CaCO3 Precipitation in Multilayered Cyanobacterial Mats: Clues to Explain the Alternation of Micrite and Sparite Layers in Calcareous Stromatolites
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples

2.2. Electron Microscope Analyses
2.3. Mineralogical Analyses
2.4. Saturation Index of Calcite and Aragonite
2.5. Carbon (δ13Ccarb) Isotopic Analysis

3. Previous Studies
3.1. Structure and Development
3.2. Microenvironmental Characteristics and Carbon Cycling
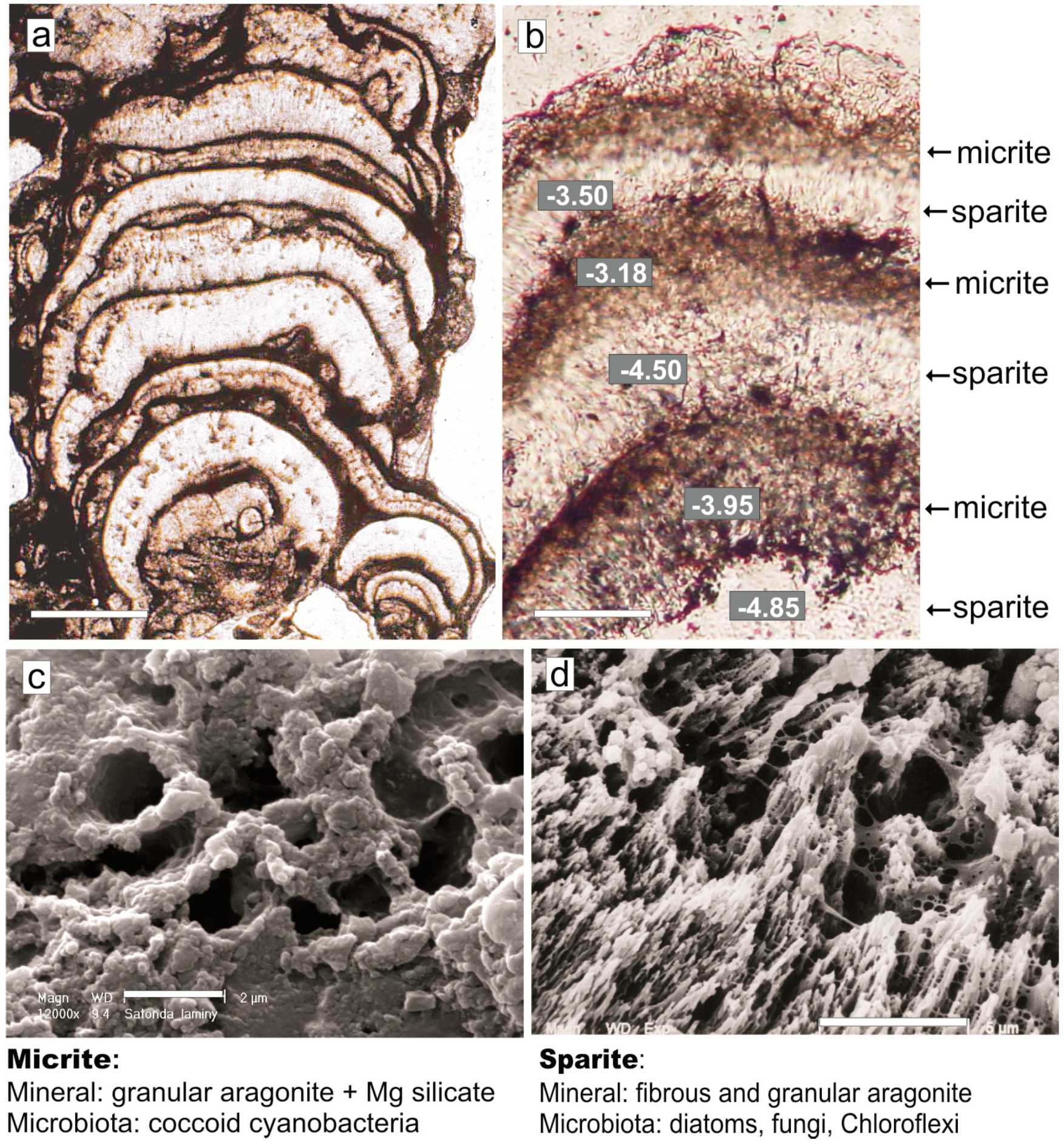
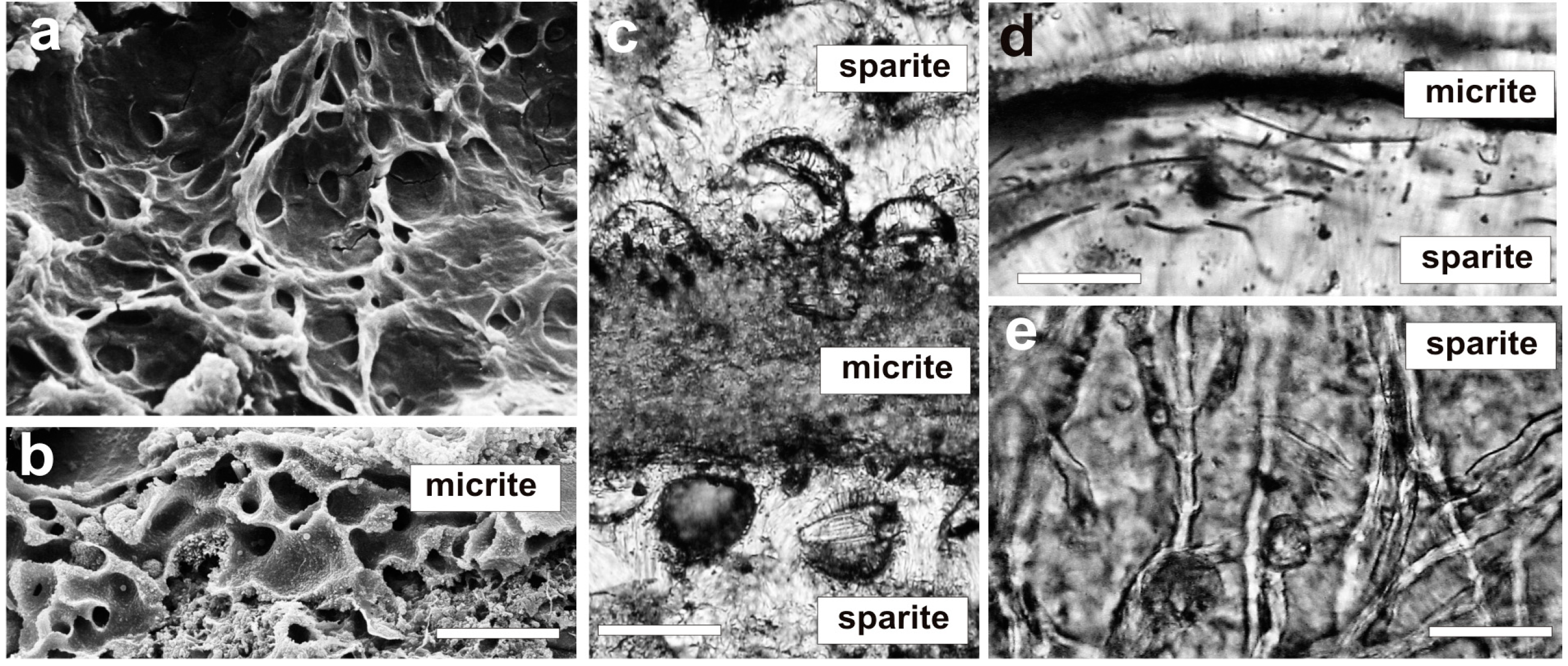
4. Mat Microbial Zones and Associated CaCO3 Morphs


- (i)
- Micrite: <1 µm-sized globular grains of Mg calcite (occasionally with an admixture of nanograins of magnesium silicate) precipitated in the mucilage of the living cyanobacterial layers, particularly dense at their basal parts.
- (ii)
- Sparite: a variety of >1 µm-sized mixed Mg calcite/aragonite particles (anhedral and subhedral grains, hemispheres, dumbbell- and peanut-like bodies, and subglobular aggregates of dagger-like and rhombic, platy crystals) precipitated in the zones of cyanobacterial necromass colonized predominantly by phototrophic purple bacteria with varying admixtures of green sulfur bacteria and heterotrophic bacteria.
5. Carbonate System in the Artificial Mat
| Nivå Bay: Calculated for S = 22‰ and T = 15°C; Ca: 6.3; Mg: 33.3; K: 6.3; Na: 295.4; SO4: 17.6 (all mmol/L) | |||||||
| Alkc | 8.49 meq/L | SI Calcite | 0.67 | ||||
| pH | 7.80 | SI Aragonite | 0.52 | ||||
| pPCO2 | 2.35 | SI Dolomite | 2.10 | ||||
| δ13C -3.09 | |||||||
| Artificial mat: Calculated for S = 21‰ and T = 20°C; Ca: 6.0; Mg: 31.8; K: 5.16; Na: 270.9; SO4: 15.6 (all mmol/L) | |||||||
| Light | Dark | ||||||
| above mat | 1 mm depth | 2 mm depth | 1 mm depth | 2 mm depth | |||
| pH | 8.54 | 8.82 | 8.73 | 8.25 | 8.26 | ||
| Alkc | 8.54 | 10.10 | 6.58 | 10.10 | 6.50 | ||
| SI Calcite | 1.48 | 1.73 | 1.48 | 1.18 | 1.00 | ||
| SI Aragonite | 1.33 | 1.58 | 1.33 | 1.03 | 8.85 | ||
| SI Dolomite | 3.79 | 4.29 | 3.79 | 3.11 | 2.76 | ||
| pPCO2 | 3.04 | 3.35 | 3.43 | 2.72 | 2.92 | ||
6. Discussion
| Satonda Crater Lake | Seawater (Satonda shore) | ||
|---|---|---|---|
| above chemocline | below chemocline ** | mean values | |
| pH | 8.42 | 7.31–6.87 | 8.20 |
| alkalt | 3.5 meq/kg | 5.6–50 meq/kg | 2.1 meq/kg |
| pCO2 | 372 ppmv | 10,000–240,000 ppmv | 309 ppmv |
| Na | 425 meq/kg | 478–560 meq/kg | 460 meq/kg |
| Mg | 86 meq/kg | 96–110 meq/kg | 102 meq/kg |
| Ca | 10 meq/kg | 12–13 meq/kg | 21 meq/kg |
| Mg/Ca | 7.4 | 7.8 *** | 4.8 |
| SICc | 0.84 | 0.09–0.61 | 0.73 |
| SIAra | 0.70 | 0.54–0.61 | 0.59 |
| δ13C | −8.4 (10 m) | −11.4 (40 m); −21.4 (60 m) | - |
| δ18O | 3.05 (10 m) | 2.99 (40 m); 2.53 (60 m) | - |

7. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Lyons, W.B.; Long, D.T.; Hines, M.E.; Gaudette, H.E.; Armstrong, P.B. Calcification of cyanobacterial mats in Solar Lake, Sinai. Geology 1984, 12, 623–626. [Google Scholar] [CrossRef]
- Caudwell, C. Étude expérimentale de la formation de micrite et de sparite dans les stromatolites d’eau douce á Rivularia. Bulletin de la Société géologique de France 1987, 3, 299–306. (In French) [Google Scholar]
- Pentecost, A.; Bauld, J. Nucleation of calcite on the sheaths of cyanobacteria using a simple diffusion cell. Geomicrobiol. J. 1988, 8, 17–26. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J. Calcium carbonate supersaturation and the formation of in situ calcified stromatolites. In Facets of Modern Biogeochemistry; Ittekkot, V., Kempe, S., Michaelis, W., Spitzy, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 255–278. [Google Scholar]
