Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Protocol
2.2. Ultrasound Technique
2.3. Ultrasound Diagnostic and Staging Criteria
2.4. Staging Criteria Included Extraluminal Free Fluid, Parietal and Villi Alterations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Catena, F.; De Simone, B.; Coccolini, F.; Di Saverio, S.; Sartelli, M.; Ansaloni, L. Bowel obstruction: A narrative review for all physicians. World J. Emerg. Surg. 2019, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Cappell, M.S.; Batke, M. Mechanical obstruction of the small bowel and colon. Med. Clin. North Am. 2008, 92, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Kulaylat, M.N.; Doerr, R.J. Small Bowel Obstruction. In Surgical Treatment: Evidence-Based and Problem-Oriented, 2nd ed.; Holzheimer, R.G., Mannick, J.A., Eds.; Zuckschwerdt: Munich, Germany, 2001. [Google Scholar]
- Long, B.; Robertson, J.; Koyfman, A. Emergency Medicine Evaluation and Management of Small Bowel Obstruction: Evidence-Based Recommendations. J. Emerg. Med. 2019, 56, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; Lalani, N. Adult small bowel obstruction. Acad. Emerg. Med. 2013, 20, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Pimenta, M.; Guimaraes, L.S. Small bowel obstruction: What to look for. Radiographics 2009, 29, 423–439. