Rapid Multiplex Real-Time PCR Method for the Detection and Quantification of Selected Cariogenic and Periodontal Bacteria
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Clinical Data Collection and Sampling
2.3. Bacterial Strains
2.4. Isolation of gDNA from Bacterial Cultures, PCR Amplification of Specific DNA Targets and Cloning
2.5. Optimization of Multiplex Real-Time PCR Conditions
2.6. Sensitivity of Multiplex Real-Time PCR Reactions
2.7. Validation of Method
2.8. Sequencing Analysis
2.9. Statistical Analysis
3. Results
3.1. Demographic Description and Clinical Data
3.2. Multiplex Real-Time PCR Conditions
3.3. Specificity of Multiplex Real-Time PCR Assays
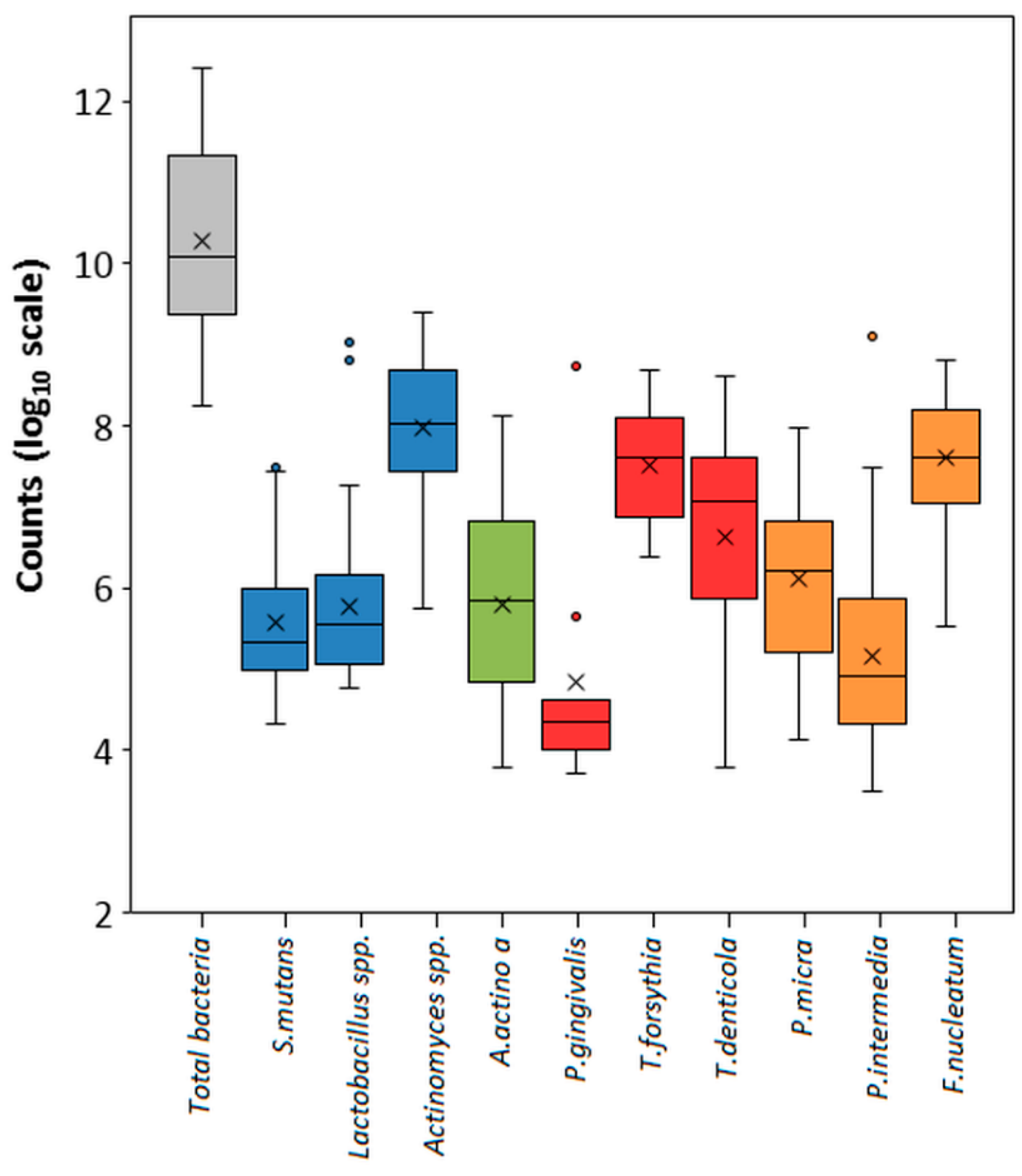
3.4. Testing of Clinical Samples
3.5. Validation of Method
3.6. Correlation between the Counts of Bacteria and Their Percentage Content in Sample
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anil, S.; Anand, P.S. Early Childhood Caries: Prevalence, Risk Factors, and Prevention. Front. Pediatr. 2017, 5, 157. [Google Scholar] [CrossRef] [Green Version]
- American Academy on Pediatric Dentistry. Policy on early childhood caries (ECC): Classifications, consequences, and preventive strategies. Pediatr. Dent. 2008, 30, 40–43. [Google Scholar]
- Kanasi, E.; Dewhirst, F.E.; Chalmers, N.I.; Kent, R.; Moore, A.; Hughes, C.V.; Pradhan, N.; Loo, C.Y.; Tanner, A.C.R. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010, 44, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Meuric, V.; Le Gall-David, S.; Boyer, E.; Acuña-Amador, L.; Martin, B.; Fong, S.B.; Barloy-Hubler, F.; Bonnaure-Mallet, M. Signature of Microbial Dysbiosis in Periodontitis. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Camelo-Castillo, A.J.; Mira, A.; Pico, A.; Nibali, L.; Henderson, B.; Donos, N.; Tomás, I. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front. Microbiol. 2015, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Nibali, L.; Henderson, B.; Sadiq, S.T.; Donos, N. Genetic dysbiosis: The role of microbial insults in chronic inflammatory diseases. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Chokshi, A.; Mahesh, P.; Sharada, P.; Chokshi, K.; Anupriya, S.; Ashwini, B. A correlative study of the levels of salivary Streptococcus mutans, lactobacilli and Actinomyces with dental caries experience in subjects with mixed and permanent dentition. J. Oral Maxillofac. Pathol. 2016, 20, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol. Oral Microbiol. 2016, 31, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Al-Hebshi, N.N.; Baraniya, D.; Chen, T.; Hill, J.; Puri, S.; Tellez, M.; Hasan, N.A.; Colwell, R.R.; Ismail, A. Metagenome sequencing-based strain-level and functional characterization of supragingival microbiome associated with dental caries in children. J. Oral Microbiol. 2018, 11. [Google Scholar] [CrossRef]
