Prediction of Antidepressant Treatment Outcome Using Event-Related Potential in Patients with Major Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Psychological Assessments
2.3. EEG Recordings and Analysis
2.3.1. Frontal Alpha Asymmetry (FAA)
2.3.2. Loudness Dependent Auditory Evoked Potentials (LDAEP)
2.3.3. Mismatch Negativity (MMN)
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
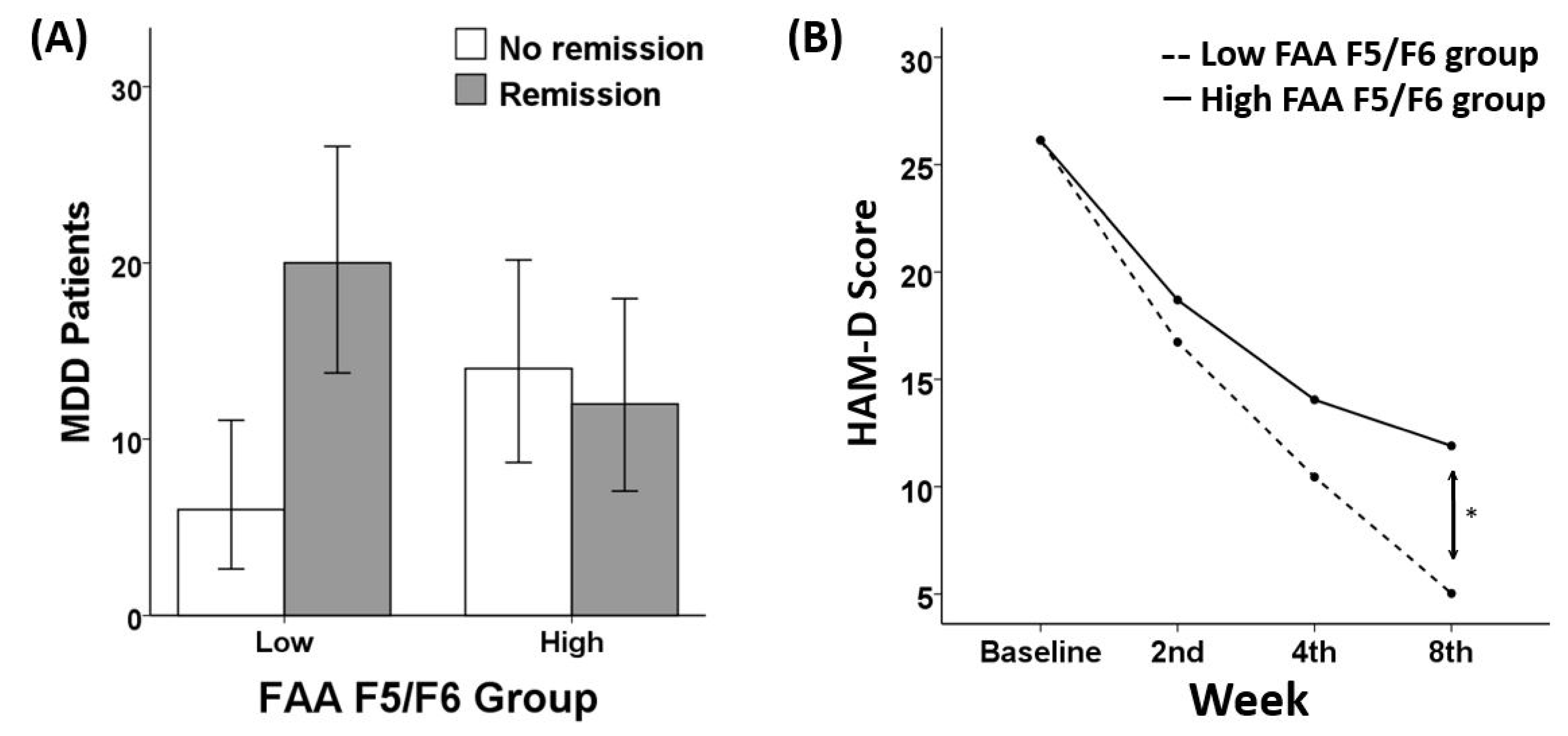
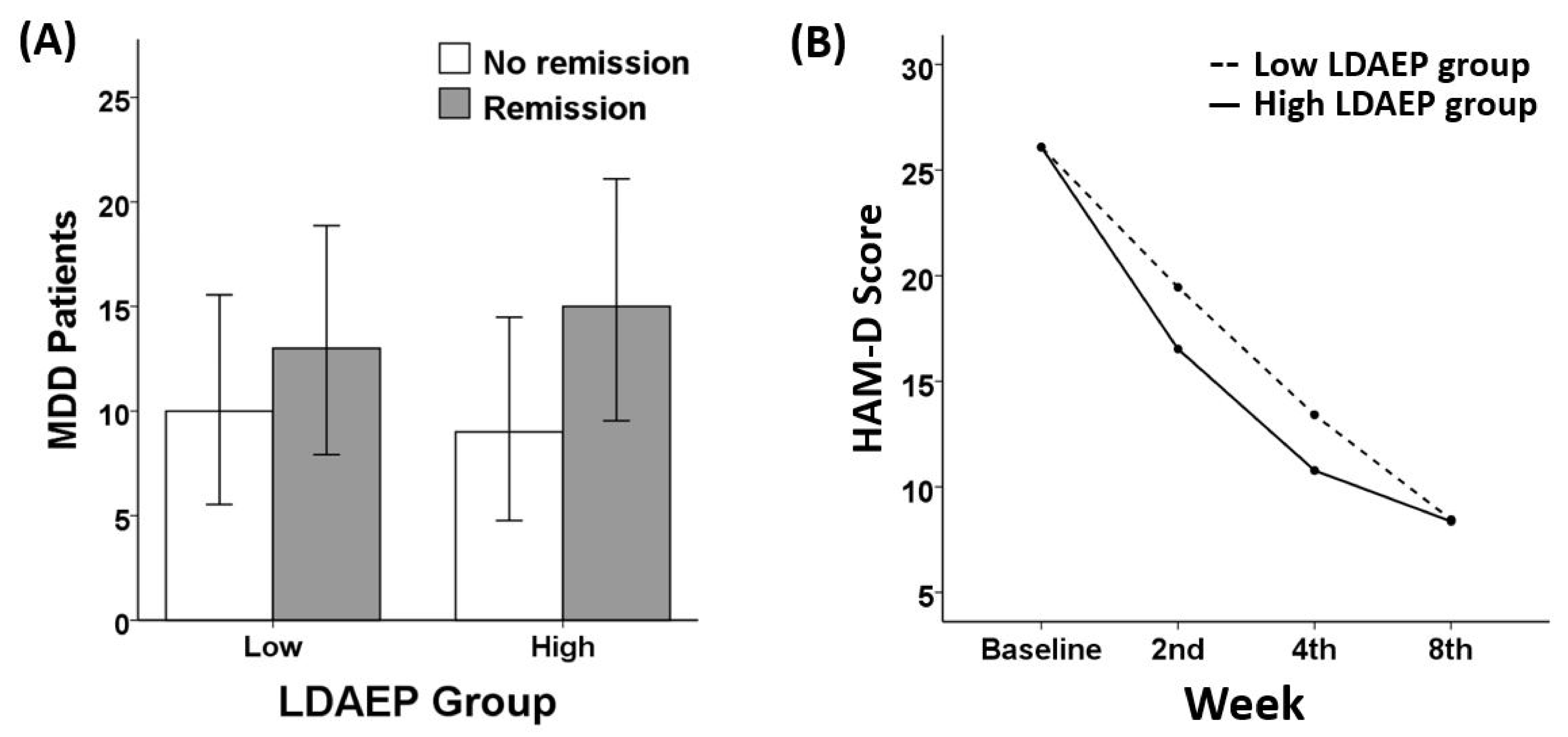
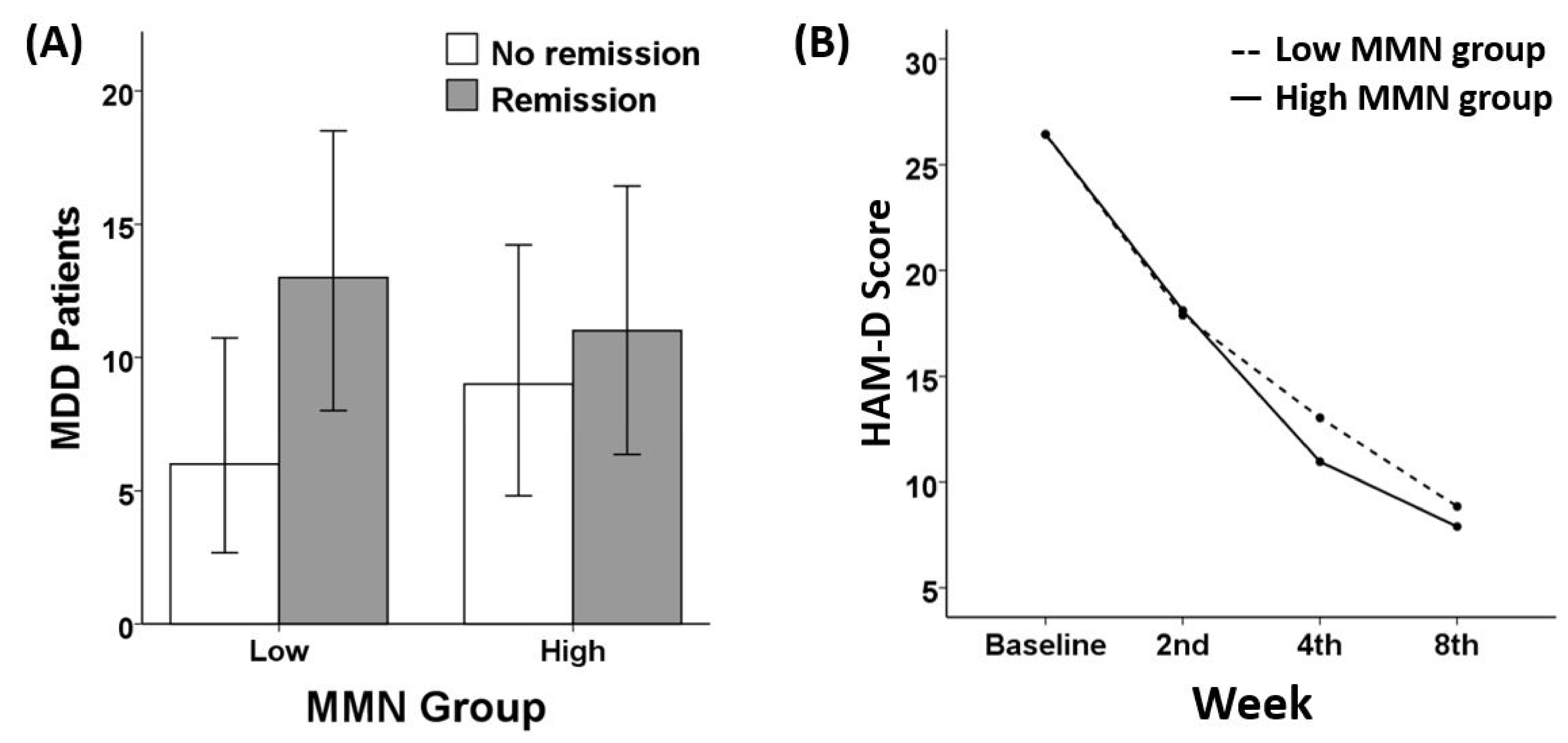
3.2. Remission and Response Ratio
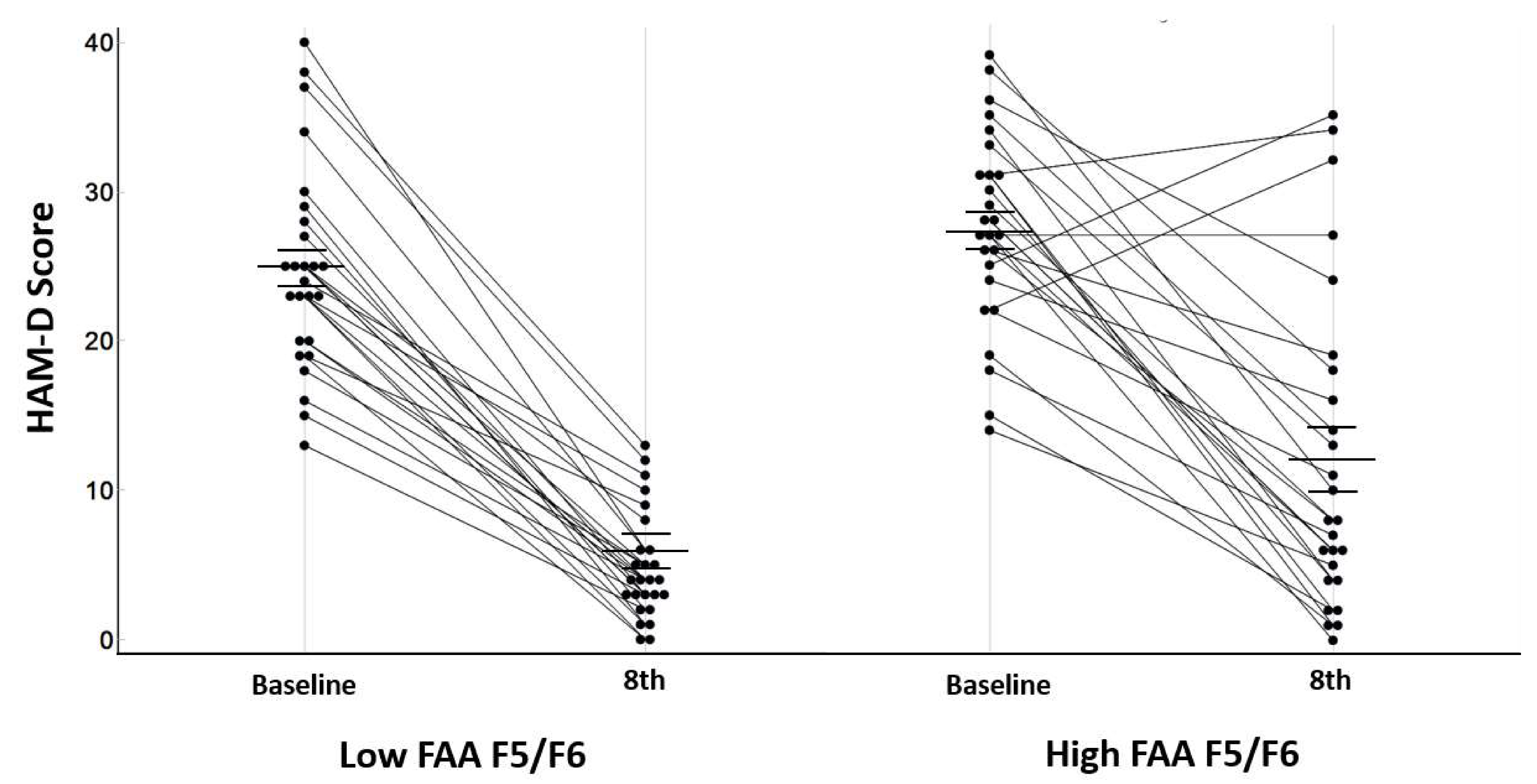
3.3. Treatment Responsiveness (HAM-D)
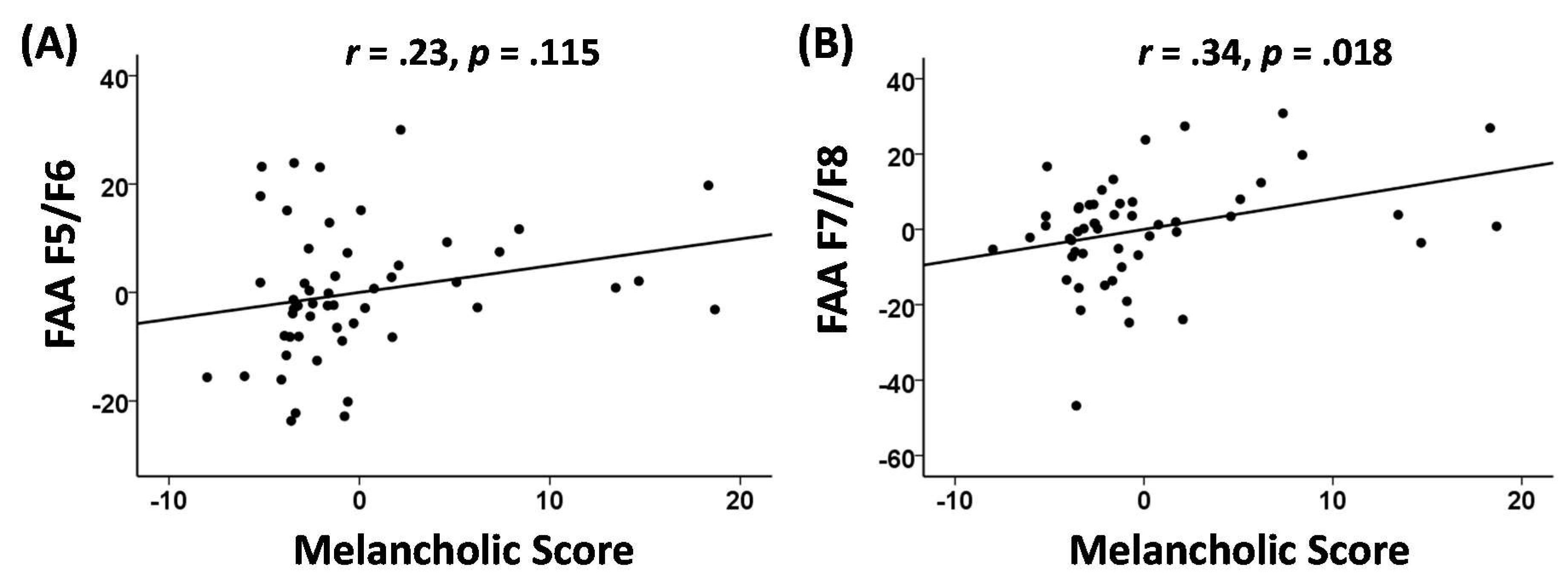
3.4. Melancholia Score Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kessler, R.C.; de Jonge, P.; Shahly, V.; van Loo, H.M.; Wang, P.S.-E.; Wilcox, M.A. Epidemiology of depression. In Handbook of Depression, 3rd ed.; Guilford Press: New York, NY, USA, 2014; pp. 7–24. [Google Scholar]
- Lépine, J.-P.; Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3. [Google Scholar]
- Goldberg, D. The heterogeneity of “major depression”. World Psychiatr. 2011, 10, 226. [Google Scholar] [CrossRef]
- Penninx, B.; Lamers, F.; Milaneschi, Y. Clinical heterogeneity in major depressive disorder. Eur. Neuropsychopharmacol. 2018, 28, S62–S63. [Google Scholar] [CrossRef]
- Han, K.-M.; De Berardis, D.; Fornaro, M.; Kim, Y.-K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 20–27. [Google Scholar] [CrossRef]
- Serafini, G.; Lamis, D.A.; Aguglia, A.; Amerio, A.; Nebbia, J.; Geoffroy, P.A.; Pompili, M.; Amore, M. Hopelessness and its correlates with clinical outcomes in an outpatient setting. J. Affect. Disord. 2020, 263, 472–479. [Google Scholar] [CrossRef]
- Orsolini, L.; Latini, R.; Pompili, M.; Serafini, G.; Volpe, U.; Vellante, F.; Fornaro, M.; Valchera, A.; Tomasetti, C.; Fraticelli, S. Understanding the complex of suicide in depression: From research to clinics. Psychiatry Investig. 2020, 17, 207. [Google Scholar] [CrossRef] [Green Version]
- Gili, M.; Roca, M.; Armengol, S.; Asensio, D.; Garcia-Campayo, J.; Parker, G. Clinical patterns and treatment outcome in patients with melancholic, atypical and non-melancholic depressions. PLoS ONE 2012, 7, e48200. [Google Scholar] [CrossRef] [Green Version]
- Quinn, C.; Harris, A.; Kemp, A. The interdependence of subtype and severity: Contributions of clinical and neuropsychological features to melancholia and non-melancholia in an outpatient sample. J. Int. Neuropsychol. Soc. 2012, 18, 361–369. [Google Scholar] [CrossRef]
- Kendler, K.S. The diagnostic validity of melancholic major depression in a population-based sample of female twins. Arch. Gen. Psychiatry 1997, 54, 299–304. [Google Scholar] [CrossRef]
- Khan, A.Y.; Carrithers, J.; Preskorn, S.H.; Lear, R.; Wisniewski, S.R.; John Rush, A.; Stegman, D.; Kelley, C.; Kreiner, K.; Nierenberg, A.A. Clinical and demographic factors associated with DSM-IV melancholic depression. Ann. Clin. Psychiatry 2006, 18, 91–98. [Google Scholar] [CrossRef]