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.A.; Meehan, C.P.; Alhajeri, A.N.; Regan, M.C.; O’Keeffe, D.P. The use of MRI to demonstrate small bowel obstruction during pregnancy. Br. J. Radiol. 2007, 80, e11–e14. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Dimbil, U.; Drake, A.; Shokoohi, H. The Accuracy of Point-of-Care Ultrasound in Detecting Small Bowel Obstruction in Emergency Department. Emerg. Med. Int. 2018, 2018, 3684081. [Google Scholar] [CrossRef] [PubMed]
- Maglinte, D.D.; Howard, T.J.; Lillemoe, K.D.; Sandrasegaran, K.; Rex, D.K. Small-bowel obstruction: State-of-the-art imaging and its role in clinical management. Clin. Gastroenterol. Hepatol. 2008, 6, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Frasure, S.E.; Hildreth, A.; Takhar, S.; Stone, M.B. Emergency department patients with small bowel obstruction: What is the anticipated clinical course? World J. Emerg. Med. 2016, 7, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasure, S.E.; Hildreth, A.F.; Seethala, R.; Kimberly, H.H. Accuracy of abdominal ultrasound for the diagnosis of small bowel obstruction in the emergency department. World J. Emerg. Med. 2018, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, S.; Lugara, M.; Iaselli, F.; Saturnino, P.P.; Liguori, C.; Carbone, R.; Vecchione, D.; Abete, R.; Tammaro, P.; Marano, I. Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction. Diagnostics 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boniface, K.S.; King, J.B.; LeSaux, M.A.; Haciski, S.C.; Shokoohi, H. Diagnostic Accuracy and Time-Saving Effects of Point-of-Care Ultrasonography in Patients With Small Bowel Obstruction: A Prospective Study. Ann. Emerg. Med. 2020, 75, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Peksa, G.D.; Pandurangadu, A.V.; Nakitende, D.; Takhar, S.; Seethala, R.R. Utilization of ultrasound for the evaluation of small bowel obstruction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2018, 36, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Romano, S.; D’Amario, F.; Giorgio Rossi, A.; Romano, L.; Pinto, F.; Di Mizio, R. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur. J. Radiol. 