- Kempe, S.; Kazmierczak, J. Hydrochemical key to the genesis of calcareous nonlaminated and laminated cyanobacterial microbialites. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 242–264. [Google Scholar]
- Kempe, S.; Kazmierczak, J. Terrestrial analogues for early planetary oceans: Niuafo’ou caldera lakes (Tonga) and their geology, water chemistry and stromatolites. In Life on Earth and other Planetary Bodies; Hanslmeier, A., Kempe, S., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 195–234. [Google Scholar]
- Kempe, S.; Kazmierczak, J.; Landmann, G.; Konuk, T.; Reimer, A.; Lipp, A. Largest known microbialites discovered in Lake Van, Turkey. Nature 1991, 349, 605–608. [Google Scholar] [CrossRef]
- Reid, R.P.; Browne, K.M. Intertidal stromatolites in a fringing Holocene reef complex, Bahamas. Geology 1991, 19, 15–18. [Google Scholar] [CrossRef]
- Défarge, C.; Trichet, J.; Couté, A. On the appearance of cyanobacterial calcification in modern stromatolites. Sediment. Geol. 1994, 94, 11–19. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Altermann, W.; Kremer, B.; Kempe, S.; Eriksson, P.G. Mass occurrence of benthic coccoid cyanobacteria and their role in the production of carbonates in Neoarchean of South Africa. Precambrian Res. 2009, 173, 79–92. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Microbialite formation in seawater of increased alkalinity. J. Sediment. Res. 2003, 73, 105–127. [Google Scholar] [CrossRef]
- Reid, R.P.; Macintyre, I.G.; Browne, K.M.; Steneck, R.S.; Miller, T. Modern marine stromatolites in the Exuma Cays, Bahamas: Uncommonly common. Facies 1995, 33, 1–17. [Google Scholar] [CrossRef]
- Reid, R.P.; James, N.P.; Macintyre, I.G.; Dupraz, C.P.; Burne, R.V. Shark Bay stromatolites: Microfabrics and reinterpretation of origins. Facies 2003, 49, 45–53. [Google Scholar]
- Ludwig, R.; Al-Horani, F.A.; De Beer, D.; Jonkers, H.M. Photosynthesis-controlled calcification in a hypersaline microbial mat. Limnol. Oceanogr. 2005, 50, 1836–1843. [Google Scholar] [CrossRef]
- López-García, P.; Kazmierczak, J.; Benzerara, K.; Kempe, S.; Guyot, F.; Moreira, D. Bacterial diversity and carbonate precipitation in the giant microbialites from the highly alkaline Lake Van, Turkey. Extremophiles 2005, 9, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sediment. Geol. 2006, 18, 175–183. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Dittrich, M.; McKenzie, J.A. Evidence of microbiocoenosis in the formation of laminae in modern stromatolites. Facies 2014, 60, 3–13. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Kempe, S. Genuine modern analogues of Precambrian stromatolites from caldera lakes of Niuafo’ou, Tonga. Naturwissenschaften 2006, 93, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, J.; Kempe, S.; Altermann, W. Microbial origin of Precambrian carbonates: Lessons from modern analogues. In The Precambrian Earth: Tempos and Events; Eriksson, P.G., Altermann, W., Nelson, D.R., Mueller, W.U., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 545–564. [Google Scholar]
- Kazmierczak, J.; Kempe, S.; Kremer, B.; Lopez-Garcia, P.; Moreira, D.; Tavera, R. Hydrochemistry and microbialites of the alkaline crater Lake Alchichica, Mexico. Facies 2011, 57, 543–570. [Google Scholar] [CrossRef]
- Bissett, A.; Reimer, A.; De Beer, D.; Shiraishi, F.; Arp, G. Metabolic microenvironmental control by photosynthetic biofilms under changing macroenvironmental temperature and pH conditions. Appl. Environ. Microbiol. 2008, 74, 6306–6312. [Google Scholar] [CrossRef] [PubMed]
- Kremer, B.; Kazmierczak, J.; Stal, L.J. Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea. Geobiology 2008, 6, 46–56. [Google Scholar] [PubMed]