- Borilova Linhartova, P.; Deissova, T.; Musilova, K.; Zackova, L.; Kukletova, M.; Kukla, L.; Izakovicova Holla, L. Lack of association between ENAM gene polymorphism and dental caries in primary and permanent teeth in Czech children. Clin. Oral Investig. 2018, 22, 1873–1877. [Google Scholar] [CrossRef]
- Petersen, P.E.; Baez, R.J.; World Health Organization. Oral Health Surveys: Basic Methods; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-154864-9. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Owczarzy, R.; Tataurov, A.V.; Wu, Y.; Manthey, J.A.; McQuisten, K.A.; Almabrazi, H.G.; Pedersen, K.F.; Lin, Y.; Garretson, J.; McEntaggart, N.O.; et al. IDT SciTools: A suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 2008, 36, W163–W169. [Google Scholar] [CrossRef]
- Byun, R.; Nadkarni, M.A.; Chhour, K.L.; Martin, F.E.; Jacques, N.A.; Hunter, N. Quantitative Analysis of Diverse Lactobacillus Species Present in Advanced Dental Caries. J. Clin. Microbiol. 2004, 42, 3128–3136. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lin, S.; Kelen, G.D.; Quinn, T.C.; Dick, J.D.; Gaydos, C.A.; Rothman, R.E. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J. Clin. Microbiol. 2002, 40, 3449–3454. [Google Scholar] [CrossRef] [Green Version]
- Coffey, J.; Choudhry, M.; Shlossman, M.; Makin, I.R.S.; Singh, V.K. Multiplex real-time PCR detection and relative quantification of periodontal pathogens. Clin. Exp. Dent. Res. 2016, 2, 185–192. [Google Scholar] [CrossRef]
- Martin, F.E.; Nadkarni, M.A.; Jacques, N.A.; Hunter, N. Quantitative Microbiological Study of Human Carious Dentine by Culture and Real-Time PCR: Association of Anaerobes with Histopathological Changes in Chronic Pulpitis. J. Clin. Microbiol. 2002, 40, 1698–1704. [Google Scholar] [CrossRef] [Green Version]
- Kuboniwa, M.; Amano, A.; Kimura, K.R.; Sekine, S.; Kato, S.; Yamamoto, Y.; Okahashi, N.; Iida, T.; Shizukuishi, S. Quantitative detection of periodontal pathogens using real-time polymerase chain reaction with TaqMan probes. Oral Microbiol. Immunol. 2004, 19, 168–176. [Google Scholar] [CrossRef]
- Costa, M.T.; Dorta, M.L.; Ribeiro-Dias, F.; Pimenta, F.C. Biofilms of black tooth stains: PCR analysis reveals presence of Streptococcus mutans. Braz. Dent. J. 2012, 23, 555–558. [Google Scholar] [CrossRef] [Green Version]
