Treatment. Eur. J. Immunol. 2024, 54, e2250281. [Google Scholar] [CrossRef]
- Su, Q.; Wang, F.; Dong, Z.; Chen, M.; Cao, R. IFN-γ Induces Apoptosis in Human Melanocytes by Activating the JAK1/STAT1 Signaling Pathway. Mol. Med. Rep. 2020, 22, 3111–3116. [Google Scholar] [CrossRef]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The Delicate Relation Between Melanocytes and Skin Immunity: A Game of Hide and Seek. Pigment. Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Dong, J.; Huang, X.; Ma, L.-P.; Qi, F.; Wang, S.-N.; Zhang, Z.-Q.; Wei, S.-N.; Gao, L.; Liu, F. Baricitinib Is Effective in Treating Progressing Vitiligo in Vivo and In Vitro. Dose Response 2022, 20, 15593258221105370. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Suzuki, T.; Sano, S.; Katayama, I. JAK Inhibitors for the Treatment of Vitiligo. J. Dermatol. Sci. 2024, 113, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Peeva, E.; Yamaguchi, Y.; Cox, L.A.; Banerjee, A.; Han, G.; Hamzavi, I.; Ganesan, A.K.; Picardo, M.; Thaçi, D.; et al. Efficacy and Safety of Oral Ritlecitinib for the Treatment of Active Nonsegmental Vitiligo: A Randomized Phase 2b Clinical Trial. J. Am. Acad. Dermatol. 2023, 88, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Du, J.; Wang, F.; Ding, X. Excellent Repigmentation of Generalized Vitiligo with Oral Baricitinib Combined with NB-UVB Phototherapy. Clin. Cosmet. Investig. Dermatol. 2023, 16, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Strassner, J.P.; Refat, M.A.; Harris, J.E.; King, B.A. Repigmentation in Vitiligo Using the Janus Kinase Inhibitor Tofacitinib May Require Concomitant Light Exposure. J. Am. Acad. Dermatol. 2017, 77, 675–682.e1. [Google Scholar] [CrossRef]
- Damsky, W.; King, B.A. JAK Inhibitors in Dermatology: The Promise of a New Drug Class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Tung, T.-H.; Wang, S.-H.; Chi, C.-C. Efficacy and Safety of Tofacitinib for Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 355–362. [Google Scholar] [CrossRef]
- Fisher, P.B.; Mufson, R.A.; Weinstein, I.B. Interferon Inhibits Melanogenesis in B-16 Mouse Melanoma Cells. Biochem. Biophys. Res. Commun. 1981, 100, 823–830. [Google Scholar] [CrossRef]
- Zhou, J.; Ling, J.; Ping, F. Interferon-γ Attenuates 5-Hydroxytryptamine-Induced Melanogenesis in Primary Melanocyte. Biol. Pharm. Bull. 2016, 39, 1091–1099. [Google Scholar] [CrossRef][Green Version]
- De, A.; Choudhary, N.; Sil, A.; Sarda, A.; Hasanoor Raja, A.H. A Cross-Sectional Study of the Levels of Cytokines IL-6, TNF-α, and IFN-γ in Blood and Skin (Lesional and Uninvolved) of Vitiligo Patients and Their Possible Role as Biomarkers. Indian J. Dermatol. 2023, 68, 67–72. [Google Scholar] [CrossRef]
- Yu, H.S.; Chang, K.L.; Yu, C.L.; Li, H.F.; Wu, M.T.; Wu, C.S.; Wu, C.S. Alterations in IL-6, IL-8, GM-CSF, TNF-Alpha, and IFN-Gamma Release by Peripheral Mononuclear Cells in Patients with Active Vitiligo. J. Investig. Dermatol. 1997, 108, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Gholijani, N.; Yazdani, M.-R.; Dastgheib, L. Predominant Role of Innate Pro-Inflammatory Cytokines in Vitiligo Disease. Arch. Dermatol. Res. 2020, 312, 123–131. [Google Scholar] [CrossRef]