- Leventhal, A.M.; Rehm, L.P. The empirical status of melancholia: Implications for psychology. Clin. Psychol. Rev. 2005, 25, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; Fink, M.; Shorter, E.; Taylor, M.A.; Akiskal, H.; Berrios, G.; Bolwig, T.; Brown, W.A.; Carroll, B.; Healy, D. Issues for DSM-5: Whither melancholia? The case for its classification as a distinct mood disorder. Am. Psychiatr. Assoc. 2010, 167, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; Hadzi-Pavlovic, D.; Wilhelm, K.; Hickie, I.; Brodaty, H.; Boyce, P.; Mitchell, P.; Eyers, K. Defining melancholia: Properties of a refined sign-based measure. Br. J. Psychiatry 1994, 164, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Weissenburger, J.E. Melancholic symptom features and DSM-IV. Am. J. Psychiatr. 1994, 151, 489–498. [Google Scholar]
- Bosaipo, N.B.; Foss, M.P.; Young, A.H.; Juruena, M.F. Neuropsychological changes in melancholic and atypical depression: A systematic review. Neurosci. Biobehav. Rev. 2017, 73, 309–325. [Google Scholar] [CrossRef]
- Fletcher, K.; Parker, G.; Paterson, A.; Fava, M.; Iosifescu, D.; Pizzagalli, D.A. Anhedonia in melancholic and non-melancholic depressive disorders. J. Affect. Disord. 2015, 184, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Gold, P.W.; Chrousos, G.P. The endocrinology of melancholic and atypical depression: Relation to neurocircuitry and somatic consequences. Proc. Assoc. Am. Physicians 1999, 111, 22–34. [Google Scholar] [CrossRef]
- Parker, G.; McCraw, S.; Blanch, B.; Hadzi-Pavlovic, D.; Synnott, H.; Rees, A.-M. Discriminating melancholic and non-melancholic depression by prototypic clinical features. J. Affect. Disord. 2013, 144, 199–207. [Google Scholar] [CrossRef]
- Withall, A.; Harris, L.M.; Cumming, S.R. A longitudinal study of cognitive function in melancholic and non-melancholic subtypes of major depressive disorder. J. Affect. Disord. 2010, 123, 150–157. [Google Scholar] [CrossRef]
- Brown, W. Treatment response in melancholia. Acta Psychiatr. Scand. 2007, 115, 125–129. [Google Scholar] [CrossRef]
- McGrath, P.J.; Khan, A.Y.; Trivedi, M.H.; Stewart, J.W.; Morris, D.W.; Wisniewski, S.R.; Miyahara, S.; Nierenberg, A.A.; Fava, M.; Rush, A.J. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: A STAR* D report. J. Clin. Psychiatry 2008, 69, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Tomasetti, C.; Pompili, M.; Serafini, G.; Vellante, F.; Fornaro, M.; Valchera, A.; Perna, G.; Volpe, U.; Martinotti, G. An update on glutamatergic system in suicidal depression and on the role of esketamine. Curr. Top. Med. Chem. 2020, 20, 554–584. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Montemitro, C.; Fiengo, A.L.; Santone, C.; Orsolini, L.; Valchera, A.; Carano, A.; Pompili, M.; Serafini, G.; Perna, G. Novel pathways in the treatment of major depression: Focus on the glutamatergic system. Curr. Pharm. Des. 2019, 25, 381–387. [Google Scholar] [CrossRef] [PubMed]