- Carrouel, F.; Viennot, S.; Santamaria, J.; Veber, P.; Bourgeois, D. Quantitative Molecular Detection of 19 Major Pathogens in the Interdental Biofilm of Periodontally Healthy Young Adults. Front. Microbiol. 2016, 7, 840. [Google Scholar] [CrossRef]
- Childers, N.K.; Osgood, R.C.; Hsu, K.L.; Manmontri, C.; Momeni, S.S.; Mahtani, H.; Cutter, G.R.; Ruby, J.D. Real-Time Quantitative Polymerase Chain Reaction for Enumeration of Streptococcus mutans from Oral Samples. Eur. J. Oral Sci. 2011, 119, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Dalwai, F.; Spratt, D.A.; Pratten, J. Use of Quantitative PCR and Culture Methods to Characterize Ecological Flux in Bacterial Biofilms. J. Clin. Microbiol. 2007, 45, 3072–3076. [Google Scholar] [CrossRef] [Green Version]
- Bořilová Linhartová, P.; Kukletová, M.; Izakovičová Hollá, L. Relationship between Breastfeeding and Severe Early Childhood Caries. Ceska Stomatol. Prakt. Zubn. Lek. 2018, 118, 58–67. [Google Scholar]
- Fernandes, T.; Bhavsar, C.; Sawarkar, S.; D’souza, A. Current and novel approaches for control of dental biofilm. Int. J. Pharm. 2018, 536, 199–210. [Google Scholar] [CrossRef]
- Maheaswari, R.; Kshirsagar, J.T.; Lavanya, N. Polymerase chain reaction: A molecular diagnostic tool in periodontology. J. Indian Soc. Periodontol. 2016, 20, 128–135. [Google Scholar] [CrossRef]
- Pretzl, B.; Sälzer, S.; Ehmke, B.; Schlagenhauf, U.; Dannewitz, B.; Dommisch, H.; Eickholz, P.; Jockel-Schneider, Y. Administration of systemic antibiotics during non-surgical periodontal therapy—A consensus report. Clin. Oral Investig. 2018, 23, 3073–3085. [Google Scholar] [CrossRef]
- McGowan, K.; McGowan, T.; Ivanovski, S. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Periodontol. 2018, 45, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J. Clin. Periodontol. 2011, 38, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Nickles, K.; Koch, R.; Harks, I.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; et al. Is furcation involvement affected by adjunctive systemic amoxicillin plus metronidazole? A clinical trials exploratory subanalysis. J. Clin. Periodontol. 2016, 43, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeld, D.; Koch, R.; Jünemann, S.; Prior, K.; Harks, I.; Eickholz, P.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; et al. Do we treat our patients or rather periodontal microbes with adjunctive antibiotics in periodontal therapy? A 16S rDNA microbial community analysis. PLoS ONE 2018, 13, e0195534. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.C.R.; Milgrom, P.M.; Kent, R.; Mokeem, S.A.; Page, R.C.; Riedy, C.A.; Weinstein, P.; Bruss, J. The microbiota of young children from tooth and tongue samples. J. Dent. Res. 2002, 81, 53–57. [Google Scholar] [CrossRef]