- Jimbo, H.; Nagai, H.; Fujiwara, S.; Shimoura, N.; Nishigori, C. Fas-FasL Interaction in Cytotoxic T Cell-Mediated Vitiligo: The Role of Lesional Expression of Tumor Necrosis Factor-α and Interferon-γ in Fas-Mediated Melanocyte Apoptosis. Exp. Dermatol. 2020, 29, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kim, M.; Lee, H.H.; Kim, K.-J.; Shin, H.; Ju, H.J.; Kim, G.M.; Park, C.J.; Park, H.J. Increased Risk of Vitiligo Following Anti-Tumor Necrosis Factor Therapy: A 10-Year Population-Based Cohort Study. J. Investig. Dermatol. 2018, 138, 768–774. [Google Scholar] [CrossRef]
- Dwivedi, M.; Laddha, N.C.; Shah, K.; Shah, B.J.; Begum, R. Involvement of Interferon-Gamma Genetic Variants and Intercellular Adhesion Molecule-1 in Onset and Progression of Generalized Vitiligo. J. Interferon Cytokine Res. 2013, 33, 646–659. [Google Scholar] [CrossRef]
- Rashighi, M.; Harris, J.E. Interfering with the IFN-γ/CXCL10 Pathway to Develop New Targeted Treatments for Vitiligo. Ann. Transl. Med. 2015, 3, 343. [Google Scholar] [CrossRef]
- Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Ananthanarayanan, P.H.; Thappa, D.M.; Negi, V.S. Polymorphisms in T Helper 1 Proinflammatory Cytokine Genes and the Risk of Psoriasis in a South Indian Tamil Cohort. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2017, 15, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Szepietowski, J.C.; Mazur, G.; Baran, E. IFN-Gamma Promoter Gene Polymorphism in Psoriasis Vulgaris. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2008, 13, 52–58. [Google Scholar] [CrossRef]
- Yen, H.; Chi, C.-C. Association Between Psoriasis and Vitiligo: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2019, 20, 31–40. [Google Scholar] [CrossRef]
- Jin, Y.; Mailloux, C.M.; Gowan, K.; Riccardi, S.L.; LaBerge, G.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. NALP1 in Vitiligo-Associated Multiple Autoimmune Disease. N. Engl. J. Med. 2007, 356, 1216–1225. [Google Scholar] [CrossRef]
- Carlström, M.; Ekman, A.-K.; Petersson, S.; Söderkvist, P.; Enerbäck, C. Genetic Support for the Role of the NLRP3 Inflammasome in Psoriasis Susceptibility. Exp. Dermatol. 2012, 21, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-J.; Lv, Y.-M.; Yin, X.-Y.; Wang, Z.-X.; Sun, L.-D.; He, S.-M.; Cheng, H.; Hu, D.-Y.; Zhang, Z.; Li, Y.; et al. Psoriasis Regression Analysis of MHC Loci Identifies Shared Genetic Variants with Vitiligo. PLoS ONE 2011, 6, e23089. [Google Scholar] [CrossRef]
- Seçkin, D.; Durusoy, C.; Sahin, S. Concomitant Vitiligo and Psoriasis in a Patient Treated with Interferon Alfa-2a for Chronic Hepatitis B Infection. Pediatr. Dermatol. 2004, 21, 577–579. [Google Scholar] [CrossRef]
- Hsueh, Y.-C. The Development of a Skin-Targeted Interferon-Gamma-Neutralizing Bispecific Antibody for Vitiligo Treatment. Ph.D. Thesis, UMass Chan Medical School, Worcester, MA, USA, 2022. [Google Scholar]
- Ng, C.Y.; Chan, Y.-P.; Chiu, Y.-C.; Shih, H.-P.; Lin, Y.-N.; Chung, P.-H.; Huang, J.-Y.; Chen, H.-K.; Chung, W.-H.; Ku, C.-L. Targeting the Elevated IFN-γ in Vitiligo Patients by Human Anti- IFN-γ Monoclonal Antibody Hampers Direct Cytotoxicity in Melanocyte. J. Dermatol. Sci. 2023, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- McCully, M.L.; Moser, B. The Human Cutaneous Chemokine System. Front. Immunol. 2011, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Gharib, K.; Gadallah, H.; Elsayed, A. Chemokines in Vitiligo Pathogenesis: CXCL10 and 12. J. Clin. Aesthetic Dermatol. 2021, 14, 27–32. [Google Scholar]