- McGorry, P.; Keshavan, M.; Goldstone, S.; Amminger, P.; Allott, K.; Berk, M.; Lavoie, S.; Pantelis, C.; Yung, A.; Wood, S. Biomarkers and clinical staging in psychiatry. World Psychiatr. 2014, 13, 211–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, W.; Malik, A.S.; Yasin, M.A.M.; Xia, L. Review on EEG and ERP predictive biomarkers for major depressive disorder. Biomed. Signal Process. Control 2015, 22, 85–98. [Google Scholar] [CrossRef]
- Olbrich, S.; Arns, M. EEG biomarkers in major depressive disorder: Discriminative power and prediction of treatment response. Int. Rev. Psychiatry 2013, 25, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Kujala, T.; Tervaniemi, M.; Schröger, E. The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biol. Psychol. 2007, 74, 1–19. [Google Scholar] [CrossRef]
- May, P.J.; Tiitinen, H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology 2010, 47, 66–122. [Google Scholar] [CrossRef]
- Qiao, Z.; Yu, Y.; Wang, L.; Yang, X.; Qiu, X.; Zhang, C.; Ning, N.; Shi, J.; Chen, L.; Li, Z. Impaired pre-attentive change detection in major depressive disorder patients revealed by auditory mismatch negativity. Psychiatry Res. Neuroimag. 2013, 211, 78–84. [Google Scholar] [CrossRef]
- Restuccia, D.; Vollono, C.; Scaloni, L.; Buccelletti, F.; Camardese, G. Abnormality of auditory mismatch negativity in depression and its dependence on stimulus intensity. Clin. Eeg Neurosci. 2016, 47, 105–112. [Google Scholar] [CrossRef]
- He, W.; Chai, H.; Zheng, L.; Yu, W.; Chen, W.; Li, J.; Chen, W.; Wang, W. Mismatch negativity in treatment-resistant depression and borderline personality disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2010, 34, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Naismith, S.L.; Mowszowski, L.; Ward, P.B.; Diamond, K.; Paradise, M.; Kaur, M.; Lewis, S.J.; Hickie, I.B.; Hermens, D.F. Reduced temporal mismatch negativity in late-life depression: An event-related potential index of cognitive deficit and functional disability? J. Affect. Disord. 2012, 138, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Juckel, G.; Pogarell, O.; Augustin, H.; Mulert, C.; Müller-Siecheneder, F.; Frodl, T.; Mavrogiorgou, P.; Hegerl, U. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J. Clin. Psychiatr. 2007, 68, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-A.; Lee, S.-H.; Lee, S.-Y.; Chae, J.-H.; Lee, C.-U.; Park, Y.-M.; Bae, S.-M. Clinical characteristics associated with different strengths of loudness dependence of auditory evoked potentials (LDAEP) in major depressive disorder. Psychiatr. Res. 2012, 200, 374–381. [Google Scholar] [CrossRef]
- Uhl, I.; Illes, F.; Graßnickel, V.; Echterhoff, S.; Norra, C.; Juckel, G. Loudness dependence of auditory evoked potentials (LDAEP) in clinical monitoring of suicidal patients with major depression: A pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 487–492. [Google Scholar] [CrossRef]