- Kremer, B.; Kazmierczak, J.; Łukomska-Kowalczyk, M.; Kempe, S. Calcification and silicification: Fossilization potential of cyanobacteria from stromatolites of Niuafoʻou’s caldera cakes (Tonga) and implications for the early fossil record. Astrobiology 2012, 12, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Havemann, S.A.; Foster, J.S. Comparative characterization of the microbial diversities of an artificial microbialite model and a natural stromatolite. Appl. Environ. Microbiol. 2008, 74, 7410–7421. [Google Scholar] [CrossRef] [PubMed]
- Arp, G.; Helms, G.; Karlinska, K.; Schumann, G.; Reimer, A.; Reitner, J.; Trichet, J. Photosynthesis versus exopolymer degradation in the formation of microbialites on the atoll of Kiritimati, Republic of Kiribati, Central Pacific. Geomicrobiol. J. 2012, 29, 29–65. [Google Scholar] [CrossRef]
- Bühring, S.I.; Smittenberg, R.H.; Sachse, D.; Lipp, J.S.; Golubic, S.; Sachs, J.P.; Hinrichs, K.-U.; Summons, R.E. A hypersaline microbial mat from the Pacific Atoll Kiritimati: Insights into composition and carbon fixation using biomarker analyses and a 13C-labelling approach. Geobiology 2009, 7, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Planavsky, N.; Reid, R.P.; Lyons, T.W.; Myshrall, K.L.; Visscher, P.T. Formation and diagenesis of modern marine calcified Cyanobacteria. Geobiology 2009, 7, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, C.; Fowler, A.; Tobiasi, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [PubMed]
- Mobberley, J.M.; Khodadad, C.L.M.; Foster, J.S. Metabolic potential of lithifying cyanobacteria-dominated thrombolytic mats. Photosynth. Res. 2013, 118, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Ahrendt, S.R.; Mobberley, J.M.; Visscher, P.T.; Koss, L.L.; Foster, J.S. Effects of elevated carbon dioxide and salinity on the microbial diversity in lithifying microbial mats. Minerals 2014, 4, 145–169. [Google Scholar] [CrossRef]
- Gomez, F.J.; Kah, L.C.; Bartley, J.K.; Astini, R.A. Microbialites in a high-altitude Andean lake: Multiple controls on carbonate precipitation and lamina accretion. Palaios 2014, 29, 233–249. [Google Scholar] [CrossRef]
- Lepot, K.; Compère, P.; Gérard, E.; Namsaraev, Z.; Verleyen, E.; Tavernier, I.; Hodgson, D.A.; Vyverman, W.; Gilbert, B.; Wilmotte, A.; et al. Organic and mineral imprints in fossil photosynthetic mats of an East Antarctic lake. Geobiology 2014, 12, 424–450. [Google Scholar] [CrossRef] [PubMed]
- Buick, R.; Dunlop, J.S.R.; Groves, D.I. Stromatolite recognition in ancient rocks: An appraisal of irregular laminated structures in an early Archean chert-barite unit from North Pole, Western Australia. Alcheringa 1981, 5, 161–181. [Google Scholar] [CrossRef]
- Walter, M.R. Archean stromatolites: Evidence of the Earth’s earliest benthos. In Earth’s Earliest Biosphere; Schopf, J.W, Ed.; Princeton University Press: Princeton, NJ, USA, 1983; pp. 187–213. [Google Scholar]
- Altermann, W.; Kazmierczak, J.; Oren, A.; Wright, D.T. Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology 2006, 4, 147–166. [Google Scholar] [CrossRef]
- Allwood, A.C.; Grotzinger, J.P.; Knoll, A.H.; Burch, I.W.; Anderson, M.S.; Coleman, M.L.; Kanik, I. Controls on development and diversity of Early Archean stromatolites. Proc. Natl. Acad. Sci. USA 2009, 106, 9548–9555. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, I.J. Origins of carbonate in Neoproterozoic stromatolites and identification of modern analogues. Precambrian Res. 1991, 53, 281–299. [Google Scholar] [CrossRef]