- Lin, F.; Hu, W.; Xu, W.; Zhou, M.; Xu, A.-E. CXCL9 as a Key Biomarker of Vitiligo Activity and Prediction of the Success of Cultured Melanocyte Transplantation. Sci. Rep. 2021, 11, 18298. [Google Scholar] [CrossRef]
- Aulakh, S.; Goel, S.; Kaur, L.; Gulati, S.; Kaur, M.; Chopra, D.; Sarangal, R.; Batra, J. Differential Expression of Serum CXCL9 and CXCL10 Levels in Vitiligo Patients and Their Correlation with Disease Severity and Stability: A Cross-Sectional Study. Indian J. Dermatol. Venereol. Leprol. 2025, 91, 9–15. [Google Scholar] [CrossRef]
- Speeckaert, R.; Caelenberg, E.V.; Belpaire, A.; Speeckaert, M.M.; van Geel, N. Vitiligo: From Pathogenesis to Treatment. J. Clin. Med. 2024, 13, 5225. [Google Scholar] [CrossRef]
- Tulic, M.K.; Cavazza, E.; Cheli, Y.; Jacquel, A.; Luci, C.; Cardot-Leccia, N.; Hadhiri-Bzioueche, H.; Abbe, P.; Gesson, M.; Sormani, L.; et al. Innate Lymphocyte-Induced CXCR3B-Mediated Melanocyte Apoptosis Is a Potential Initiator of T-Cell Autoreactivity in Vitiligo. Nat. Commun. 2019, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Kim, T.; Pang, J.; Sun, W.; Yang, X.; Wang, J.; Song, Y.; Zhang, H.; Sun, H.; Rangan, V.; et al. A Novel Function of CXCL10 in Mediating Monocyte Production of Proinflammatory Cytokines. J. Leukoc. Biol. 2017, 102, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 Axis for Immune Activation—A Target for Novel Cancer Therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, K.J.; Strassner, J.P.; Essien, K.; Refat, M.A.; Murphy, R.L.; Coffin-Schmitt, A.; Pandya, A.G.; Tovar-Garza, A.; Frisoli, M.L.; Fan, X.; et al. scRNA-Seq of Human Vitiligo Reveals Complex Networks of Subclinical Immune Activation and a Role for CCR5 in Treg Function. Sci. Transl. Med. 2021, 13, eabd8995. [Google Scholar] [CrossRef]
- Rezk, A.F.; Kemp, D.M.; El-Domyati, M.; El-Din, W.H.; Lee, J.B.; Uitto, J.; Igoucheva, O.; Alexeev, V. Misbalanced CXCL12 and CCL5 Chemotactic Signals in Vitiligo Onset and Progression. J. Investig. Dermatol. 2017, 137, 1126–1134. [Google Scholar] [CrossRef]
- Grayson, M.H.; Holtzman, M.J. Chemokine Complexity: The Case for CCL5. Am. J. Respir. Cell Mol. Biol. 2006, 35, 143–146. [Google Scholar] [CrossRef]
- Martins, C.; Migayron, L.; Drullion, C.; Jacquemin, C.; Lucchese, F.; Rambert, J.; Merhi, R.; Michon, P.; Taieb, A.; Rezvani, H.-R.; et al. Vitiligo Skin T Cells Are Prone to Produce Type 1 and Type 2 Cytokines to Induce Melanocyte Dysfunction and Epidermal Inflammatory Response Through Jak Signaling. J. Investig. Dermatol. 2022, 142, 1194–1205.e7. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 Axis in Human Diseases and Related Treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef]
- Essien, K.I.; Katz, E.L.; Strassner, J.P.; Harris, J.E. Regulatory T Cells Require CCR6 for Skin Migration and Local Suppression of Vitiligo. J. Investig. Dermatol. 2022, 142, 3158–3166.e7. [Google Scholar] [CrossRef]