- Graßnickel, V.; Illes, F.; Juckel, G.; Uhl, I. Loudness dependence of auditory evoked potentials (LDAEP) in clinical monitoring of suicidal patients with major depression in comparison with non-suicidal depressed patients and healthy volunteers: A follow-up-study. J. Affect. Disord. 2015, 184, 299–304. [Google Scholar] [CrossRef]
- Wyss, C. The LDAEP as a Potential Biomarker for Central Serotonergic Activity: Challenges to Overcome; University of Zurich: Zurich, Switzerland, 2015. [Google Scholar]
- Allen, J.J.; Reznik, S.J. Frontal EEG asymmetry as a promising marker of depression vulnerability: Summary and methodological considerations. Curr. Opin. Psychol. 2015, 4, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Gold, C.; Fachner, J.; Erkkilä, J. Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scand. J. Psychol. 2013, 54, 118–126. [Google Scholar] [CrossRef]
- Gordon, E.; Palmer, D.M.; Cooper, N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin. Eeg Neurosci. 2010, 41, 178–183. [Google Scholar] [CrossRef]
- Leiser, S.C.; Dunlop, J.; Bowlby, M.R.; Devilbiss, D.M. Aligning strategies for using EEG as a surrogate biomarker: A review of preclinical and clinical research. Biochem. Pharmacol. 2011, 81, 1408–1421. [Google Scholar] [CrossRef]
- Kaiser, A.K.; Gnjezda, M.-T.; Knasmüller, S.; Aichhorn, W. Electroencephalogram alpha asymmetry in patients with depressive disorders: Current perspectives. Neuropsychiatr. Dis. Treat. 2018, 14, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Vinne, N.; Vollebregt, M.A.; Van Putten, M.J.; Arns, M. Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. Neuroimage: Clin. 2017, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bruder, G.E.; Tenke, C.E.; Warner, V.; Nomura, Y.; Grillon, C.; Hille, J.; Leite, P.; Weissman, M.M. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol. Psychiatr. 2005, 57, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.L.; Shankman, S.A.; Kujawa, A.; Torpey-Newman, D.C.; Olino, T.M.; Klein, D.N. Developmental changes in electroencephalographic frontal asymmetry in young children at risk for depression. J. Child Psychol. Psychiatr. 2016, 57, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.L.; Bismark, A.W.; Towers, D.N.; Coan, J.A.; Allen, J.J. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010, 119, 502. [Google Scholar] [CrossRef] [Green Version]
- Fingelkurts, A.A.; Fingelkurts, A.A. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol. Psychiatr. 2015, 77, 1050–1060. [Google Scholar] [CrossRef]
- Jaworska, N.; Blier, P.; Fusee, W.; Knott, V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J. Psychiatr. Res. 2012, 46, 1483–1491. [Google Scholar] [CrossRef] [Green Version]
- Kemp, A.; Griffiths, K.; Felmingham, K.; Shankman, S.A.; Drinkenburg, W.; Arns, M.; Clark, C.R.; Bryant, R. Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biol. Psychol. 2010, 85, 350–354. [Google Scholar] [CrossRef]