- Schopf, J.W. Fossil evidence of Archaean life. Philos. Trans. R. Soc. B 2006, 361, 869–885. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kudryavtsev, A.B.; Czaja, A.D.; Tripathi, A.B. Evidence of Archean life: Stromatolites and microfossils. Precambrian Res. 2007, 158, 141–155. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Altermann, W.; Kremer, B.; Kempe, S.; Eriksson, P.G. Mass occurrence of benthic coccoid cyanobacteria and their role in the production of carbonates in Neoarchean of South Africa. Precambrian Res. 2009, 79, 79–92. [Google Scholar] [CrossRef]
- Kremer, B.; Kazmierczak, J. Cyanobacterial mats from Silurian black radiolarian cherts: Phototrophic life at the edge of darkness? J. Sediment. Res. 2005, 75, 897–906. [Google Scholar] [CrossRef]
- Kremer, B. Mat-forming coccoid cyanobacteria from early Silurian marine deposits of Sudetes, Poland. Acta Palaeontol. Polonica 2006, 51, 143–154. [Google Scholar]
- Giani, D.; Seeler, J.; Giani, L.; Krumbein, W.E. Microbial mats and physicochemistry in a saltern in the Bretagne (France) and in a laboratory scale saltern. FEMS Microbiol. Ecol. 1989, 62, 151–162. [Google Scholar] [CrossRef]
- Pringault, O.; Garcia-Pichel, F. Monitoring of oxygenic and anoxygenic photosynthesis in a unicyanobacterial biofilm, grown in a benthic gradient chamber. FEMS Microbiol. Ecol. 2000, 33, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.; Garcia-Pichel, F. Long-term compositional changes after transplant in a microbial mat cyanobacterial community revealed with a polyphasic approach. Environ. Microbiol. 2001, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T. Formation of laminated cyanobacterial mats in the absence of benthic fauna. Aquat. Microb. Ecol. 1998, 14, 235–240. [Google Scholar] [CrossRef]
- Fenchel, T. Artificial cyanobacterial mats: structure and composition of the biota. Aquat. Microb. Ecol. 1998, 14, 241–251. [Google Scholar] [CrossRef]
- Fenchel, T. Artificial cyanobacterial mats: Cycling of C, O, and S. Aquat. Microb. Ecol. 1998, 14, 253–259. [Google Scholar] [CrossRef]
- Kühl, M.; Fenchel, T. Bio-optical characteristics and the vertical distribution of photosynthetic pigments and photosynthesis in an artificial cyanobacterial mat. Microb. Ecol. 2000, 40, 94–103. [Google Scholar] [PubMed]
- Kühl, M.; Fenchel, T.; Kazmierczak, J. Growth, structure and calcification potential of an artificial cyanobacterial mat. In Fossil and Recent Biofilms: A Natural History of Life on Earth; Krumbein, W.E., Paterson, D.M., Zavarzin, G.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 77–102. [Google Scholar]
- Goldsmith, J.R.; Donald, L.G.; Heard, H.C. Lattice constants of the calcium-magnesium carbonates. Am. Mineral. 1961, 46, 453–457. [Google Scholar]
- Tarutani, T.; Clayton, R.N.; Mayeda, T.K. The effect of polymorphism and magnesium substitution on oxygen isotope fractionation between calcium carbonate and water. Geochim. Cosmochim. Acta 1969, 33, 987–996. [Google Scholar] [CrossRef]
- Smalley, P.C.; Maile, C.N.; Coleman, M.L.; Rouse, J.E. LASSIE (laser ablation sampler for stable isotope extraction). Chem. Geol. 1992, 101, 43–52. [Google Scholar]
- Fenchel, T.; Kühl, M. Artificial cyanobacterial mats: growth, structure, and vertical zonation patterns. Microb. Ecol. 2000, 40, 85–93. [Google Scholar] [PubMed]
- Stal, L.J. Cyanobacterial mats and stromatolites. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 61–120. [Google Scholar]
- Parkhurst, D.L.; Thorstenson, D.C.; Plummer, L.N. PHREEQE, A Computer Program for Geochemical Calculations; Water-Resources Investigations 80–96; U.S. Geological Survey: Reston, VA, USA, 1980. [Google Scholar]
- Cölfen, H.; Antonietti, M. Crystal design of calcium carbonate microparticles using double-hydrophilic block copolymers. Langmuir 1998, 4, 582–589. [Google Scholar] [CrossRef]