- Hess, C.; Means, T.K.; Autissier, P.; Woodberry, T.; Altfeld, M.; Addo, M.M.; Frahm, N.; Brander, C.; Walker, B.D.; Luster, A.D. IL-8 Responsiveness Defines a Subset of CD8 T Cells Poised to Kill. Blood 2004, 104, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lee, K.M.; Vujkovic-Cvijin, I.; Ucmak, D.; Farahnik, B.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Bhutani, T.; Wei, M.; et al. The Role of IL-17 in Vitiligo: A Review. Autoimmun. Rev. 2016, 15, 397–404. [Google Scholar] [CrossRef]
- Yamada, T.; Hasegawa, S.; Hasebe, Y.; Kawagishi-Hotta, M.; Arima, M.; Iwata, Y.; Kobayashi, T.; Numata, S.; Yamamoto, N.; Nakata, S.; et al. CXCL12 Regulates Differentiation of Human Immature Melanocyte Precursors as Well as Their Migration. Arch. Dermatol. Res. 2019, 311, 55–62. [Google Scholar] [CrossRef]
- Liao, Z.-K.; Hu, S.-H.; Han, B.-Y.; Qiu, X.; Jiang, S.; Lei, T.-C. Pro-Pigmentary Action of 5-Fluorouracil through the Stimulated Secretion of CXCL12 by Dermal Fibroblasts. Chin. Med. J. Engl. 2021, 134, 2475–2482. [Google Scholar] [CrossRef]
- Li, S.; Zhu, G.; Yang, Y.; Jian, Z.; Guo, S.; Dai, W.; Shi, Q.; Ge, R.; Ma, J.; Liu, L.; et al. Oxidative Stress Drives CD8+ T-Cell Skin Trafficking in Patients with Vitiligo through CXCL16 Upregulation by Activating the Unfolded Protein Response in Keratinocytes. J. Allergy Clin. Immunol. 2017, 140, 177–189.e9. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Essien, K.I.; Harris, J.E. T-Cell Positioning by Chemokines in Autoimmune Skin Diseases. Immunol. Rev. 2019, 289, 186–204. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, J.; Wu, J. The Promising Role of Chemokines in Vitiligo: From Oxidative Stress to the Autoimmune Response. Oxid. Med. Cell. Longev. 2022, 2022, 8796735. [Google Scholar] [CrossRef]
- Roca, H.; Varsos, Z.S.; Sud, S.; Craig, M.J.; Ying, C.; Pienta, K.J. CCL2 and Interleukin-6 Promote Survival of Human CD11b+ Peripheral Blood Mononuclear Cells and Induce M2-Type Macrophage Polarization. J. Biol. Chem. 2009, 284, 34342–34354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, X.; Chen, S.; Kang, Y.; Wang, X.; Zhang, C.; Xiang, L. Increased Circulating CXCL10 in Non-Segmental Vitiligo Concomitant with Autoimmune Thyroid Disease and Alopecia Areata. Ann. Dermatol. 2019, 31, 393–402. [Google Scholar] [CrossRef]
- Tam, I.; Dzierżęga-Lęcznar, A.; Stępień, K. Differential Expression of Inflammatory Cytokines and Chemokines in Lipopolysaccharide-Stimulated Melanocytes from Lightly and Darkly Pigmented Skin. Exp. Dermatol. 2019, 28, 551–560. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 Alpha)/CCL3: As a Biomarker. In General Methods in Biomarker Research and Their Applications; Preedy, V.R., Patel, V.B., Eds.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 223–249. ISBN 978-94-007-7695-1. [Google Scholar]
- Bhardwaj, S.; Bhatia, A.; Kumaran, M.S.; Parsad, D. Role of IL-17A Receptor Blocking in Melanocyte Survival: A Strategic Intervention against Vitiligo. Exp. Dermatol. 2019, 28, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, R.A.; Fawzy, M.M.; Gawdat, H.I.; Samir, N.; Rashed, L.A. T Helper 17 and T Regs: A Novel Proposed Mechanism for NB-UVB in Vitiligo. Exp. Dermatol. 2014, 23, 283–286. [Google Scholar] [CrossRef]