- Allen, J.J.; Cohen, M.X. Deconstructing the “resting” state: Exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Front. Hum. Neurosci. 2010, 4, 232. [Google Scholar] [CrossRef] [Green Version]
- Deslandes, A.C.; De Moraes, H.; Pompeu, F.A.; Ribeiro, P.; Cagy, M.; Capitão, C.; Alves, H.; Piedade, R.A.; Laks, J. Electroencephalographic frontal asymmetry and depressive symptoms in the elderly. Biol. Psychol. 2008, 79, 317–322. [Google Scholar] [CrossRef]
- Stewart, J.L.; Coan, J.A.; Towers, D.N.; Allen, J.J. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J. Affect. Disord. 2011, 129, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aftanas, L.; Golocheikine, S. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci. Lett. 2001, 310, 57–60. [Google Scholar] [CrossRef]
- Stipacek, A.; Grabner, R.; Neuper, C.; Fink, A.; Neubauer, A. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci. Lett. 2003, 353, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.Y.; Kim, C.; Kim, S.; Yook, D.-W.; Kim, H.S.; Chang, H.; Lee, S.-H. The moderating effect of heart rate variability on the relationship between alpha asymmetry and depressive symptoms. Heliyon 2019, 5, e01290. [Google Scholar] [CrossRef] [Green Version]
- Fink, A.; Grabner, R.; Neuper, C.; Neubauer, A. EEG alpha band dissociation with increasing task demands. Cogn. Brain Res. 2005, 24, 252–259. [Google Scholar] [CrossRef]
- Park, Y.; Jung, W.; Kim, S.; Jeon, H.; Lee, S.-H. Frontal Alpha Asymmetry Correlates with Suicidal Behavior in Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 377. [Google Scholar] [CrossRef] [Green Version]
- Dharmadhikari, A.S.; Jaiswal, S.V.; Tandle, A.L.; Sinha, D.; Jog, N. Study of frontal alpha asymmetry in mild depression: A potential biomarker or not? J. Neurosci. Rural Pract. 2019, 10, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Gollan, J.K.; Hoxha, D.; Chihade, D.; Pflieger, M.E.; Rosebrock, L.; Cacioppo, J. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol. Psychol. 2014, 99, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Duran, N.L.; Nusslock, R.; George, C.; Kovacs, M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology 2012, 49, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Roh, S.-C.; Kim, J.S.; Kim, S.; Kim, Y.; Lee, S.-H. Frontal Alpha Asymmetry Moderated by Suicidal Ideation in Patients with Major Depressive Disorder: A Comparison with Healthy Individuals. Clin. Psychopharmacol. Neurosci. 2020, 18, 58. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.E.; Reznik, S.J.; Stewart, J.L.; Allen, J.J. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017, 111, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-H.; Na, S.H.; Kim, S.Y.; Ham, B.-J.; Lee, D.H.; Lee, J.H.; Lee, H. Hemispheric laterality and dimensional complexity in schizophrenia under sound and light stimulation. Int. J. Psychophysiol. 2003, 49, 1–15. [Google Scholar] [CrossRef]