- Cölfen, H.; Qi, L. A systematic examination of the morphogenesis of calcium carbonate in the presence of a double block copolymer. Chem. Eur. J. 2001, 7, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.L.; Tanaka, K.; Erbs, J. Size dependent kinetics of oriented aggregation. J. Cryst. Growth 2007, 309, 97–102. [Google Scholar] [CrossRef]
- Meldrum, F.S.; Cölfen, H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.U.T.; Killian, C.E.; Olson, I.C.; Appathurai, N.P.; Amasino, A.L.; Martin, M.C.; Holt, L.J.; Wilt, F.H.; Gilbert, P.U.P.A. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2012, 109, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Merz, M. The biology of carbonate precipitation by cyanobacteria. Facies 1992, 26, 81–102. [Google Scholar] [CrossRef]
- Merz, M.; Zankl, H. The influence of the sheath on carbonate precipitation by Cyanobacteria. In Studies on Fossil Benthic Algae; Mucchi: Modena, Italy, 1993; pp. 325–331. [Google Scholar]
- Merz-Preiβ, M.; Riding, R. Cyanobacterial tufa calcification in two freshwater streams: Ambient environment, chemical thresholds and biological processes. Sediment. Geol. 1999, 126, 103–124. [Google Scholar] [CrossRef]
- Ferris, F.G.; Wiese, R.G.; Fyfe, W.S. Precipitation of carbonate minerals by microorganisms: Implications for silicate weathering and the global carbon dioxide budget. Geomicrobiol. J. 1994, 12, 1–13. [Google Scholar] [CrossRef]
- Ferris, F.G.; Thompson, J.B.; Beveridge, T.J. Modern freshwater microbialites from Kelly Lake, British Columbia, Canada. Palaios 1997, 12, 213–219. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Ferris, F.G.; Sherwood-Lollar, B.; Gerits, J.P. Ultrastructure and seasonal growth patterns of microbial mats in temperate climate saline-alkaline lake: Goddenough Lake, British Columbia, Canada. Can. J. Microbiol. 1996, 42, 147–161. [Google Scholar] [CrossRef]
- Ries, J.B.; Anderson, M.A.; Hill, R.T. Seawater Mg/Ca controls polymorph mineralogy of microbial CaCO3: A potential proxy for calcite-aragonite seas in Precambrian time. Geobiology 2008, 6, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, F. Chemical conditions favoring photosynthesis induced CaCO3 precipitation and implications for microbial carbonate formation in the ancient ocean. Geochim. Cosmochim. Acta 2012, 77, 157–174. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Riding, R.; Liang, L. Geobiology of microbial carbonates: Metazoan and seawater saturation state influences on secular trends during the Phanerozoic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 101–115. [Google Scholar] [CrossRef]
- Kennard, J.M.; James, N.P. Thrombolites and stromatolites: Two distinct types of microbial structures. Palaios 1986, 1, 492–503. [Google Scholar] [CrossRef]
- Burne, R.V.; Moore, L.S. Microbialites: organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Coleman, M.L.; Gruszczynski, M.; Kempe, S. Cyanobacterial key to the genesis of micritic and peloidal limestones in ancient seas. Acta Palaeontol. Polonica 1996, 41, 319–338. [Google Scholar]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Ajo-Franklin, C.M.; Northen, T.; Jansson, C. Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J. Satonda Crater Lake, Indonesia: Hydrogeochemistry and biocarbonates. Facies 1993, 28, 1–31. [Google Scholar] [CrossRef]
- Benzerara, K.; Meibom, A.; Gautier, Q.; Kaźmierczak, J.; Stolarski, J.; Menguy, N.; Brown, G.E., Jr. Nanotextures of aragonite in stromatolites from the quasi-marine Satonda crater lake, Indonesia. In Tufas and Speleothems: Unravelling the Microbial and Physical Controls; Pedley, H.M., Rogerson, M., Eds.; Geological Society of London: London, UK, 2010; pp. 211–224. [Google Scholar]
- Calder, J.A.; Parker, P.L. Geochemical implications of induced changes in 13C fractionation by blue-green algae. Geochim. Cosmochim. Acta 1973, 37, 133–140. [Google Scholar] [CrossRef]