| Bacterial Strain | Target Gene | Sequence of Primers or Probe | Fluorophore | Amplicon Size (bp) | References |
|---|---|---|---|---|---|
| Cariogenic Pathogens and Total Bacteria Content | |||||
| S. mutans | gtfB | Forward: CCTACAGCTCAGAGATGCTAT | 113 | This study | |
| Reverse: GCCATACACCACTCATGAATT | This study | ||||
| Probe: TGGAAATGACGGTCGCCGTTAT | FAM | This study | |||
| Lactobacillus spp. | 16S | Forward: TGGAAACAGGTGCTAATACC | 232 | [18] | |
| Reverse: GTCCATTGTGGAAGATTCCC | [18] | ||||
| Probe: CCACATTGGGACTGAGACACGG | HEX | This study | |||
| Actinomyces spp. | 16S | Forward: CCTCTGGCTTAACTGGGG | 88 | [19] | |
| Reverse: CATTCCACCGCTACACCA | [19] | ||||
| Probe: TCCAGTCTCCCCTACCGCAC | Cy5 | This study | |||
| Total bacteria content | 16S | Forward: TGGAGCATGTGGTTTAATTCGA | 130 | [20] | |
| Reverse: ACGAGCTGACGACAACCATG | This study | ||||
| Probe: TGG+TAAGG+TTCTTCGCGT | Texas Red | This study | |||
| Orange Complex and A. actinomycetemcomitans | |||||
| A. actinomycetemcomitans | 23S | Forward: GCGAAACGAAGAGAAGCAAG | 111 | [21] | |
| Reverse: CCTACCCAACAGGCGTATCA | [21] | ||||
| Probe: AAGTGCGGTTGGGAATTGAGGA | FAM | This study | |||
| P. micra | 16S | Forward: AGTGGGATAGCCGTTGGAAA | 100 | [22] | |
| Reverse: GACGCGAGCCCTTCTTACAC | [22] | ||||
| Probe: ACCGCATGAGACCACAGAATCGC | Texas Red | This study | |||
| P. intermedia | 16S | Forward: TCCACCGATGAATCTTTGGTC | 103 | [23] | |
| Reverse: ATCCAACCTTCCCTCCACTC | [23] | ||||
| Probe: CGTCAGATGCCATATGTGGACAAC | Cy5 | This study | |||
| F. nucleatum | 16S | Forward: GGCTTCCCCATCGGCATTCC | 123 | [21] | |
| Reverse: AATGCAGGGCTCAACTCTGT | [21] | ||||
| Probe: AGTTCCGCTTACCTCTCCAGTAC | HEX | This study | |||
| Red Complex and Total Bacteria Content | |||||
| P. gingivalis | 23S | Forward: CTGCGTATCCGACATATC | 134 | [21] | |
| Reverse: GGTACTGGTTCACTATCG | [21] | ||||
| Probe: AGACATCCTGTGTGAATTGGCG | FAM | This study | |||
| T. forsythia | groL | Forward: GAGGTTGTGGAAGGTATG | 108 | [21] | |
| Reverse: GTAGATCAGAATGTACGGATT | [21] | ||||
| Probe: TCCGCTTATTTCGTGACCGAT | Cy5 | This study | |||
| T. denticola | 16S | Forward: GTTGTTCGGAATTATTGG | 109 | [21] | |
| Reverse: GATTCAAGTCAAGCAGTA | [21] | ||||
| Probe: AGGCGGTTAGGTAAGCCTG | HEX | This study | |||
| Total bacteria content | 16S | Forward: TGGAGCATGTGGTTTAATTCGA | 130 | [20] | |
| Reverse: ACGAGCTGACGACAACCATG | This study | ||||
| Probe: TGG+TAAGG+TTCTTCGCGT | Texas Red | This study | |||
| Bacterial Strain | LOD90 (Log10cop./μL) | PCR Efficiency | Calibration Fit (R2) |