- Kumaran, M.S.; Bishnoi, A.; Srivastava, N.; Tekumalla, S.; Vinay, K.; Bhatia, A.; Parsad, D. Significant Reduction in the Expression of Interleukins-17A, 22 and 23A, Forkhead Box P3 and Interferon Gamma Delineates Lichen Planus Pigmentosus from Lichen Planus. Arch. Dermatol. Res. 2019, 311, 519–527. [Google Scholar] [CrossRef]
- Su, H.-J.; Chan, Y.-P.; Shen, P.-C.; Ku, C.-L.; Ng, C.Y. Anti-IL-17A Antibody-Associated de Novo Vitiligo: Case Report and Review of Literature. Front. Immunol. 2022, 13, 1077681. [Google Scholar] [CrossRef]
- Pirro, F.; Caldarola, G.; De Simone, C.; Moretta, G.; Giovanardi, G.; Peris, K. Multiple Paradoxical Reactions During Ixekizumab Therapy. Dermatol. Ther. 2019, 32, e12852. [Google Scholar] [CrossRef]
- Méry-Bossard, L.; Bagny, K.; Chaby, G.; Khemis, A.; Maccari, F.; Marotte, H.; Perrot, J.L.; Reguiai, Z.; Sigal, M.L.; Avenel-Audran, M.; et al. New-Onset Vitiligo and Progression of Pre-Existing Vitiligo During Treatment with Biological Agents in Chronic Inflammatory Diseases. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF? And the TNF Receptor Superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.C.; Tung, R.; Winterfield, L.S.; Gottlieb, A.B.; Eby, J.M.; Henning, S.W.; Le Poole, I.C. Tumour Necrosis Factor-α Inhibition Can Stabilize Disease in Progressive Vitiligo. Br. J. Dermatol. 2015, 173, 641–650. [Google Scholar] [CrossRef]
- Singh, M.; Mansuri, M.S.; Kadam, A.; Palit, S.P.; Dwivedi, M.; Laddha, N.C.; Begum, R. Tumor Necrosis Factor-Alpha Affects Melanocyte Survival and Melanin Synthesis via Multiple Pathways in Vitiligo. Cytokine 2021, 140, 155432. [Google Scholar] [CrossRef]
- Kądziela, M.; Kutwin, M.; Karp, P.; Woźniacka, A. Role of Cytokines and Chemokines in Vitiligo and Their Therapeutic Implications. J. Clin. Med. 2024, 13, 4919. [Google Scholar] [CrossRef]
- Riding, R.L.; Harris, J.E. The Role of Memory CD8+ T Cells in Vitiligo. J. Immunol. Baltim. Md 1950 2019, 203, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.; Kemp, E.H.; Gawkrodger, D.J. Patho-Immunological Mechanisms of Vitiligo: The Role of the Innate and Adaptive Immunities and Environmental Stress Factors. Clin. Exp. Immunol. 2022, 207, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Karam, R.A.; Zidan, H.E.; Khater, M.H. Genetic Variants of Interferon-Gamma and Its mRNA Expression and Inflammatory Parameters in the Pathogenesis of Vitiligo. Biochem. Cell Biol. Biochim. Biol. Cell. 2017, 95, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M.; Devaraju, P. Association Analysis of Tumor Necrosis Factor Alpha Promoter Polymorphisms and Vitiligo Susceptibility in South Indian Tamils. Dermatol. Basel Switz. 2020, 236, 554–564. [Google Scholar] [CrossRef]
- Aydıngöz, I.E.; Kanmaz-Özer, M.; Gedikbaşi, A.; Vural, P.; Doğru-Abbasoğlu, S.; Uysal, M. The Combination of Tumour Necrosis Factor-α -308A and Interleukin-10 -1082G Gene Polymorphisms and Increased Serum Levels of Related Cytokines: Susceptibility to Vitiligo. Clin. Exp. Dermatol. 2015, 40, 71–77. [Google Scholar] [CrossRef]
