Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches
Abstract
1. Introduction
2. Mass-Spectrometry-Based Omics for Biomarker Discovery
3. Review of MS-Based Biomarker Studies for T21
3.1. Approach for Literature Review: Search Strategy and Criteria for Inclusion/Exclusion
3.2. Proteomic Signature of Prenatal Down Syndrome
3.2.1. Proteomic Analysis of Down Syndrome Amniotic Fluid Samples
3.2.2. Proteomic Analysis of Down Syndrome Maternal Serum Samples
3.2.3. Proteomic Analysis of Down Syndrome Maternal Plasma Samples
3.2.4. Proteomic Analysis of Down Syndrome Maternal Urine Samples
3.3. Metabolomic Signature of Prenatal Down Syndrome
3.3.1. Metabolomic Analysis of Down Syndrome Amniotic Fluid Samples
3.3.2. Metabolomic Analysis of Down Syndrome Maternal Plasma and Serum Samples
3.3.3. Metabolomic Analysis of Down Syndrome Maternal Urine Samples
4. Assessment of Modified Pathways and Their Importance in Down Syndrome Pathology—A Sample-Type-Based Assessment Using Differentially Expressed Proteins Reported in the Literature
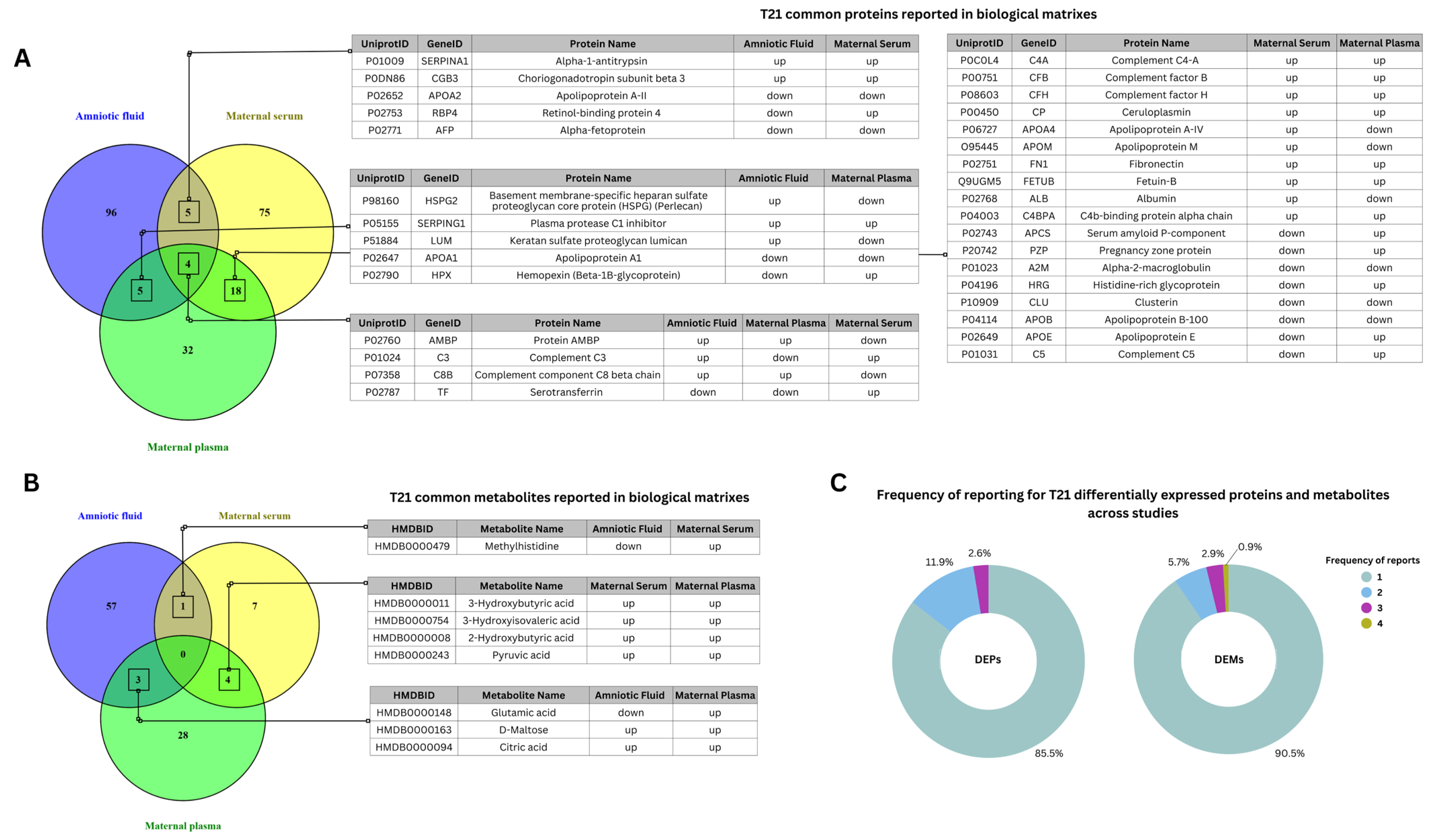
4.1. Differential Expression of Proteins and Metabolites in Trisomy 21 Across Biological Matrices
4.2. Methodology Implemented for Enrichment Analysis of Literature-Reported Differentially Expressed Proteins
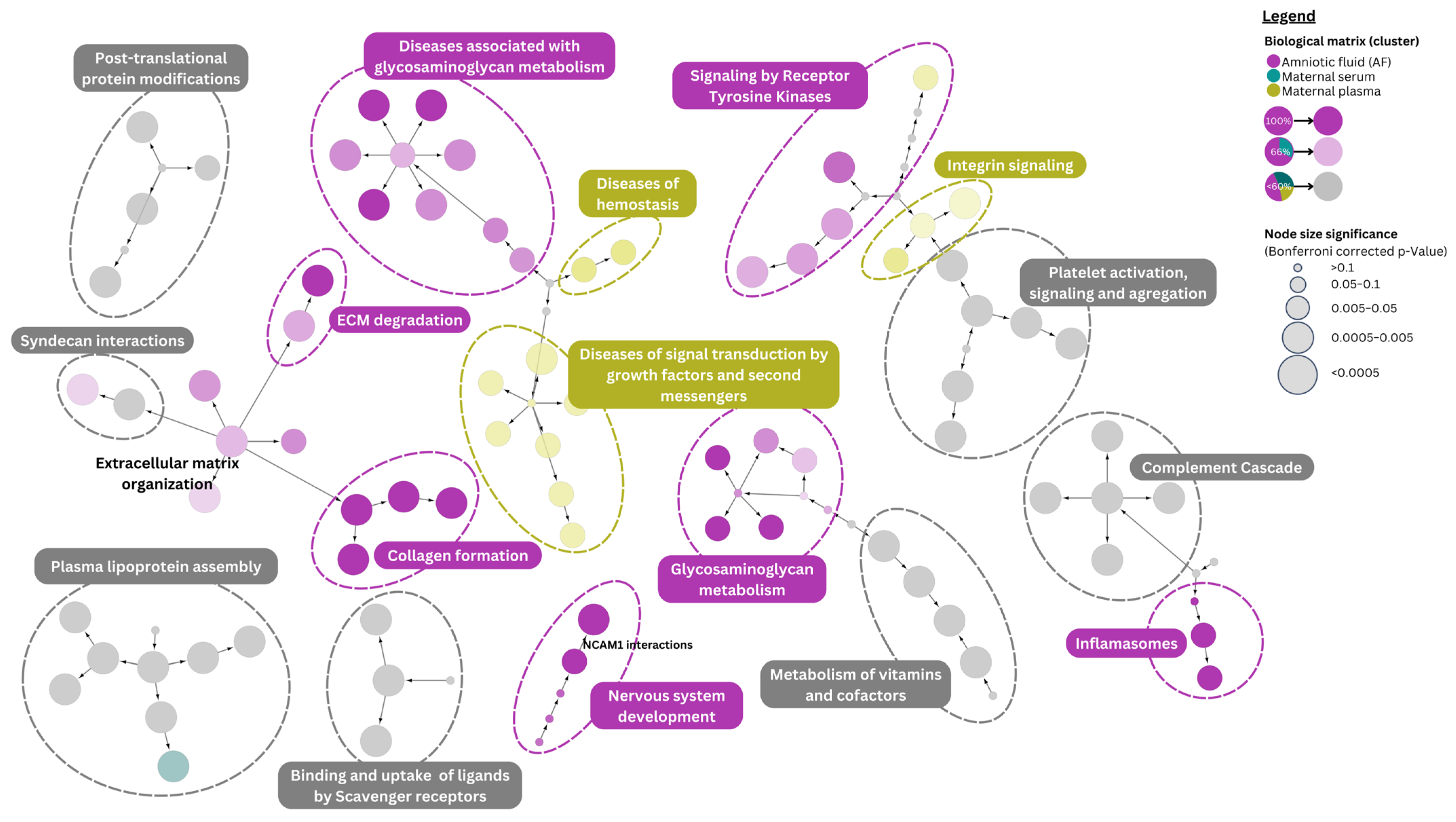
4.3. Pathway Analysis of Down Syndrome Differentially Expressed Proteins
4.4. Pathway Associations of Literature-Reported Differentially Expressed Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the Number of People with Down Syndrome in Europe. Eur. J. Hum. Genet. 2021, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- CDC. Living with Down Syndrome. Available online: https://www.cdc.gov/birth-defects/living-with-down-syndrome/index.html (accessed on 17 January 2025).