|---|---|---|---|
| S. mutans | 3.8 | 0.93 | 1.00 |
| Lactobacillus spp. | 4.5 | 0.95 | 1.00 |
| Actinomyces spp. | 3.8 | 1.04 | 0.99 |
| A. actinomycetemcomitans | 3.0 | 1.08 | 0.99 |
| P. gingivalis | 3.5 | 1.11 | 0.99 |
| T. forsythia | 3.3 | 1.08 | 0.98 |
| T. denticola | 5.2 | 1.10 | 0.98 |
| P. micra | 2.9 | 1.07 | 1.00 |
| P. intermedia | 3.2 | 1.16 | 1.00 |
| F. nucleatum | 3.3 | 1.07 | 1.00 |
| Prevalence | Percentage Content | |||||
|---|---|---|---|---|---|---|
| Bacterial Strain | AT (%) | HT (%) | p Value * | AT (%) | HT (%) | p Value † |
| S. mutans | 63.3 | 46.7 | 0.24 | 0.36 | 0.37 | 0.67 |
| Lactobacillus spp. | 83.3 | 83.3 | 0.22 | 0.36 | 0.40 | 0.33 |
| Actinomyces spp. | 100.0 | 100.0 | 0.90 | 0.57 | 0.63 | 0.87 |
| A. actinomycetemcomitans | 43.3 | 66.7 | 0.17 | 0.51 | 0.26 | <0.01 |
| P. gingivalis | 6.7 | 0.0 | 0.60 | 0.49 | 0.29 | 0.44 |
| T. forsythia | 16.7 | 6.7 | 0.37 | 0.26 | 0.50 | 0.85 |
| T. denticola | 13.3 | 3.3 | 0.67 | 0.79 | 0.29 | 0.03 |
| P. micra | 70.0 | 50.0 | 0.09 | 0.29 | 0.49 | 0.44 |
| P. intermedia | 36.7 | 26.7 | 0.74 | 0.26 | 0.23 | 0.31 |
| F. nucleatum | 96.7 | 96.7 | 0.58 | 0.98 | 0.43 | <0.01 |
| Bacterial Strain | Really Positive | False-Positive | False-Negative | Really Negative |
|---|---|---|---|---|
| S. mutans | 33 | 0 | 0 | 27 |
| Lactobacillus spp. | 50 | 0 | 0 | 10 |
| Actinomyces spp. | 60 | 0 | 0 | 0 |
| A. actinomycetemcomitans | 33 | 0 | 0 | 27 |
| P. gingivalis | 2 | 0 | 0 | 58 |
| T. forsythia | 7 | 0 | 0 | 53 |
| T. denticola | 5 | 0 | 0 | 55 |
| P. micra | 36 | 0 | 0 | 24 |
| P. intermedia | 19 | 0 | 0 | 41 |
| F. nucleatum | 58 | 0 | 0 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lochman, J.; Zapletalova, M.; Poskerova, H.; Izakovicova Holla, L.; Borilova Linhartova, P. Rapid Multiplex Real-Time PCR Method for the Detection and Quantification of Selected Cariogenic and Periodontal Bacteria. Diagnostics 2020, 10, 8. https://doi.org/10.3390/diagnostics10010008
Lochman J, Zapletalova M, Poskerova H, Izakovicova Holla L, Borilova Linhartova P. Rapid Multiplex Real-Time PCR Method for the Detection and Quantification of Selected Cariogenic and Periodontal Bacteria. Diagnostics. 2020; 10(1):8. https://doi.org/10.3390/diagnostics10010008
Chicago/Turabian StyleLochman, Jan, Martina Zapletalova, Hana Poskerova, Lydie Izakovicova Holla, and Petra Borilova Linhartova. 2020. "Rapid Multiplex Real-Time PCR Method for the Detection and Quantification of Selected Cariogenic and Periodontal Bacteria" Diagnostics 10, no. 1: 8. https://doi.org/10.3390/diagnostics10010008