- Laddha, N.C.; Dwivedi, M.; Begum, R. Increased Tumor Necrosis Factor (TNF)-α and Its Promoter Polymorphisms Correlate with Disease Progression and Higher Susceptibility Towards Vitiligo. PLoS ONE 2012, 7, e52298. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative Stress-Induced Calreticulin Expression and Translocation: New Insights into the Destruction of Melanocytes. J. Investig. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef]
- Mitra, S.; De Sarkar, S.; Pradhan, A.; Pati, A.K.; Pradhan, R.; Mondal, D.; Sen, S.; Ghosh, A.; Chatterjee, S.; Chatterjee, M. Levels of Oxidative Damage and Proinflammatory Cytokines Are Enhanced in Patients with Active Vitiligo. Free Radic. Res. 2017, 51, 986–994. [Google Scholar] [CrossRef]
- Shao, X.; Chen, T.; Pan, X.; Chen, S.; Chen, Y.; Chen, J. Biologic Drugs Induced Vitiligo: Case Reports and Review of Literature. Front. Immunol. 2024, 15, 1455050. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Scala, E.; Caiazzo, G.; Lembo, S.; Marino, R.; Megna, M.; Patrì, A.; Di Caprio, R.; Balato, A. Possible Role of BMP-4 in the Hyper-Pigmentation of Psoriatic Plaques After Anti-TNF-α Treatment. Exp. Ther. Med. 2019, 18, 4120–4124. [Google Scholar] [CrossRef]
- Tu, C.-X.; Jin, W.-W.; Lin, M.; Wang, Z.-H.; Man, M.-Q. Levels of TGF-β(1) in Serum and Culture Supernatants of CD4(+)CD25 (+) T Cells from Patients with Non-Segmental Vitiligo. Arch. Dermatol. Res. 2011, 303, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, M.C.; Lutfalla, G.; Forlenza, M. IL10, A Tale of an Evolutionarily Conserved Cytokine across Vertebrates. Crit. Rev. Immunol. 2016, 36, 99–129. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Gomes, I.A.; de Carvalho, F.O.; de Menezes, A.F.; Almeida, F.M.; Shanmugam, S.; de Souza Siqueira Quintans, J.; Quintans-Júnior, L.J.; de Moura, T.R.; Oliveira, P.D.; de Souza Araújo, A.A. The Role of Interleukins in Vitiligo: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 2097–2111. [Google Scholar] [CrossRef]
- Rätsep, R.; Kingo, K.; Karelson, M.; Reimann, E.; Raud, K.; Silm, H.; Vasar, E.; Kõks, S. Gene Expression Study of IL10 Family Genes in Vitiligo Skin Biopsies, Peripheral Blood Mononuclear Cells and Sera. Br. J. Dermatol. 2008, 159, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Seeger, P.; Musso, T.; Sozzani, S. The TGF-β Superfamily in Dendritic Cell Biology. Cytokine Growth Factor Rev. 2015, 26, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Branton, M.H.; Kopp, J.B. TGF-Beta and Fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M. Tr1 Cells and the Counter-Regulation of Immunity: Natural Mechanisms and Therapeutic Applications. Curr. Top. Microbiol. Immunol. 2014, 380, 39–68. [Google Scholar] [CrossRef]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef]
- Jin, R.; Xu, H.; Zhou, M.; Lin, F.; Xu, W.; Xu, A. EGR1 Mediated Reduction of Fibroblast Secreted-TGF-Β1 Exacerbated CD8+ T Cell Inflammation and Migration in Vitiligo. Inflammation 2024, 47, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shi, Y.; Li, K.; Hamzavi, I.; Gao, T.; Huggins, R.H.; Lim, H.W.; Mi, Q. Increased Circulating Th17 Cells and Elevated Serum Levels of TGF-beta and IL-21 Are Correlated with Human Non-segmental Vitiligo Development. Pigment. Cell Melanoma Res. 2015, 28, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kidir, M.; Karabulut, A.A.; Ercin, M.E.; Atasoy, P. Regulatory T-cell Cytokines in Patients with Nonsegmental Vitiligo. Int. J. Dermatol. 2017, 56, 581–588. [Google Scholar] [CrossRef]