- Arumugam, A.; Raja, K.; Venugopalan, M.; Chandrasekaran, B.; Kovanur Sampath, K.; Muthusamy, H.; Shanmugam, N. Down Syndrome—A Narrative Review with a Focus on Anatomical Features. Clin. Anat. 2016, 29, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef]
- Spencer, K. Screening for Down Syndrome. Scand. J. Clin. Lab. Investig. 2014, 74, 41–47. [Google Scholar] [CrossRef]
- Wald, N.J.; Hackshaw, A.K. Advances in Antenatal Screening for Down Syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 563–580. [Google Scholar] [CrossRef]
- Benn, P.A. Advances in Prenatal Screening for Down Syndrome: II First Trimester Testing, Integrated Testing, and Future Directions. Clin. Chim. Acta 2002, 324, 1–11. [Google Scholar] [CrossRef]
- Visootsak, J.; Smith, M.M. Noninvasive Screening Tools for Down Syndrome: A Review. Int. J. Women’s Health 2013, 5, 125–131. [Google Scholar] [CrossRef]
- Yao, Y.; Liao, Y.; Han, M.; Li, S.-L.; Luo, J.; Zhang, B. Two Kinds of Common Prenatal Screening Tests for Down’s Syndrome: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 18866. [Google Scholar] [CrossRef]
- Natoli, J.L.; Ackerman, D.L.; Mcdermott, S.; Edwards, J.G. Prenatal Diagnosis of Down Syndrome: A Systematic Review of Termination Rates (1995–2011). Prenat. Diagn. 2012, 32, 142–153. [Google Scholar] [CrossRef]
- Skjøth, M.M.; Draborg, E.; Pedersen, C.D.; Hansen, H.P.; Lamont, R.F.; Jørgensen, J.S. Providing Information About Prenatal Screening for Down Syndrome: A Systematic Review. Acta Obs. Gynecol. Scand. 2015, 94, 125–132. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Trochimiuk, A.; Ławicki, S.; Krętowski, A.J.; Zbucka-Krętowska, M. Novel Approaches to an Integrated Route for Trisomy 21 Evaluation. Biomolecules 2021, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Di Mattei, V.; Ferrari, F.; Perego, G.; Tobia, V.; Mauro, F.; Candiani, M. Decision-Making Factors in Prenatal Testing: A Systematic Review. Health Psychol. Open 2021, 8, 2055102920987455. [Google Scholar] [CrossRef] [PubMed]
- Messerlian, G.M.; Farina, A.; Palomaki, G. First-Trimester Combined Test and Integrated Tests for Screening for Down Syndrome and Trisomy 18. Available online: https://www.uptodate.com/contents/first-trimester-combined-test-and-integrated-tests-for-screening-for-down-syndrome-and-trisomy-18 (accessed on 17 January 2025).
- Alfirevic, Z.; Navaratnam, K.; Mujezinovic, F. Amniocentesis and Chorionic Villus Sampling for Prenatal Diagnosis. Cochrane Database Syst. Rev. 2017, 9, 1–116. [Google Scholar] [CrossRef]
- Cuckle, H.S.; Canick, J.A.; Kellner, L.H. Collaborative Study of Maternal Urine β-Core Human Chorionic Gonadotrophin Screening for Down Syndrome. Prenat. Diagn. 1999, 19, 911–917. [Google Scholar] [CrossRef]
- Franchi, P.G.; Palka, C.; Morizio, E.; Sabbatinelli, G.; Alfonsi, M.; Fantasia, D.; Sitar, G.; Benn, P.; Calabrese, G. Sequential Combined Test, Second Trimester Maternal Serum Markers, and Circulating Fetal Cells to Select Women for Invasive Prenatal Diagnosis. PLoS ONE 2017, 12, e0189235. [Google Scholar] [CrossRef]
- Sekizawa, A.; Purwosunu, Y.; Matsuoka, R.; Koide, K.; Okazaki, S.; Farina, A.; Saito, H.; Okai, T. Recent Advances in Non-Invasive Prenatal DNA Diagnosis Through Analysis of Maternal Blood. J. Obstet. Gynaecol. Res. 2007, 33, 747–764. [Google Scholar] [CrossRef]
- Wilmot, H.C.; de Graaf, G.; van Casteren, P.; Buckley, F.; Skotko, B.G. Down Syndrome Screening and Diagnosis Practices in Europe, United States, Australia, and New Zealand from 1990–2021. Eur. J. Hum. Genet. 2023, 31, 497–503. [Google Scholar] [CrossRef]
- Liehr, T. False-Positives and False-Negatives in Non-Invasive Prenatal Testing (NIPT): What Can We Learn from a Meta-Analyses on >750,000 Tests? Mol. Cytogenet. 2022, 15, 36. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, F.; Jiang, Z.; Li, L. Improving Deep Proteome and PTMome Coverage Using Tandem HILIC-HPRP Peptide Fractionation Strategy. Anal. Bioanal. Chem. 2019, 411, 459–469. [Google Scholar] [CrossRef]
- Kota, U.; Stolowitz, M.L. Improving Proteome Coverage by Reducing Sample Complexity via Chromatography. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Gargano, A.F.G.; Roca, L.S.; Fellers, R.T.; Bocxe, M.; Domínguez-Vega, E.; Somsen, G.W. Capillary HILIC-MS: A New Tool for Sensitive Top-Down Proteomics. Anal. Chem. 2018, 90, 6601–6609. [Google Scholar] [CrossRef]
- Boersema, P.J.; Mohammed, S.; Heck, A.J.R. Hydrophilic Interaction Liquid Chromatography (HILIC) in Proteomics. Anal. Bioanal. Chem. 2008, 391, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Smith, A.J.; Astarita, G. Ion Mobility Mass Spectrometry in the Omics Era: Challenges and Opportunities for Metabolomics and Lipidomics. Mass. Spectrom. Rev. 2022, 41, 722–765. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Fountoulakis, M.; Juranville, J.; Rosner, M.; Hengstschlaeger, M.; Lubec, G. Proteomic Determination of Metabolic Enzymes of the Amnion Cell: Basis for a Possible Diagnostic Tool? Proteomics 2004, 4, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-K.J.; Drabovich, A.P.; Karagiannis, G.S.; Martínez-Morillo, E.; Dason, S.; Dimitromanolakis, A.; Diamandis, E.P. Quantitative Proteomic Analysis of Amniocytes Reveals Potentially Dysregulated Molecular Networks in Down Syndrome. Clin. Proteom. 2013, 10, 2. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhu, H.; Zhang, H.; Liu, S. Preliminary Study of Protein Changes in Trisomy 21 Fetus by Proteomics Analysis in Amniocyte. Prenat. Diagn. 2018, 38, 435–444. [Google Scholar] [CrossRef]
- Tsangaris, G.T.h.; Karamessinis, P.; Kolialexi, A.; Garbis, S.D.; Antsaklis, A.; Mavrou, A.; Fountoulakis, M. Proteomic Analysis of Amniotic Fluid in Pregnancies with Down Syndrome. Proteomics 2006, 6, 4410–4419. [Google Scholar] [CrossRef]
- Wang, T.-H.; Chao, A.-S.; Chen, J.-K.; Chao, A.; Chang, Y.-L.; Cheng, P.-J.; Chang, S.-D.; Wang, H.-S. Network Analyses of Differentially Expressed Proteins in Amniotic Fluid Supernatant Associated with Abnormal Human Karyotypes. Fertil. Steril. 2009, 92, 96–107. [Google Scholar] [CrossRef]
- Kim, Y. Comparative Proteomic Analysis of Human Amniotic Fluid Supernatants with Down Syndrome Using Mass Spectrometry. J. Microbiol. Biotechnol. 2010, 20, 959–967. [Google Scholar] [CrossRef]
- Cho, C.-K.J.; Smith, C.R.; Diamandis, E.P. Amniotic Fluid Proteome Analysis from Down Syndrome Pregnancies for Biomarker Discovery. J. Proteome Res. 2010, 9, 3574–3582. [Google Scholar] [CrossRef]
- Cho, C.-K.J.; Drabovich, A.P.; Batruch, I.; Diamandis, E.P. Verification of a Biomarker Discovery Approach for Detection of Down Syndrome in Amniotic Fluid via Multiplex Selected Reaction Monitoring (SRM) Assay. J. Proteom. 2011, 74, 2052–2059. [Google Scholar] [CrossRef]