- Liu, H.; Sarapas, C.; Shankman, S.A. Anticipatory reward deficits in melancholia. J. Abnorm. Psychol. 2016, 125, 631. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Nitschke, J.B.; Oakes, T.R.; Hendrick, A.M.; Horras, K.A.; Larson, C.L.; Abercrombie, H.C.; Schaefer, S.M.; Koger, J.V.; Benca, R.M. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biol. Psychiatr. 2002, 52, 73–85. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef]
- Parker, G.; Hadzi-Pavlovic, D.; Boyce, P.; Wilhelm, K.; Brodaty, H.; Mitchell, P.; Hickie, I.; Eyers, K. Classifying depression by mental state signs. Br. J. Psychiatr. 1990, 157, 55–65. [Google Scholar] [CrossRef]
- Semlitsch, H.V.; Anderer, P.; Schuster, P.; Presslich, O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 1986, 23, 695–703. [Google Scholar] [CrossRef]
- Cherbuin, N.; Luders, E.; Chou, Y.Y.; Thompson, P.M.; Toga, A.W.; Anstey, K.J. Right, left, and center: How does cerebral asymmetry mix with callosal connectivity? Hum. Brain Mapp. 2013, 34, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Kim, S.; Jung, W.; Im, C.-H.; Lee, S.-H. Auditory evoked potential could reflect emotional sensitivity and impulsivity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kwon, A.; Min, D.; Kim, S.; Jin, M.J.; Lee, S.-H. Neurophysiological and Psychological Predictors of Social Functioning in Patients with Schizophrenia and Bipolar Disorder. Psychiatry Investig. 2019, 16, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nierenberg, A.; Husain, M.; Trivedi, M.; Fava, M.; Warden, D.; Wisniewski, S.; Miyahara, S.; Rush, A. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR* D report. Psychol. Med. 2010, 40, 41–50. [Google Scholar] [CrossRef]
- Nelson, J.C.; Delucchi, K.; Schneider, L.S. Efficacy of second generation antidepressants in late-life depression: A meta-analysis of the evidence. Focus 2010, 8, 605–613. [Google Scholar] [CrossRef]
- Quitkin, F.M.; McGrath, P.J.; Stewart, J.W.; Deliyannides, D.; Taylor, B.P.; Davies, C.A.; Klein, D.F. Remission rates with 3 consecutive antidepressant trials: Effectiveness for depressed outpatients. J. Clin. Psychiatr. 2005, 66, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Nierenberg, A.A.; Vrijland, P.; van Oers, H.J.; Schutte, A.-J.; Simmons, J.H. Remission with mirtazapine and selective serotonin reuptake inhibitors: A meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int. Clin. Psychopharmacol. 2010, 25, 189–198. [Google Scholar] [CrossRef]
- Tullett, A.M.; Harmon-Jones, E.; Inzlicht, M. Right frontal cortical asymmetry predicts empathic reactions: Support for a link between withdrawal motivation and empathy. Psychophysiology 2012, 49, 1145–1153. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004, 67, 7–50. [Google Scholar] [CrossRef]
- Davidson, R.J. Affective style and affective disorders: Perspectives from affective neuroscience. Cogn. Emot. 1998, 12, 307–330. [Google Scholar] [CrossRef]