- Pentecost, A.; Spiro, B. Stable carbon and oxygen isotope composition of calcites associated with modern freshwater cyanobacteria and algae. Geomicrobiol. J. 1990, 8, 17–26. [Google Scholar] [CrossRef]
- Staal, M.; Thar, R.; Kühl, M.; van Loosdrecht, M.C.M.; Wolf, G.; De Brouwer, J.F.C.; Rijstenbil, J.W. Different carbon isotope fractionation patterns during the development of phototrophic freshwater and marine biofilms. Biogeosciences 2007, 4, 613–626. [Google Scholar] [CrossRef]
- Bathurst, R.G.C. Carbonate Sediments and Their Diagenesis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Walter, M.R. Stromatolites; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Monty, C. Phanerozoic Stromatolites: Case Histories; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Bertrand-Sarfati, J.; Monty, C. Phanerozoic Stromatolites II; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Riding, R. Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Jonkers, H.M.; Ludwig, R.; de Wit, R.; Pringault, O.; Muyzer, G.; Niemann, H.; Finke, N.; de Beer, D. Structural and functional analysis of a microbial mat ecosystem from a unique permanent hypersaline inland lake: ‘La Salada de Chiprana’ (NE Spain). FEMS Microbiol. Ecol. 2003, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Boomer, S.M.; Noll, K.L.; Geesey, G.G.; Dutton, B.E. Formation of multilayered photosynthetic biofilms in an alkaline thermal spring in Yellowstone National Park, Wyoming. Appl. Environ. Microbiol. 2009, 75, 2464–2475. [Google Scholar] [CrossRef] [PubMed]
- Bosak, T.; Greene, S.E.; Newman, D.K. A likely role for anoxygenic photosynthetic microbes in the formation of ancient stromatolites. Geobiology 2007, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bundeleva, I.A.; Shirokova, L.S.; Bénézeth, P.; Pokrovsky, O.S.; Kompantseva, E.I.; Balor, S. Calcium carbonate precipitation by anoxygenic phototrophic bacteria. Chem. Geol. 2012, 291, 116–131. [Google Scholar] [CrossRef]
- Given, R.K.; Wilkinson, B.H. Kinetic control of morphology, composition, and mineralogy of abiotic sedimentary carbonates. J. Sediment. Petrol. 1985, 55, 109–119. [Google Scholar]
- Loste, E.; Meldrum, F.C. Control of calcium carbonate morphology by transformation of an amorphous precursor in a constrained volume. Chem. Commun. 2001, 10, 901–902. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chen, F.-B. Polymorphism of CaCO3 precipitated in a constant-composition environment. AIChE J. 1998, 44, 1790–1798. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotech. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Buczynski, C.; Chafetz, H.S. Habit of bacterially induced precipitates of calcium carbonate and the influence of medium viscosity on mineralogy. J. Sediment. Petrol. 1990, 61, 226–233. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Buczynski, C. Bacterially induced lithification of microbial mats. Palaios 1992, 7, 277–293. [Google Scholar] [CrossRef]
- Guo, X.; Chafetz, H.S. Large tufa mounds, Searles Lake, California. Sedimentology 2012, 59, 1509–1535. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Torrent-Burgués, J.; López-Macipe, A.; Rodríguez-Clemente, R. Precipitation of calcium carbonate from solutions with varying Ca2+/carbonate ratios. J. Cryst. Growth 1996, 166, 1020–1026. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Rodriguez-Navarro, C.; Putnis, A. Effect of pH on calcite growth at constant aCa2+/aCO32− ratio and supersaturation. Geochim. Cosmochim. Acta 2011, 75, 284–296. [Google Scholar] [CrossRef]