- Perluigi, M.; di Domenico, F.; Fiorini, A.; Cocciolo, A.; Giorgi, A.; Foppoli, C.; Butterfield, D.A.; Giorlandino, M.; Giorlandino, C.; Eugenia Schininà, M.; et al. Oxidative Stress Occurs Early in Down Syndrome Pregnancy: A Redox Proteomics Analysis of Amniotic Fluid. Proteom. Clin. Appl. 2011, 5, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Morillo, E.; Cho, C.-K.J.; Drabovich, A.P.; Shaw, J.L.V.; Soosaipillai, A.; Diamandis, E.P. Development of a Multiplex Selected Reaction Monitoring Assay for Quantification of Biochemical Markers of Down Syndrome in Amniotic Fluid Samples. J. Proteome Res. 2012, 11, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.P.H.; Pennings, J.L.A.; Imholz, S.; Rodenburg, W.; Visser, G.H.A.; de Vries, A.; Schielen, P.C.J.I. Bead-Based Multiplexed Immunoassays to Identify New Biomarkers in Maternal Serum to Improve First Trimester Down Syndrome Screening. Prenat. Diagn. 2009, 29, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.P.H.; Pennings, J.L.A.; Imholz, S.; Rodenburg, W.; Visser, G.H.A.; de Vries, A.; Schielen, P.C.J.I. Proteomics and Down Syndrome Screening: A Validation Study. Prenat. Diagn. 2010, 30, 1039–1043. [Google Scholar] [CrossRef]
- Lopez, M.F.; Kuppusamy, R.; Sarracino, D.A.; Prakash, A.; Athanas, M.; Krastins, B.; Rezai, T.; Sutton, J.N.; Peterman, S.; Nicolaides, K. Mass Spectrometric Discovery and Selective Reaction Monitoring (SRM) of Putative Protein Biomarker Candidates in First Trimester Trisomy 21 Maternal Serum. J. Proteome Res. 2011, 10, 133–142. [Google Scholar] [CrossRef]
- Mastricci, A.L.; Akolekar, R.; Kuppusamy, R.; Ahmed, M.; Nicolaides, K.H. Are Serum Protein Biomarkers Derived from Proteomic Analysis Useful in Screening for Trisomy 21 at 11–13 Weeks? Fetal Diagn. Ther. 2011, 30, 53–59. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, B.; Wang, J.; Wang, Q.; Huang, R.; Yang, Y.; Shao, S. Preliminary Proteomic-Based Identification of a Novel Protein for Down’s Syndrome in Maternal Serum. Exp. Biol. Med. 2012, 237, 530–539. [Google Scholar] [CrossRef]
- Kolialexi, A.; Tsangaris, G.T.h.; Papantoniou, N.; Anagnostopoulos, A.K.; Vougas, K.; Bagiokos, V.; Antsaklis, A.; Mavrou, A. Application of Proteomics for the Identification of Differentially Expressed Protein Markers for Down Syndrome in Maternal Plasma. Prenat. Diagn. 2008, 28, 691–698. [Google Scholar] [CrossRef]
- Kolla, V.; Jenö, P.; Moes, S.; Tercanli, S.; Lapaire, O.; Choolani, M.; Hahn, S. Quantitative Proteomics Analysis of Maternal Plasma in Down Syndrome Pregnancies Using Isobaric Tagging Reagent (ITRAQ). J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Heywood, W.E.; Madgett, T.E.; Wang, D.; Wallington, A.; Hogg, J.; Mills, K.; Avent, N.D. 2D DIGE Analysis of Maternal Plasma for Potential Biomarkers of Down Syndrome. Proteome Sci. 2011, 9, 56. [Google Scholar] [CrossRef]
- Heywood, W.; Wang, D.; Madgett, T.E.; Avent, N.D.; Eaton, S.; Chitty, L.S.; Mills, K. The Development of a Peptide SRM-Based Tandem Mass Spectrometry Assay for Prenatal Screening of Down Syndrome. J. Proteom. 2012, 75, 3248–3257. [Google Scholar] [CrossRef] [PubMed]
- Heywood, W.; Mills, K.; Wang, D.; Hogg, J.; Madgett, T.E.; Avent, N.D.; SChitty, L. Identification of New Biomarkers for Down’s Syndrome in Maternal Plasma. J. Proteom. 2012, 75, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Zhang, R.; Chen, J.; He, H.; Cui, Z.; Ou, M.; Li, W.; Qi, S.; Wen, J.; Lin, X.; et al. Quantitative Proteomic Analysis of Down Syndrome in the Umbilical Cord Blood Using ITRAQ. Mol. Med. Rep. 2015, 11, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.J.; Yan, L.Y.; Wang, W.; Yu, S.; Wang, X.; Zhang, W.Y. Proteomic analysis of the alteration of protein expression in the placenta of Down syndrome. Chin. Med. J. 2011, 124, 3738–3745. [Google Scholar] [CrossRef]
- Baggot, P.J.; Eliseo, A.J.Y.; DeNicola, N.G.; Kalamarides, J.A.; Shoemaker, J.D. Organic Acid Concentrations in Amniotic Fluid Found in Normal and Down Syndrome Pregnancies. Fetal Diagn. Ther. 2008, 23, 245–248. [Google Scholar] [CrossRef]
- Huang, J.; Mo, J.; Zhao, G.; Lin, Q.; Wei, G.; Deng, W.; Chen, D.; Yu, B. Application of the Amniotic Fluid Metabolome to the Study of Fetal Malformations, Using Down Syndrome as a Specific Model. Mol. Med. Rep. 2017, 16, 7405–7415. [Google Scholar] [CrossRef]
- Liu, X.; Quan, S.; Fu, Y.; Wang, W.; Zhang, W.; Wang, X.; Zhang, C.; Xiang, D.; Zhang, L.; Wang, C. Study on Amniotic Fluid Metabolism in the Second Trimester of Trisomy 21. J. Clin. Lab. Anal. 2020, 34, e23089. [Google Scholar] [CrossRef]
- Chen, X.; Hu, L.; Su, J.; Liu, X.; Luo, X.; Pei, Y.; Gao, Y.; Wei, F. Amniotic Fluid and Urine Metabolomic Alterations Associated with Pregnant Women with Down Syndrome Fetuses. J. Matern. Neonatal Med. 2022, 35, 7882–7889. [Google Scholar] [CrossRef]
- Parfieniuk, E.; Pietrowska, K.; Samczuk, P.; Kretowski, A.; Ciborowski, M.; Zbucka-Kretowska, M. Amniotic Fluid Metabolic Fingerprinting Indicated Metabolites Which May Play a Role in the Pathogenesis of Foetal Down Syndrome—A Preliminary Report. Ginekol. Pol. 2021, 92, 188–194. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Akolekar, R.; Mandal, R.; Dong, E.; Xia, J.; Kruger, M.; Wishart, D.S.; Nicolaides, K. Metabolomic Analysis for First-Trimester Down Syndrome Prediction. Am. J. Obs. Gynecol. 2013, 208, 371.e1–371.e8. [Google Scholar] [CrossRef]
- Parfieniuk, E.; Samczuk, P.; Kowalczyk, T.; Pietrowska, K.; Niemira, M.; Paczkowska-Abdulsalam, M.; Wolczynski, S.; Kretowski, A.; Ciborowski, M.; Zbucka-Kretowska, M. Maternal Plasma Metabolic Fingerprint Indicative for Fetal Down Syndrome. Prenat. Diagn. 2018, 38, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Nemutlu, E.; Orgul, G.; Recber, T.; Aydin, E.; Ozkan, E.; Turgal, M.; Alikasifoglu, M.; Kir, S.; Beksac, M.S. Metabolic Infrastructure of Pregnant Women with Trisomy 21 Fetuses; Metabolomic Analysis. Z. Geburtshilfe Neonatol. 2019, 223, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Özkan, E.; Nemutlu, E.; Beksac, M.S.; Kır, S. GC–MS Analysis of Seven Metabolites for the Screening of Pregnant Women with Down Syndrome Fetuses. J. Pharm. Biomed. Anal. 2020, 188, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reçber, T.; Özkan, E.; Nemutlu, E.; Beksac, M.S.; Kir, S. Analysis of 3-Hydroxyisovaleric Acid and 3-Hydroxybutyric Acid in Plasma Samples by LC-MS/MS. J. Res. Pharm. 2022, 26, 136–144. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Iles, R.K. Shotgun Metabolomic Profiles in Maternal Urine Identify Potential Mass Spectral Markers of Abnormal Fetal Biochemistry—Dihydrouracil and Progesterone in the Metabolism of Down Syndrome. Biomed. Chromatogr. 2015, 29, 1173–1183. [Google Scholar] [CrossRef]
- Cho, C.-K.J.; Shan, S.J.; Winsor, E.J.; Diamandis, E.P. Proteomics Analysis of Human Amniotic Fluid. Mol. Cell. Proteom. 2007, 6, 1406–1415. [Google Scholar] [CrossRef]