- Papatheodorou, S.I.; Evangelou, E. Umbrella Reviews: What They Are and Why We Need Them. In Meta-Research; Evangelou, E., Veroniki, A.A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2345, pp. 135–146. ISBN 978-1-07-161565-2. [Google Scholar]
- Azimi, A.; Lo, K.; Kim, J.; Fernandez-Penas, P. Investigating Proteome Changes between Primary and Metastatic Cutaneous Squamous Cell Carcinoma Using SWATH Mass Spectrometry. J. Dermatol. Sci. 2020, 99, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, T. Beyond Skin White Spots: Vitiligo and Associated Comorbidities. Front. Med. 2023, 10, 1072837. [Google Scholar] [CrossRef]
- Almasi-Nasrabadi, M.; Amoli, M.M.; Robati, R.M.; Rajabi, F.; Ghalamkarpour, F.; Gauthier, Y. CDH1 and DDR1 Common Variants Confer Risk to Vitiligo and Autoimmune Comorbidities. Gene 2019, 700, 17–22. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, F.; Tan, Y.; Zhang, Y.; Geng, Y.; Yang, G.; Wang, S. Causal Relationship between Inflammatory Cytokines and Autoimmune Thyroid Disease: A Bidirectional Two-Sample Mendelian Randomization Analysis. Front. Immunol. 2024, 15, 1334772. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, Y.; Hou, Y.; Liu, Y.; Liu, T.; Zhang, H.; Fan, C.; Guan, H.; Li, Y.; Shan, Z.; et al. Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated with Autoimmune Thyroiditis. Front. Immunol. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Duarte-García, A.; Leung, Y.Y.; Coates, L.C.; Beaton, D.; Christensen, R.; Craig, E.T.; De Wit, M.; Eder, L.; Fallon, L.; FitzGerald, O.; et al. Endorsement of the 66/68 Joint Count for the Measurement of Musculoskeletal Disease Activity: OMERACT 2018 Psoriatic Arthritis Workshop Report. J. Rheumatol. 2019, 46, 996–1005. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Abudoureyimu, D.; Wang, M.; Yu, S.-R.; Kang, X.-J. Association between Celiac Disease and Vitiligo: A Review of the Literature. World J. Clin. Cases 2021, 9, 10430–10437. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Skodje, G.I.; Sarna, V.K.; Dzuris, J.L.; Russell, A.K.; Goel, G.; Wang, S.; Goldstein, K.E.; Williams, L.J.; Sollid, L.M.; et al. Cytokine Release After Gluten Ingestion Differentiates Coeliac Disease from Self-Reported Gluten Sensitivity. United Eur. Gastroenterol. J. 2020, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Tye-Din, J.A.; Qiao, S.-W.; Russell, A.K.; Mayassi, T.; Ciszewski, C.; Sarna, V.K.; Wang, S.; Goldstein, K.E.; Dzuris, J.L.; et al. Cytokine Release and Gastrointestinal Symptoms After Gluten Challenge in Celiac Disease. Sci. Adv. 2019, 5, eaaw7756. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Inflammatory Cytokines in Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2011, 2011, 432595. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.-K. Role of Cytokines in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Pathogenesis of Alopecia Areata and Vitiligo: Commonalities and Differences. Int. J. Mol. Sci. 2024, 25, 4409. [Google Scholar] [CrossRef]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the Significance of Cytokines and Chemokines in the Pathogenesis of Alopecia Areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef]