- Sutton, S.K.; Davidson, R.J. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia 2000, 38, 1723–1733. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology 1998, 35, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Gómez-Ariza, C. Dorsolateral prefrontal cortex and dual mechanisms of cognitive control: A tDCS study. Brain Stimul.: Basic Transl. Clin. Res. Neuromodulation 2015, 8, 347. [Google Scholar] [CrossRef]
- Ravizza, S.M.; Carter, C.S. Shifting set about task switching: Behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia 2008, 46, 2924–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notzon, S.; Steinberg, C.; Zwanzger, P.; Junghöfer, M. Modulating emotion perception: Opposing effects of inhibitory and excitatory prefrontal cortex stimulation. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2018, 3, 329–336. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, R.; Leyman, L.; Baeken, C.; Van Schuerbeek, P.; Luypaert, R.; Vanderhasselt, M.-A.; Dannlowski, U. Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: An event-related fMRI study. Biol. Psychol. 2010, 85, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Mars, R.B.; Grol, M.J. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J. Neurosci. 2007, 27, 1801–1802. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Davidson, R.J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004, 67, 219–234. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Ottowitz, W.E.; Tondo, L.; Dougherty, D.D.; Savage, C.R. The neural network basis for abnormalities of attention and executive function in major depressive disorder: Implications for application of the medical disease model to psychiatric disorders. Harv. Rev. Psychiatr. 2002, 10, 86–99. [Google Scholar] [CrossRef]
- Bracht, T.; Horn, H.; Strik, W.; Federspiel, A.; Schnell, S.; Höfle, O.; Stegmayer, K.; Wiest, R.; Dierks, T.; Müller, T.J. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J. Affect. Disord. 2014, 155, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Korgaonkar, M.S.; Grieve, S.M.; Koslow, S.H.; Gabrieli, J.D.; Gordon, E.; Williams, L.M. Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011, 32, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.; Oakes, T.; Fox, A.; Chung, M.; Larson, C.; Abercrombie, H.; Schaefer, S.; Benca, R.; Davidson, R. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol. Psychiatr. 2004, 9, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.; Bellgrove, M.A.; Chiu, E.; Mileshkin, C.; Bradshaw, J.L. Response selection deficits in melancholic but not nonmelancholic unipolar major depression. J. Clin. Exp. Neuropsychol. 2004, 26, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Day, C.V.; Gatt, J.M.; Etkin, A.; DeBattista, C.; Schatzberg, A.F.; Williams, L.M. Cognitive and emotional biomarkers of melancholic depression: An iSPOT-D report. J. Affect. Disord. 2015, 176, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Foti, D.; Carlson, J.M.; Sauder, C.L.; Proudfit, G.H. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage 2014, 101, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, M.-P.; Mitchell, P.; Wilhelm, K.; Parker, G.; Hickie, I.; Brodaty, H.; Chan, J.; Eyers, K.; Milic, M.; Hadzi-Pavlovic, D. Cognitive function in depression: A distinct pattern of frontal impairment in melancholia? Psychol. Med. 1999, 29, 73–85. [Google Scholar] [CrossRef]