- Busch, A.; Michel, S.; Hoppe, C.; Driesch, D.; Claussen, U.; von Eggeling, F. Proteome Analysis of Maternal Serum Samples for Trisomy 21 Pregnancies Using ProteinChip Arrays and Bioinformatics. J. Histochem. Cytochem. 2005, 53, 341–343. [Google Scholar] [CrossRef]
- Kang, Y.; Dong, X.; Zhou, Q.; Zhang, Y.; Cheng, Y.; Hu, R.; Su, C.; Jin, H.; Liu, X.; Ma, D.; et al. Identification of Novel Candidate Maternal Serum Protein Markers for Down Syndrome by Integrated Proteomic and Bioinformatic Analysis. Prenat. Diagn. 2012, 32, 284–292. [Google Scholar] [CrossRef]
- Narasimhan, K.; Lin, S.L.; Tong, T.; Baig, S.; Ho, S.; Sukumar, P.; Biswas, A.; Hahn, S.; Bajic, V.B.; Choolani, M. Maternal Serum Protein Profile and Immune Response Protein Subunits as Markers for Non-Invasive Prenatal Diagnosis of Trisomy 21, 18, and 13. Prenat. Diagn. 2013, 33, 223–231. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, Y.; Han, M.; Xia, Y.; He, Y.; Wang, Y.; Luo, Y.; Zhang, B. Screening and Identification of Potential Predictive Biomarkers for Down’s Syndrome from Second Trimester Maternal Serum. Expert. Rev. Proteom. 2015, 12, 97–107. [Google Scholar] [CrossRef]
- Uriarte, G.A.L.; Flores, C.H.B.; De La Cruz, V.M.T.; Aguado, M.M.M.; Puente, V.M.G.; Gutiérrez, L.N.R.; De Villarreal, L.E.M. Proteomic Profile of Serum of Pregnant Women Carring a Fetus with Down Syndrome Using Nano Uplc Q-Tof Ms/Ms Technology. J. Matern.-Fetal Neonatal Med. 2018, 31, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Wang, H.; Khatri, P.; Niu, Y.; Song, W.; Zhao, S.; Jiang, Y.; Ma, Q.; Liu, X.; Zhang, R.; et al. The Urinary Peptidome as a Noninvasive Biomarker Development Strategy for Prenatal Screening of Down’s Syndrome. OMICS A J. Integr. Biol. 2019, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Iles, R.K.; Shahpari, M.E.; Cuckle, H.; Butler, S.A. Direct and Rapid Mass Spectral Fingerprinting of Maternal Urine for the Detection of Down Syndrome Pregnancy. Clin. Proteom. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, Glycine and One-Carbon Units: Cancer Metabolism in Full Circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Zhang, Q.; Ford, L.A.; Goodman, K.D.; Freed, T.A.; Hauser, D.M.; Conner, J.K.; Vroom, K.E.T.; Toal, D.R. LC-MS/MS Method for Quantitation of Seven Biomarkers in Human Plasma for the Assessment of Insulin Resistance and Impaired Glucose Tolerance. J. Chromatogr. Anal. Technol. Biomed. Life Sci. 2016, 1038, 101–108. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Iles, R.K. HILIC-MS-Based Shotgun Metabolomic Profiling of Maternal Urine at 9–23 Weeks of Gestation—Establishing the Baseline Changes in the Maternal Metabolome. Biomed. Chromatogr. 2015, 29, 240–245. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and in Silico Data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Adesina, A.; Ahmad, S.; Bowler-Barnett, E.H.; Bye-A-Jee, H.; Carpentier, D.; Denny, P.; et al. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, 609–617. [Google Scholar] [CrossRef]
- Kinjo, T.; Inoue, H.; Kusuda, T.; Fujiyoshi, J.; Ochiai, M.; Takahata, Y.; Honjo, S.; Koga, Y.; Hara, T.; Ohga, S. Chemokine Levels Predict Progressive Liver Disease in Down Syndrome Patients with Transient Abnormal Myelopoiesis. Pediatr. Neonatol. 2019, 60, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sui, W.; Li, W.; Tan, Q.; Chen, J.; Lin, X.; Guo, H.; Ou, M.; Xue, W.; Zhang, R.; et al. Integrated MicroRNA and Protein Expression Analysis Reveals Novel MicroRNA Regulation of Targets in Fetal down Syndrome. Mol. Med. Rep. 2016, 14, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Damar, İ.H.; Eröz, R.; Kiliçaslan, Ö. Frequency of Hereditary Prothrombotic Risk Factors in Patients with Down Syndrome. Konuralp Med. J. 2021, 13, 89–93. [Google Scholar] [CrossRef]
- Kurokami, T.; Takasawa, R.; Takeda, S.; Kurobe, M.; Takasawa, K.; Nishioka, M.; Shimohira, M. Venous Thromboembolism in Two Adolescents with Down Syndrome. Turk. J. Pediatr. 2018, 60, 429–432. [Google Scholar] [CrossRef]
- Nadeau, V.; Charron, J. Essential Role of the ERK/MAPK Pathway in Blood-Placental Barrier Formation. Development 2014, 141, 2825–2837. [Google Scholar] [CrossRef]
- Huang, R.; Yuan, D.-J.; Li, S.; Liang, X.-S.; Gao, Y.; Lan, X.-Y.; Qin, H.-M.; Ma, Y.-F.; Xu, G.-Y.; Schachner, M.; et al. NCAM Regulates Temporal Specification of Neural Progenitor Cells via Profilin2 During Corticogenesis. J. Cell Biol. 2020, 219, e201902164. [Google Scholar] [CrossRef]
- Ma, Z.; Deng, C.; Hu, W.; Zhou, J.; Fan, C.; Di, S.; Liu, D.; Yang, Y.; Wang, D. Liver X Receptors and Their Agonists: Targeting for Cholesterol Homeostasis and Cardiovascular Diseases. Curr. Issues Mol. Biol. 2017, 22, 41–64. [Google Scholar] [CrossRef]
- Mollo, N.; Aurilia, M.; Scognamiglio, R.; Zerillo, L.; Cicatiello, R.; Bonfiglio, F.; Pagano, P.; Paladino, S.; Conti, A.; Nitsch, L.; et al. Overexpression of the Hsa21 Transcription Factor RUNX1 Modulates the Extracellular Matrix in Trisomy 21 Cells. Front. Genet. 2022, 13, 824922. [Google Scholar] [CrossRef]
- Mollo, N.; Scognamiglio, R.; Conti, A.; Paladino, S.; Nitsch, L.; Izzo, A. Genetics and Molecular Basis of Congenital Heart Defects in Down Syndrome: Role of Extracellular Matrix Regulation. Int. J. Mol. Sci. 2023, 24, 2918. [Google Scholar] [CrossRef]
- Gittenberger-De Groot, A.C.; Bartram, U.; Oosthoek, P.W.; Bartelings, M.M.; Hogers, B.; Poelmann, R.E.; Jongewaard, I.N.; Klewer, S.E. Collagen Type VI Expression During Cardiac Development and in Human Fetuses with Trisomy 21. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bhowmik, K.; Chatterjee, A.; Chakrabarty, P.B.; Sinha, S.; Mukhopadhyay, K. Down Syndrome Related Muscle Hypotonia: Association with COL6A3 Functional SNP Rs2270669. Front. Genet. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Quarello, E.; Guimiot, F.; Moalic, J.M.; Simoneau, M.; Ville, Y.; Delezoide, A.L. Quantitative Evaluation of Collagen Type VI and SOD Gene Expression in the Nuchal Skin of Human Fetuses with Trisomy 21. Prenat. Diagn. 2007, 27, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Annus, T.; Wilson, L.R.; Hong, Y.T.; Acosta-Cabronero, J.; Fryer, T.D.; Cardenas-Blanco, A.; Smith, R.; Boros, I.; Coles, J.P.; Aigbirhio, F.I.; et al. The Pattern of Amyloid Accumulation in the Brains of Adults with Down Syndrome. Alzheimer’s Dement. 2016, 12, 538–545. [Google Scholar] [CrossRef]
- Zammit, M.D.; Laymon, C.M.; Betthauser, T.J.; Cody, K.A.; Tudorascu, D.L.; Minhas, D.S.; Sabbagh, M.N.; Johnson, S.C.; Zaman, S.H.; Mathis, C.A.; et al. Amyloid Accumulation in Down Syndrome Measured with Amyloid Load. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12020. [Google Scholar] [CrossRef]
- Head, E.; Helman, A.M.; Powell, D.; Schmitt, F.A. Down Syndrome, Beta-Amyloid and Neuroimaging. Free Radic. Biol. Med. 2017, 114, 102. [Google Scholar] [CrossRef]