- Perri, E.; Manzo, E.; Tucker, M.E. Multi-scale study of the role of the biofilm in the formation of minerals and fabrics in calcareous tufa. Sediment. Geol. 2012, 263, 16–29. [Google Scholar] [CrossRef]
- Chafetz, H.S. Bacterially induced precipitates of calcium carbonate and lithification of microbial mats. In Biostabilization of Sediments; Krumbein, W.E., Paterson, D.M., Stal, L.J., Eds.; BIS-Verlag: Oldenburg, Germany, 1994; pp. 149–163. [Google Scholar]
- Jada, A.; Pefferkorn, E. Smooth and rough spherical calcium carbonate particles. J. Mater. Sci. Lett. 2000, 19, 2077–2079. [Google Scholar] [CrossRef]
- Riege, H.; Gerdes, G.; Krumbein, W.E. Contribution of heterotrophic bacteria to the formation of CaCO3-aggregates in hypersaline microbial mats. Kieler Meeresforschung 1991, 8, 168–172. [Google Scholar]
- Kozánková, J.; Longauerová, D. Participation of bacterial culture in extracellular crystallization of CaCO3. Biologia 1997, 52, 373–378. [Google Scholar]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas euryhalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Knorre, H.V.; Krumbein, W.E. Bacterial calcification. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 25–31. [Google Scholar]
- González-Muñoz, M.T.; Ben Chekroun, K.; Ben Aboud, A.; Arias, J.M.; Rodriguez-Gallego, M. Bacterially induced Mg-calcite formation: role of Mg2+ in development of crystal morphology. J. Sediment. Res. 2000, 70, 559–564. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acid. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Warren, L.A.; Maurice, P.A.; Parmar, N.; Ferris, F.G. Microbially mediated calcium carbonate precipitation: Implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar] [CrossRef]
- Sugihara, H.; Ono, K.; Adachi, K.; Setoguchi, Y.; Ishihara, T.; Takita, Y. Synthesis of disk-like calcium carbonate (Part I)—Effect of various organic compounds on the carbonation of the basic calcium carbonate. J. Ceram. Soc. Jpn 1996, 104, 826–831. [Google Scholar] [CrossRef]
- Tracy, S.L.; Williams, D.A.; Jennings, H.M. The growth of calcite spherulites from solution II. Kinetics of formation. J. Cryst. Growth 1998, 193, 382–388. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Ma, J. Morphological control of CaCO3 particles by a double-hydrophilic block copolymer in mixed alcohol-water solvents. Chem. J. Chin. Univ. 2002, 23, 1595–1597. [Google Scholar]
- Nayar, S.; Sinha, A. Protein induced morphosynthesis of calcium carbonate. J. Mater. Sci. Lett. 2003, 22, 167–170. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Hyde, S.T. Morphological influence of magnesium and organic additives on the precipitation of calcite. J. Cryst. Growth 2001, 231, 544–558. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Astilleros, J.M.; Pina, C.M. The morphology of calcite crystals grown in porous medium doped with divalent cations. Chem. Geol. 2006, 225, 314–321. [Google Scholar] [CrossRef]
- Jiao, Y.; Feng, Q.; Li, X. The co-effect of collagen and magnesium ions on calcium carbonate biomineralization. Mater. Sci. Eng. C 2006, 26, 648–652. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, D.; Zhao, H.; Xie, C.; Guan, G.; Wang, D.; Yu, S.-H. Biomimetic assembly of polypeptide-stabilized CaCO3 nanoparticles. J. Phys. Chem. B 2006, 110, 8613–8618. [Google Scholar] [CrossRef] [PubMed]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Reddy, M.M.; Hoch, A. Calcium carbonate nucleation in an alkaline lake surface water, Pyramid Lake, Nevada, USA. Aquat. Geochem. 2012, 18, 95–113. [Google Scholar] [CrossRef]
- Verch, D.; Morrison, I.E.G.; van de Locht, R.; Kroger, R. In situ electron microscopy studies of calcium carbonate precipitation from aqueous solution with and without organic additives. J. Struct. Biol. 2013, 183, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, W.E. On the precipitation of aragonite on the surface of marine bacteria. Naturwissenschaften 1974, 61. [Google Scholar] [CrossRef]