- Roca, M.; Monzón, S.; Vives, M.; López-Navarro, E.; Garcia-Toro, M.; Vicens, C.; Garcia-Campayo, J.; Harrison, J.; Gili, M. Cognitive function after clinical remission in patients with melancholic and non-melancholic depression: A 6 month follow-up study. J. Affect. Disord. 2015, 171, 85–92. [Google Scholar] [CrossRef]
- Weinberg, A.; Liu, H.; Shankman, S.A. Blunted neural response to errors as a trait marker of melancholic depression. Biol. Psychol. 2016, 113, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Romans, S.E.; Tyas, J.; Cohen, M.M.; Silverstone, T. Gender differences in the symptoms of major depressive disorder. J. Nerv. Ment. Dis. 2007, 195, 905–911. [Google Scholar] [CrossRef]

| Variable | Patient (n = 52) |
|---|---|
| REMISSION STATUS | N (%) |
| Yes | 32 (61.54) |
| No | 20 (38.46) |
| TREATMENT RESPONSE | |
| Successful | 40 (76.92) |
| Unsuccessful | 12 (23.08) |
| PSYCHOLOGICAL MEASURE | |
| Age (year) | 45.87 ± 11.69 |
| Sex | |
| Male | 4 (7.69) |
| Female | 48 (92.31) |
| Education (year) | 13.42 ± 2.89 |
| HAM-D (baseline) | 26.13 ± 6.80 |
| HAM-A (baseline) | 25.13 ± 6.02 |
| CORE (baseline) | 4.35 ± 5.98 |
| NEUROPHYSIOLOGICAL MEASURE | |
| LDAEP (μV/10 dB) | 1.55 ± 2.85 |
| MMN (μV) | −2.97 ± 1.34 |
| Alpha asymmetry index of F5/F6 | |
| Low alpha band | 0.03 ± 14.13 |
| High alpha band | −1.29 ± 13.16 |
| Alpha band | −1.05 ± 12.73 |
| Alpha asymmetry index of F7/F8 | |
| Low alpha band | −1.73 ± 15.81 |
| High alpha band | −2.96 ± 13.56 |
| Alpha band | −2.80 ± 14.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Baik, S.Y.; Kim, Y.-W.; Kim, J.-Y.; Lee, S.-H. Prediction of Antidepressant Treatment Outcome Using Event-Related Potential in Patients with Major Depressive Disorder. Diagnostics 2020, 10, 276. https://doi.org/10.3390/diagnostics10050276
Lee HS, Baik SY, Kim Y-W, Kim J-Y, Lee S-H. Prediction of Antidepressant Treatment Outcome Using Event-Related Potential in Patients with Major Depressive Disorder. Diagnostics. 2020; 10(5):276. https://doi.org/10.3390/diagnostics10050276
Chicago/Turabian StyleLee, Hyun Seo, Seung Yeon Baik, Yong-Wook Kim, Jeong-Youn Kim, and Seung-Hwan Lee. 2020. "Prediction of Antidepressant Treatment Outcome Using Event-Related Potential in Patients with Major Depressive Disorder" Diagnostics 10, no. 5: 276. https://doi.org/10.3390/diagnostics10050276
APA StyleLee, H. S., Baik, S. Y., Kim, Y.-W., Kim, J.-Y., & Lee, S.-H. (2020). Prediction of Antidepressant Treatment Outcome Using Event-Related Potential in Patients with Major Depressive Disorder. Diagnostics, 10(5), 276. https://doi.org/10.3390/diagnostics10050276