- Busciglio, J.; Pelsman, A.; Wong, C.; Pigino, G.; Yuan, M.; Mori, H.; Yankner, B.A. Altered Metabolism of the Amyloid β Precursor Protein Is Associated with Mitochondrial Dysfunction in Down’s Syndrome. Neuron 2002, 33, 677–688. [Google Scholar] [CrossRef]
- Lai, G.; Zhang, Y.; Wang, L.; Zhang, B.; Zheng, G.; Wang, X.; Zhao, X.; Gao, H.; Zhao, Y. Differences in IGF Axis-related Proteins in Amniotic Fluid of Trisomy 21 and Trisomy 18 Using a Multiple Reaction Monitoring Approach. Prenat. Diagn. 2014, 34, 1146–1152. [Google Scholar] [CrossRef]
- Araya, P.; Kinning, K.T.; Coughlan, C.; Smith, K.P.; Granrath, R.E.; Enriquez-Estrada, B.A.; Worek, K.; Sullivan, K.D.; Rachubinski, A.L.; Wolter-Warmerdam, K.; et al. IGF1 Deficiency Integrates Stunted Growth and Neurodegeneration in Down Syndrome. Cell Rep. 2022, 41, 111883. [Google Scholar] [CrossRef]
- Shaki, D.; Hershkovitz, E.; Tamam, S.; Bollotin, A.; David, O.; Yalovitsky, G.; Loewenthal, N.; Carmon, L.; Walker, D.; Nowak, R.; et al. GH Treatment in Pediatric Down Syndrome: A Systematic Review and Mini Meta-Analysis. Front. Endocrinol. 2023, 14, 1135768. [Google Scholar] [CrossRef]
- Veteleanu, A.; Pape, S.; Davies, K.; Kodosaki, E.; Hye, A.; Zelek, W.M.; Strydom, A.; Morgan, B.P. Complement Dysregulation and Alzheimer’s Disease in Down Syndrome. Alzheimer’s Dement. 2023, 19, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Doherty, D.G.; Molloy, E.J. Immune Dysregulation in Children with Down Syndrome. Front. Pediatr. 2020, 8, 73. [Google Scholar] [CrossRef]
- Huggard, D.; Kelly, L.; Ryan, E.; McGrane, F.; Lagan, N.; Roche, E.; Balfe, J.; Leahy, T.R.; Franklin, O.; Doherty, D.G.; et al. Increased Systemic Inflammation in Children with Down Syndrome. Cytokine 2020, 127, 154938. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Ławicki, S.; Krętowski, A.; Zbucka-Krętowska, M. The Significance of Apolipoprotein E Measurement in the Screening of Fetal Down Syndrome. J. Clin. Med. 2020, 9, 3995. [Google Scholar] [CrossRef]
- Bhaumik, P.; Ghosh, P.; Ghosh, S.; Feingold, E.; Ozbek, U.; Sarkar, B.; Dey, S.K. Combined Association of Presenilin-1 and Apolipoprotein E Polymorphisms with Maternal Meiosis II Error in Down Syndrome Births. Genet. Mol. Biol. 2017, 40, 577–585. [Google Scholar] [CrossRef]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of Retinoid Metabolism and Function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef]
- Ferraz, I.S.; Vieira, D.M.C.; Ciampo, L.A.D.e.l.; Ued Fda, V.; Almeida, A.C.F.; Jordão, A.A.; Aragon, D.C.; Martinez, E.Z.; Martinelli, C.E.; Nogueira-de-Almeida, C.A. Vitamin A Deficiency and Association between Serum Retinol and IGF-1 Concentrations in Brazilian Children with Down Syndrome. J. Pediatr. 2022, 98, 76–83. [Google Scholar] [CrossRef]
- Goodman, A.B.; Pardee, A.B. Evidence for Defective Retinoid Transport and Function in Late Onset Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2901–2905. [Google Scholar] [CrossRef]
- Pasmatzi, E.; Vlastos, D.; Monastirli, A.; Stephanou, G.; Georgious, S.; Sakkis, T.; Tsambaos, D. Ehlers-Danlos Type IV Syndrome in a Patient with Down Syndrome: Simple Co-Occurrence or True Association? Am. J. Med. Sci. 2006, 331, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.P.; Siret, A.; Ignacimouttou, C.; Panchal, K.; Diop, M.; Jenni, S.; Tsai, Y.C.; Roos-Weil, D.; Aid, Z.; Prade, N.; et al. Constitutive Activation of RAS/MAPK Pathway Cooperates with Trisomy 21 and Is Therapeutically Exploitable in down Syndrome b-Cell Leukemia. Clin. Cancer Res. 2020, 26, 3307–3318. [Google Scholar] [CrossRef]
- Swatton, J.E.; Sellers, L.A.; Faull, R.L.M.; Holland, A.; Iritani, S.; Bahn, S. Increased MAP Kinase Activity in Alzheimer’s and Down Syndrome but Not in Schizophrenia Human Brain. Eur. J. Neurosci. 2004, 19, 2711–2719. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, 622–631. [Google Scholar] [CrossRef]
| Biological Matrix Studied | Study Design | Methods Implemented | Main Results (in T21) | Ref. |
|---|---|---|---|---|
| Proteomics studies | ||||
| Amniotic fluid cells (amniocytes) | T21 = 3; Control = 4 [16–18 gestational weeks] | In vitro culturing of amnion cells 2-DE + MALDI-MS analysis | 99 proteins identified; aberrant expression linked to purine, carbohydrate, intermediary, and amino acid metabolism in amnion cells from T21 patients. | [26] |
| T21 = 3; Control = 5 [15–21 gestational weeks] | SCX fractionation via 2D-LC-MS/MS RP-nanoLC-Orbitrap-MS Protein quantification via RP-nanoLC-TQ-MS (SRM) | Over 4900 proteins identified from primary amniocytes (proteomic discovery), with at least 900 dysregulated in T21 (quantitative analysis). Dysregulated proteins in T21 were linked to cell morphology, hematological development, immune response, lipid metabolism, cardiovascular disease, genetic and metabolic disorders, protein degradation, embryonic development, cancer, neurological diseases, and tissue development. | [27] | |
| T21 = 18; Control = 20 [18–22 gestational weeks] | 2-DE + MALDI-TOF-MS Western Blot (WB) analysis | Six proteins were significantly upregulated in T21 amniocytes: calumenin, nucleophosmin, elongation factor 1-beta, cathepsin D, platelet-activating factor acetylhydrolase IB subunit beta, and 14-3-3 protein beta/alpha. Western Blot (WB) analysis confirmed alterations in nucleophosmin and cathepsin D. | [28] | |
| Amniotic fluid supernatants (AFS) | T21 = 6; Control = 12 [17 gestational weeks] | 2-DE + MALDI-MS analysis Nano-ESI-MS/MS Western blot (WB) | Seven proteins were differentially expressed in pregnancies with T21 fetuses. Five of these proteins were upregulated in T21 cases, SFRS-4 was detected only in T21, and a 40% decrease in IBP-1 concentration was observed in amniotic fluid (AFS) from T21 cases. | [29] |
| T21 = 19; T18 = 17; Control = 34 [15–20 gestational weeks] | 2D chromatography separation and fraction selection In-gel and in-solution digestion + MALDI-TOF-MS analysis Western blot (WB) analysis | Proteins with significant differential expressions in T21 included apolipoprotein A1, antitrypsin, prealbumin (transthyretin), and transferrin. Apolipoprotein A1 levels were significantly decreased in amniotic fluid (AFS) of both T18 and T21, while antitrypsin, transferrin, and prealbumin levels were increased in T21 AFS. Functional network analysis linked dysfunction of cholesterol metabolism to T21. | [30] | |
| T21 = 4; Control = 6 [15–18 gestational weeks] | Affinity chromatography to remove albumin and immunoglobulin G In-gel digestion + LC-ESI-MS/MS analysis Western blot (WB) analysis | Forty-four AFS proteins were differentially expressed between T21 and normal cases, with six unique to T21. Western blot (WB) analysis confirmed apolipoprotein A-II (apo-II) and alpha-fetoprotein (AFP) as potential diagnostic tools for T21. | [31] | |