- Schuler, C.F.; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel Insights into Atopic Dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Interleukin-13: Targeting an Underestimated Cytokine in Atopic Dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Zhou, P.-L.; Yin, X.-Y.; Wang, A.-X.; Wang, D.-H.; Yang, Y.; Liu, Q. Circulating Inflammatory Cytokines and Psoriasis Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0293327. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Boehncke, W.-H. Revisiting the Interleukin 17 Family of Cytokines in Psoriasis: Pathogenesis and Potential Targets for Innovative Therapies. Front. Immunol. 2023, 14, 1186455. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics Targeting the IL-23 and IL-17 Pathway in Psoriasis. Lancet Lond. Engl. 2021, 397, 754–766. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Year | 1st Author | Implicated Cytokine/Chemokine | Main Findings | Available Drugs |
|---|---|---|---|---|---|
| [21] | 2020 | Acharya P | IL-17 | Secukinumab, Ixekizumab, Bimekizumab, Brodalumab | |
| [22] | 2022 | Giri PS | IL-10, TGF-β | reduction of suppressive molecules (FOXP3, IL-10, and TGF-β) | NA |
| [23] | 2015 | Wu D | TNF-α | TNF-α-308 G/A polymorphism is not a genetic risk factor for vitiligo | Infliximab, Etanercept, Adalimumab, Golimumab, Certolizumab pegol |
| [24] | 2015 | Lee YH | TNF-α -308 A/G polymorphism may be a significant risk factor for vitiligo in Middle Eastern populations | ||
| [25] | 2015 | Nie G | TNF-α-308G/A polymorphism may not be associated with vitiligo risk | ||
| [26] | 2024 | Dutta T | TNF-α gene: rs1800629 was found to be associated with vitiligo risk in Asian population | ||
| [27] | 2022 | Giri PS | TNFA-308(G > A) SNP and vitiligo susceptibility | ||
| [28] | 2016 | Lee YH | IFN-γ | no association between IFN-γ +874 T/A polymorphism and vitiligo | NA |
| [29] | 2023 | Speeckaert R | blood CCL5, CXCL8, CXCL12, and CXCL16 levels were significantly elevated (Th1 response) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganelli, A.; Cristofoletti, C.; Moro, F.; Corrente, A.; Colonna, L.; Scala, E.; Picardo, M. Comprehensive Overview of Cytokine Interplay in Vitiligo: A Decade of Meta-Analyses Systematically Reviewed. Life 2025, 15, 684. https://doi.org/10.3390/life15050684
Paganelli A, Cristofoletti C, Moro F, Corrente A, Colonna L, Scala E, Picardo M. Comprehensive Overview of Cytokine Interplay in Vitiligo: A Decade of Meta-Analyses Systematically Reviewed. Life. 2025; 15(5):684. https://doi.org/10.3390/life15050684
Chicago/Turabian StylePaganelli, Alessia, Cristina Cristofoletti, Francesco Moro, Alessandra Corrente, Laura Colonna, Emanuele Scala, and Mauro Picardo. 2025. "Comprehensive Overview of Cytokine Interplay in Vitiligo: A Decade of Meta-Analyses Systematically Reviewed" Life 15, no. 5: 684. https://doi.org/10.3390/life15050684
APA StylePaganelli, A., Cristofoletti, C., Moro, F., Corrente, A., Colonna, L., Scala, E., & Picardo, M. (2025). Comprehensive Overview of Cytokine Interplay in Vitiligo: A Decade of Meta-Analyses Systematically Reviewed. Life, 15(5), 684. https://doi.org/10.3390/life15050684