- Krumbein, W.E. Photolithotrophic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (Gulf of Aqaba, Sinai). Geomicrobiol. J. 1979, 1, 139–203. [Google Scholar] [CrossRef]
- Morita, R.Y. Calcite precipitation by marine bacteria. Geomicrobiol. J. 1980, 2, 63–82. [Google Scholar] [CrossRef]
- Novitsky, J.A. Calcium carbonate precipitation by marine bacteria. Geomicrobiol. J. 1981, 2, 375–388. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Bacterial roles in the precipitation of carbonate minerals. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. [Google Scholar]
- Obst, M.; Wehrli, B.; Dittrich, M. CaCO3 nucleation by cyanobacteria: Laboratory evidence for a passive, surface-induced mechanism. Geobiology 2009, 7, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.E.; Pokrovsky, O.S.; Schott, J.; Oelkers, E.H. Surface charge and zeta potential of metabolically active and dead cyanobacteria. J. Colloid Interface Sci. 2008, 323, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M. Lysis of blue-green algae by myxobacter. J. Bacteriol. 1977, 104, 453–461. [Google Scholar]
- Warren, R.A.J. Microbial hydrolysis of polysaccharides. Annu. Rev. Microbiol. 1996, 50, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinkney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, A.I.; Kawaguchi, T.; Decho, A.W. Quantifying CaCO3 microprecipitates within developing surface mats of marine stromatolites using GIS and digital image analysis. Geomicrobiol. J. 2004, 21, 491–496. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbial lithifications in marine stromatolites and hypersaline mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Liebetrau, V.; Gorb, S.; Sánchez-Román, M.; McKenzie, J.; Treude, N. Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma. Geology 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Saunders, P.; Rogerson, M.; Wadhavan, J.D.; Greenway, G.; Pedley, H.M. Mg/Ca ratios in freshwater microbial carbonates: Thermodynamic, kinetic and vital effects. Geochim. Cosmochim. Acta 2014, 147, 107–118. [Google Scholar] [CrossRef]
- Meister, P. Two opposing effects of sulfate reduction on carbonate precipitation in normal marine, hypersaline, and alkaline environments. Geology 2013, 41, 499–502. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak, J.; Fenchel, T.; Kühl, M.; Kempe, S.; Kremer, B.; Łącka, B.; Małkowski, K. CaCO3 Precipitation in Multilayered Cyanobacterial Mats: Clues to Explain the Alternation of Micrite and Sparite Layers in Calcareous Stromatolites. Life 2015, 5, 744-769. https://doi.org/10.3390/life5010744
Kaźmierczak J, Fenchel T, Kühl M, Kempe S, Kremer B, Łącka B, Małkowski K. CaCO3 Precipitation in Multilayered Cyanobacterial Mats: Clues to Explain the Alternation of Micrite and Sparite Layers in Calcareous Stromatolites. Life. 2015; 5(1):744-769. https://doi.org/10.3390/life5010744
Chicago/Turabian StyleKaźmierczak, Józef, Tom Fenchel, Michael Kühl, Stephan Kempe, Barbara Kremer, Bożena Łącka, and Krzysztof Małkowski. 2015. "CaCO3 Precipitation in Multilayered Cyanobacterial Mats: Clues to Explain the Alternation of Micrite and Sparite Layers in Calcareous Stromatolites" Life 5, no. 1: 744-769. https://doi.org/10.3390/life5010744
APA StyleKaźmierczak, J., Fenchel, T., Kühl, M., Kempe, S., Kremer, B., Łącka, B., & Małkowski, K. (2015). CaCO3 Precipitation in Multilayered Cyanobacterial Mats: Clues to Explain the Alternation of Micrite and Sparite Layers in Calcareous Stromatolites. Life, 5(1), 744-769. https://doi.org/10.3390/life5010744