| T21 = 10; Control = 15 [16–20 gestational weeks] | Immunoglobulin depletion + 2D-LC fractionation followed by MS/MS (LTQ-Orbitrap MS) ELISA for candidate biomarkers on maternal serum samples | Sixty proteins showed greater than 2-fold changes in T21. Top pathways for decreased proteins in T21 were associated with organ morphology and reproductive system development and function. Amyloid precursor protein and tenascin-C were evaluated via ELISA in serum samples, showing increased levels in T21 cases. | [32] | |
| T21 = 9 Control = 9 [15–17 gestational weeks] | 2-DE Western blot (WB) analysis LC-MS/MS for protein identification | Proteins involved in iron homeostasis (ceruloplasmin and transferrin), lipid metabolism (zinc-alpha-2-glycoprotein, retinol-binding protein 4, and apolipoprotein A1), and inflammation (complement C9, α-1B-glycoprotein, collagen α-1V chain) were identified as critically relevant to the clinical outcome of T21. | [33] | |
| T21 = 10; Control = 10 [16–20 gestational weeks] | SRM assay developed to test thirteen previously identified candidate proteins in amniotic fluid ELISA for candidate biomarkers | Bile-salt-activated lipase, mucin-13, carboxypeptidase A1, and dipeptidyl peptidase 4 showed decreased levels in amniotic fluid of T21 cases, while matrix metalloproteinase-2 levels were significantly increased. In serum samples, matrix metalloproteinase-2 levels showed no significant difference between control and T21 groups. | [34] | |
| T21 = 17; Control = 37 [15–22 gestational weeks] | LC-SRM-MS | Five proteins (bile salt-activated lipase, carboxypeptidase A1, mucin-13, chloride channel accessory 1, and mucin-5AC) were significantly downregulated in T21 cases, while one protein (hyaluronan and proteoglycan link protein 1) was upregulated. | [35] | |
| Maternal serum | T21 = 14; Control = 15 [8–13 gestational weeks] | Bead-based multiplexed immunoassays | Seven potential biomarkers were selected for further analysis: alpha fetoprotein, epidermal growth factor, extracellular rage binding protein, eotaxin, haptoglobin, insulin, and lipoprotein A. None of these biomarkers were fully discriminatory between T21 cases and controls. | [36] |
| T21 = 27; Control = 27 [12 gestational weeks] | Bead-based multiplexed immunoassays | Prediction values were obtained for current screening markers (pregnancy-associated plasma protein A, free beta human chorionic gonadotrophin, and nuchal translucency) and seven previously identified markers based on concentration ratios between T21 and controls. Validation of these biomarkers confirmed epidermal growth factor for further consideration as a T21 screening marker. | [37] | |
| T21 = 24; Control = 21 [first trimester pregnancies] | LC-MS/MS; multiplexed SRM assay | Over 300 proteins were identified, with 12 selected for further development into multiplexed SRM assays. IPA analysis revealed that differentially expressed proteins are implicated in humoral immune response, cardiovascular system development, cellular growth and proliferation, and lipid metabolism. | [38] | |
| T21 = 50; Control = 25 [11–13 gestational week] | ELISA | In pregnancies with fetal T21, maternal age, fetal nuchal translucency thickness, and serum free beta human chorionic gonadotrophin were increased, while serum pregnancy-associated plasma protein A was decreased. No significant differences were found between T21 cases and controls in any of the biomarkers. | [39] | |
| T21 = 6; Control = 6 [16–19 gestational weeks] | 2-DE and MALDI-MS; ELISA for candidate biomarkers | Twenty-nine proteins were identified in maternal serum from pregnancies with T21-affected fetuses. These proteins were involved in biological regulation, metabolic processes, cellular processes, and response to stimulus. Ceruloplasmin and complement factor B expression were confirmed using ELISA. | [40] | |
| Maternal plasma | T21 = 8; Control = 12 [16–18 gestational weeks] | 2-DE + MALDI-TOF-MS Western blot (WB) confirmation | Nine DEPs were identified in maternal plasma of women with T21 fetuses, associated with fetal growth and development: transthyretin, ceruloplasmin, afamin, alpha-1-microglobulin, apolipoprotein E, serum amyloid P-component, histidine-rich glycoprotein, and alpha-1-antitrypsin (upregulated), and clusterin (downregulated). Apolipoprotein E and serum amyloid P-component levels were confirmed via WB analysis. | [41] |
| T21 = 6; Control = 6 [11–14 gestational weeks] | Immunodepletion of high-abundance plasma proteins SCX fractionation + Nano-LC MALDI-MS analysis | A total of 178 proteins were quantified. Twenty-eight proteins were upregulated in T21, linked to signaling and immunity, while 22 were downregulated, related to cell adhesion and the extracellular matrix. Panther analysis showed 13.3% of proteins in T21 samples are involved in Alzheimer’s disease pathways, and over 40% are linked to integrin signaling. | [42] | |
| T21 = 14; Control = 14 [10–14 gestational weeks] | 2D DIGE + MALDI-TOF-MS ESI Q-TOF MS/MS Western blot (WB) analysis | No DEPs were observed in the first trimester. In the second trimester, increased levels of ceruloplasmin, inter-alpha-trypsin inhibitor heavy chain H4, complement proteins (C1s subcomponent, C4-A, C5, C9), and kininogen 1 were detected in T21 maternal plasma. Ceruloplasmin expression in maternal plasma was confirmed via WB. | [43] | |
| T21 = 19; Control = 19 [10–20 gestational weeks] | SRM assay development for the quantification of two biomarkers | Significant differences in maternal plasma levels of serum amyloid-P and C1-inhibitor were observed between T21-affected and high-risk normal pregnancies in both the first and second trimesters. | [44] | |
| T21 = 28; Control = 53 [10–20 gestational weeks] | SELDI-TOF analysis ProteinChip® Q spin columns SDS-PAGE nano-LC-QTOF-MS analysis Western blot (WB) analysis | Plasma C1-inhibitor was significantly elevated in T21 vs. control (10–14 weeks) via SELDI-TOF MS analysis. Transthyretin, serum amyloid P, and complement C3 showed statistically significant changes in T21 vs. control (14–20 weeks). | [45] | |
| Umbilical cord blood | T21 = 6; Control = 11 | iTRAQ, SCX fractionation + MALDI TOF/TOF | A total of 505 proteins were identified, with 13 upregulated and 6 downregulated in T21. Apolipoprotein E, complement factor B, amyloid P component, matrin-3, and osteopontin were found to be relevant to T21, with the first three notably upregulated. The panel was proposed as potential T21 biomarkers. | [46] |
| Placenta | T21 = 19; Control = 17 [18–24 gestational weeks] | 2D-DIGE + MALDI TOF/TOF MS analysis | Annexin A2, endoplasmic reticulum protein, copper-zinc superoxide dismutase, proteasome subunit alpha type-2, heat shock protein beta-1, peptidyl-prolyl cis-trans isomerase, and fibrinogen beta chain were upregulated in T21 placenta. Copper-zinc superoxide dismutase, endoplasmic reticulum protein, and heat shock protein beta-1 were linked to reactive oxygen species damage resistance and neurogenesis. Peroxiredoxin-6, enoyl-CoA hydratase, and protein disulfide isomerase A3 were downregulated. | [47] |
| Metabolomics studies | ||||
| Amniotic fluid supernatants (AFS) | T21 = 22; Control = 41 [15–17 gestational weeks] | GC-MS analysis | 28 organic acid metabolites were quantified via GC-MS in T21 vs. control samples. Increased markers of riboflavin deficiency (5-hydroxycaproate, methylsuccinate, α-ketoglutarate, adipate) were found in T21 AFS. Elevated phenylpyruvate levels in T21 indicated involvement in neurotransmitter metabolism. | [48] |
| discovery set: T21 = 10; Control = 10; validation set: T21 = 15; Control = 15; [17 gestational weeks] | LC-HRMS (Q-TOF) in four conditions (HILIC and RP, positive and negative) Metabolites validated using standards | Notable alterations in the metabolites of coproporphyrin III, pregnenolone sulfate, taurochenodeoxycholate, L-arginine, taurocholate, hydrocortisone, L-histidine, glycocholic acid, L-glutamate, and L-glutamine. The primary pathway modifications in T21 fetuses were related to amino acid metabolism, bile secretion, neuroactive ligand-receptor interactions, and galactose metabolism. | [49] | |
| T21 = 21; Control = 21; [18 gestational weeks] | UPLC-MS/MS (Orbitrap) in four conditions (HILIC and RP, positive and negative) | Key metabolites associated with four primary metabolic pathways were identified as significant in differentiating T21: gamma-glutamyl amino acids, steroid hormone derivatives, polyamines (notably N1, N12-diacetylspermine), and glycerol derivatives from phospholipid breakdown. In T21 cases, steroid hormone and gamma-glutamyl amino acid levels were generally decreased, whereas N1, N12-diacetylspermine, and phospholipid derivatives were elevated. | [50] | |
| T21 = 20; Control = 20; [17–24 gestational weeks] | 2D LC-MS/MS analysis | Significant alterations were observed in metabolites, particularly lipid molecules, organic acids, and nucleotides. These changes were associated with pathways related to energy metabolism, amino acid metabolism, organic acid metabolism, and steroid hormone synthesis. | [51] | |
| T21 = 13; Control = 13; [15–17 gestational weeks] | UHPLC-MS (Q-TOF)—RP-C18—in positive and negative modes | An increase in diacetylspermine levels was observed in T21, alongside a significant decrease in p-cresol sulfate, methylhistidine, and hexanoylcarnitine. | [52] | |
| Maternal serum | T21 = 30; Control = 60; [11–13 gestational weeks] | NMR spectroscopy | VIP analysis identified 3-hydroxybutyrate, 3-hydroxyisovalerate, and 2-hydroxybutyrate as the most effective metabolites for distinguishing T21 cases from controls. These metabolites are linked to oxidative stress, impaired myelination, and neurotoxicity in T21 individuals. | [53] |
| Maternal plasma | T21 = 12; Control = 15; [15–18 gestational weeks] | UHPLC-Q-TOF-MS, with ESI | The concentrations of five fatty acid amide metabolites were significantly reduced in the plasma of pregnancies with T21. Most of these metabolites were linked to fetal brain and central nervous system development. The study suggests these metabolites as potential new markers for non-invasive prenatal diagnosis of fetal T21. | [54] |
| T21 = 21; Control = 32 [17–19.4 gestational weeks] | GC-MS and LC-Q-TOF-MS | Two complementary MS-driven techniques were used to profile the metabolites in T21 and normal maternal plasma samples. Elevated levels of 3-hydroxybutyric acid and 2-ketoisocaproic acid were observed in the T21 group, while beta-alanine, threonic acid, oxalic acid, alpha-tocopherol, uracil, 2-piperidone, and creatinine showed reduced levels. The study also revealed a decrease in lipid-related metabolites in women carrying T21 fetuses. | [55] | |
| T21 = 21; Control = 32 [11–15 gestational weeks] | GC-MS | Metabolites, such as 2-hydroxybutyric acid, 3-hydroxybutyric acid, β-hydroxyisovaleric acid, uracil, glutamic acid, maltose, and melezitose, were identified as potential biomarkers for prenatal T21 screening. | [56] | |
| T21 = 17; Control = 30 [gestational age not mentioned] | LC-MS/MS | An LC-MS/MS technique was developed to analyze two potential biomarkers: 3-hydroxybutyric acid and 3-hydroxyisovaleric acid. Increased levels of both biomarkers were found in T21 pregnancies. | [57] | |
| Maternal urine | T21 = 23; non-T21 aneuploidies= 6; Control = 93; [11–17 gestational weeks] | ZIC-HILIC and RPLC coupled to hybrid ion trap- TOF MS | Two stationary phases were tested for urinary metabolome coverage, with ZIC-HILIC-MS outperforming RPLC-MS. Significant alterations in maternal urinary concentrations of progesterone and dihydrouracil were linked to the presence of a T21 fetus. A screening test based on metabolomics profiling successfully identified approximately 87% of T21 pregnancies between 9 and 23 weeks of gestation using HILIC-MS, with no false positives reported. | [58] |
| T21 = 20; Control = 20; [17–24 gestational weeks] | 2D LC-MS/MS analyses | The study aimed to explore metabolomic changes in the amniotic fluid and urine of pregnant women carrying fetuses with T21. The analysis revealed a significantly lower number of differential metabolites in urine compared to amniotic fluid, with phenylalanine and glycerophospholipid metabolism pathways being enriched among the urine metabolites. | [51] | |
| Pathway Group | Pathway/Process | Implications in T21 |
|---|---|---|
| Signal transduction pathways | Signaling by platelet-derived growth factor (PDGF) | Direct evidence: no Normal function: stimulates growth and motility of connective tissue cells, neurons, and capillary endothelial cells. Key findings related to T21: linked to transient abnormal myelopoiesis in T21 newborns, which can progress to hepatic necrosis and failure. Elevated PDGF expression observed in megakaryoblasts of T21 patients with hepatic disease. |
| Signaling by MET | [Through integrins interactions e.g., PTK2] Direct evidence: no Normal function: plays a key role in promoting cell motility and migration, particularly in neuronal and connective tissue processes. Key findings related to T21: altered PTK2 expression in T21 has been documented, particularly in nervous system and connective tissue processes. | |
| Integrin signaling | Direct Evidence: yes Normal function: regulates key cellular processes, such as proliferation, differentiation, and migration, essential for embryonic and placental development. Key findings related to T21: disruptions in integrin–MAPK signaling may contribute to placental insufficiency and associated complications. | |
| Neural cell adhesion molecule (NCAM) signaling for neurite out-growth | Direct evidence: no Normal function: essential for nervous system development and synaptic plasticity. Key findings related to T21: disruptions in NCAM signaling pathways may be linked to neurodevelopmental delays in T21. | |
| Regulation of NR1H2 and NR1H3 | Direct evidence: no Normal function: regulates cholesterol efflux, lipid metabolism, and anti-inflammatory responses. Protects against atherosclerosis and neurodegeneration. Key findings related to T21: altered cholesterol transport and efflux in T21 may contribute to cardiovascular dysfunction and neurodegeneration. | |
| Hemostasis | Platelet activation, signaling, and clotting cascade | Direct evidence: yes Normal function: involved in blood coagulation, wound healing, and maintaining hemostasis. Key findings related to T21: elevated thrombosis risk in T21 patients, suggesting a prothrombotic state. |
| Extracellular matrix (ECM) organization | Collagen formation, collagen degradation, ECM proteoglycans | Direct evidence: yes Normal function: ECM is crucial for structural support, tissue integrity, and signaling processes. Key findings related to T21: disturbances in ECM contribute to congenital anomalies, like heart defects and hypotonia. Overexpression of collagen VI has been observed in T21 fetal hearts. |
| Protein metabolism | Amyloid formation | Direct evidence: yes Normal function: amyloid proteins are involved in protein folding and function, but accumulation leads to neurodegenerative diseases. Key findings related to T21: increased risk of Alzheimer’s disease (AD) in T21, with evidence of fibrillar β-amyloid peptide deposits. |
| Post-translational protein modifications | [Through phosphorylation pathways] Direct evidence: no Normal function: phosphorylation regulates protein function, including activity, localization, and interactions. Key findings related to T21: disruptions in phosphorylation-related pathways may contribute to protein dysfunction in T21 | |
| Regulation of insulin-like growth factor (IGF) transport and uptake by insulin-like growth factor binding proteins (IGFBPs) | Direct evidence: no Normal function: IGF signaling regulates growth, development, and apoptosis. Key findings related to T21: reduced IGF1R expression may impair cardiomyocyte proliferation, contributing to congenital heart defects and short stature in T21. Lower IGF1 signaling is linked to inflammation, neurodegeneration, and short stature. Correlations have been found between neurodegeneration biomarkers (e.g., NfL, UCHL1, GFAP) and IGF signaling disruptions. | |
| Innate immune system | Complement cascade pathways | Direct evidence: yes Normal function: the complement system is crucial for immune responses, inflammation, and pathogen clearance. Key findings related to T21: altered complement protein expression in T21 may be relevant to early-onset AD and is associated with chronic infections, inflammation, accelerated aging, obesity, and cognitive decline. |
| NLRP3 inflammasome | Direct evidence: no Normal function: NLRP3 activation promotes the release of pro-inflammatory cytokines (IL-1β, IL-18), contributing to immune responses. Key findings related to T21: NLRP3 activation promotes pro-inflammatory cytokine release (IL-1β, IL-18), contributing to inflammatory and autoimmune diseases. T21 patients exhibit an inflammatory profile with elevated IL-1β levels. | |
| Transport of small molecules | Plasma lipoprotein metabolism [APOA1 and APOE expression] | Direct evidence: no Normal function: APOA1 is involved in lipid metabolism and cholesterol efflux, while APOE influences lipid transport and brain function. Key findings related to T21: APOA1 is linked to amyloidosis. Higher APOE expression observed in T21 maternal plasma. The presence of the ApoE-ε4 allele in T21 pregnancies is linked to increased blood cholesterol levels, which may impair ovarian follicle microcirculation and fertility. |
| Vesicle-mediated transport | Ligand binding and uptake by scavenger receptors | Direct evidence: no Normal function: scavenger receptors mediate the clearance of oxidized LDL and pathogens, contributing to immune defense and cellular homeostasis. They are expressed in multiple tissues, including the placenta and heart. Key findings related to T21: altered scavenger receptor expression in T21 may impact immune function and oxidative stress management. |
| Retinoid metabolism | Retinoid transport and signaling | Direct evidence: no Normal function: retinoids play a role in vision, embryonic development, immune function, and cellular growth. Retinoic acid stimulates growth hormone secretion, affecting IGF1 levels. Retinoids influence neuronal function, memory, and plasticity. Key findings related to T21: vitamin A deficiency (VAD) is common in T21. Reduced retinoic acid levels have been reported in AD, which is highly prevalent in T21. |
| Disease-associated pathways | Disease of glycosaminoglycan (GAG) metabolism | Direct evidence: no Normal function: GAGs are essential for angiogenesis, coagulation, and ECM integrity. Key findings related to T21: modifications in GAG biosynthesis enzymes are linked to conditions, such as Ehlers–Danlos syndrome, congenital heart defects, and skeletal abnormalities observed in T21. |
| Diseases of hemostasis | Direct evidence: no Normal function: involves the regulation of blood coagulation and prevention of excessive bleeding. Key findings related to T21: T21 is associated with altered clotting pathways, contributing to an increased thrombotic risk. | |
| Oncogenic MAPK signaling | Direct evidence: no Normal function: MAPK signaling is essential for cell growth, differentiation, and survival. Key findings related to T21: studied in T21 patients for its role in leukemia and neurodevelopment. MAPK activity is linked to acute lymphoblastic leukemia in T21. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurca, R.L.; Pralea, I.-E.; Iacobescu, M.; Rus, I.; Iuga, C.-A.; Stamatian, F. Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches. Life 2025, 15, 695. https://doi.org/10.3390/life15050695
Jurca RL, Pralea I-E, Iacobescu M, Rus I, Iuga C-A, Stamatian F. Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches. Life. 2025; 15(5):695. https://doi.org/10.3390/life15050695
Chicago/Turabian StyleJurca, Răzvan Lucian, Ioana-Ecaterina Pralea, Maria Iacobescu, Iulia Rus, Cristina-Adela Iuga, and Florin Stamatian. 2025. "Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches" Life 15, no. 5: 695. https://doi.org/10.3390/life15050695
APA StyleJurca, R. L., Pralea, I.-E., Iacobescu, M., Rus, I., Iuga, C.-A., & Stamatian, F. (2025). Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches. Life, 15(5), 695. https://doi.org/10.3390/life15050695