2004, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.A.; Lahham, S.; Gonzales, M.A.; Nomura, J.T.; Bui, M.K.; Truong, T.A.; Stahlman, B.A.; Fox, J.C.; Kehrl, T. A Prospective, Multicenter Evaluation of Point-of-care Ultrasound for Small-bowel Obstruction in the Emergency Department. Acad. Emerg. Med. 2019, 26, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wale, A.; Pilcher, J. Current Role of Ultrasound in Small Bowel Imaging. Semin Ultrasound CT MR 2016, 37, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018, 39, 304–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, N.S.S.; Bryant, R.V.; Dong, Y.; Maaser, C.; Kucharzik, T.; Maconi, G.; Asthana, A.K.; Blaivas, M.; Goudie, A.; Gilja, O.H.; et al. How to perform gastrointestinal ultrasound: Anatomy and normal findings. World J. Gastroenterol. 2017, 23, 6931–6941. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.C.; Dowling, A.D.; Weinstein, M.L.; James, A.E., Jr. Sonographic patterns of distended, fluid-filled bowel. Radiology 1979, 133, 681–685. [Google Scholar] [CrossRef] [PubMed]
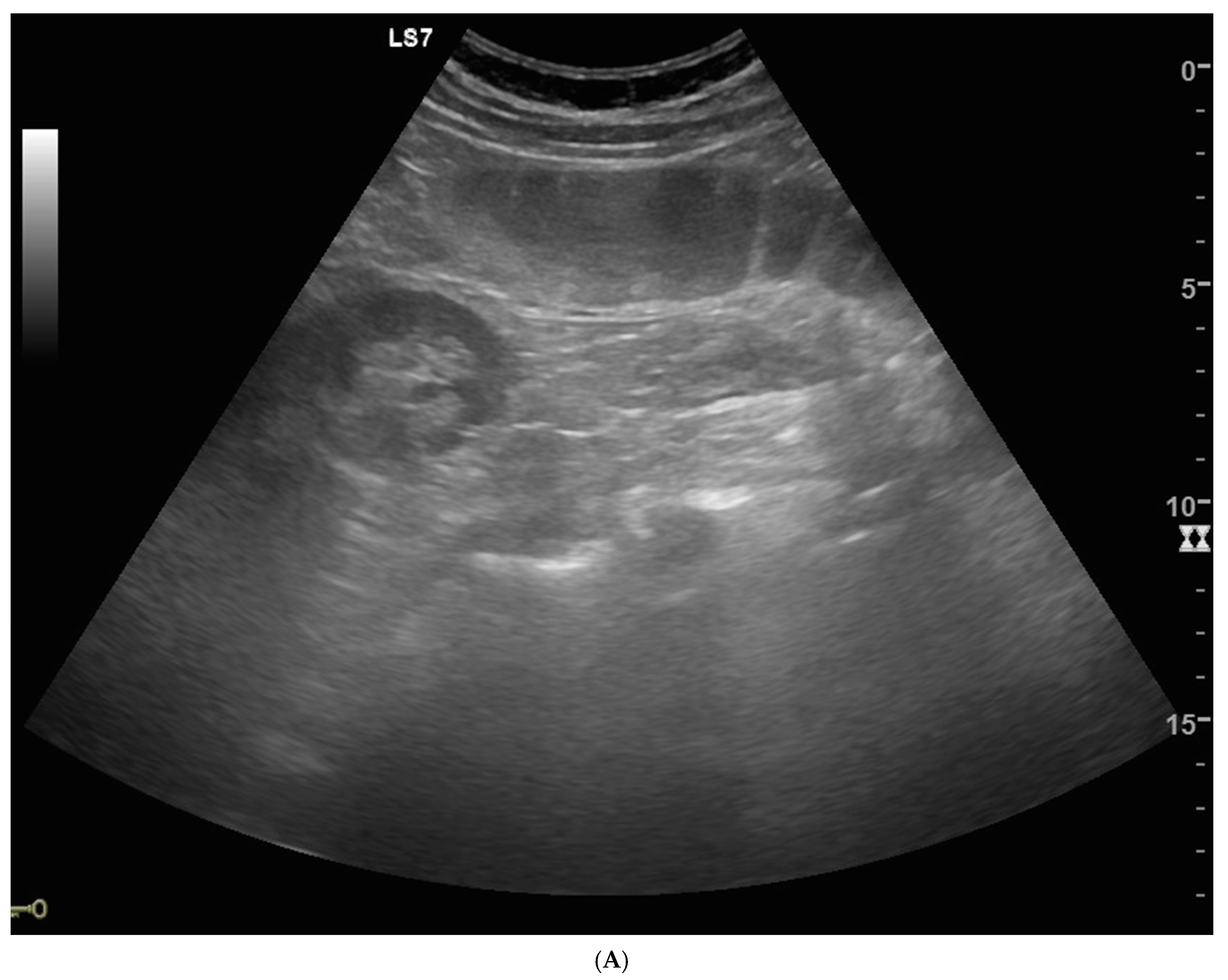
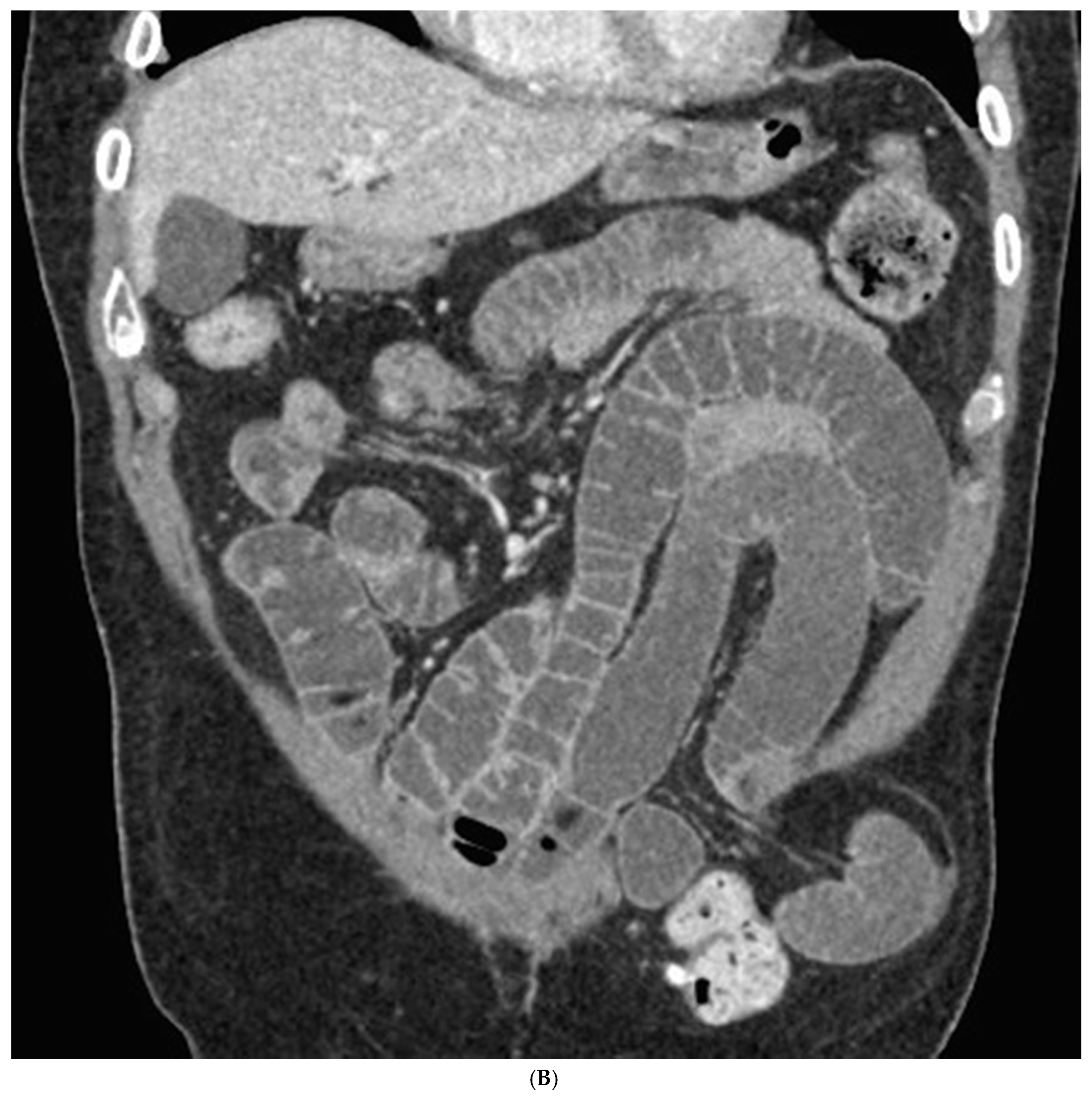

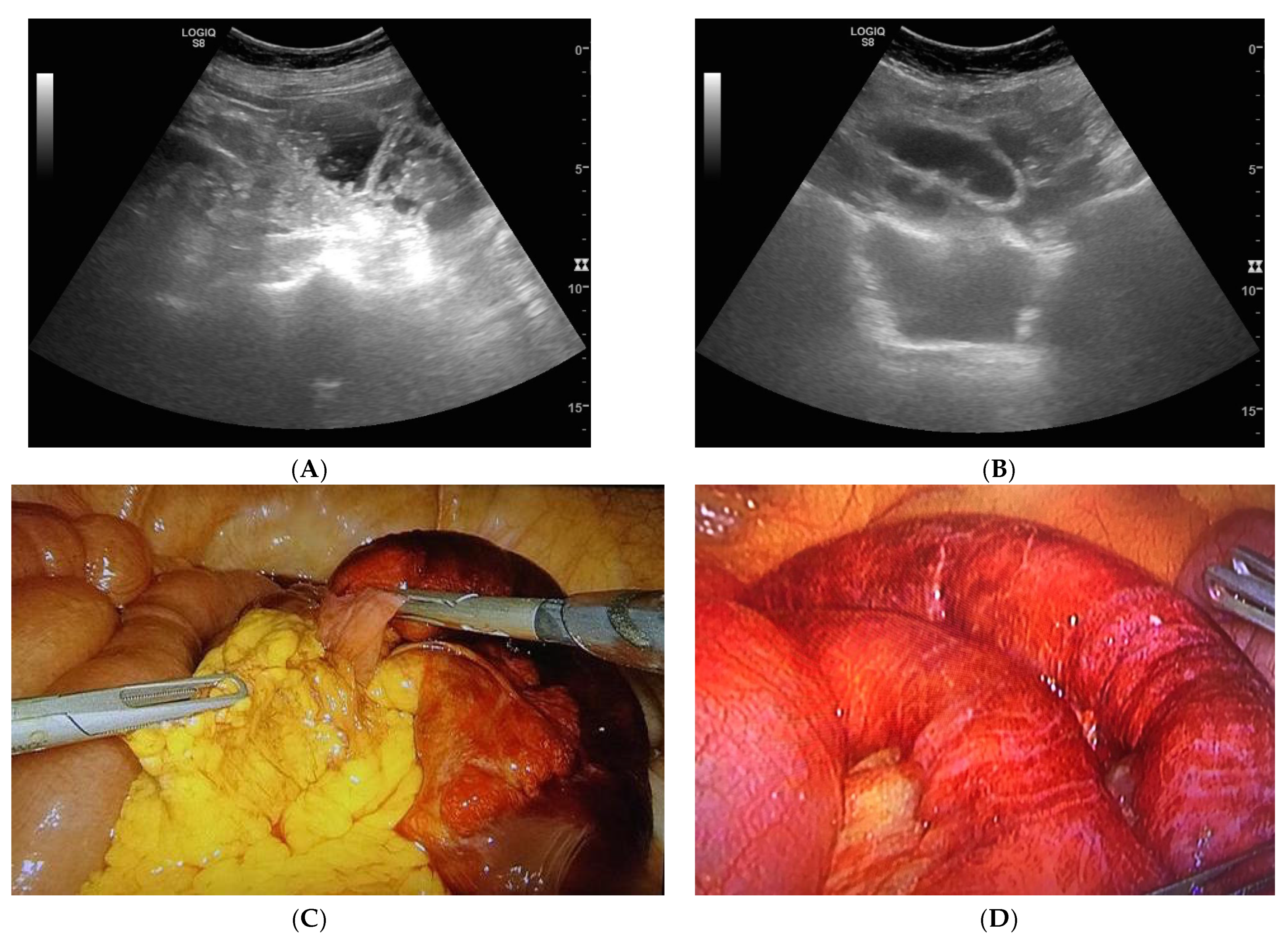
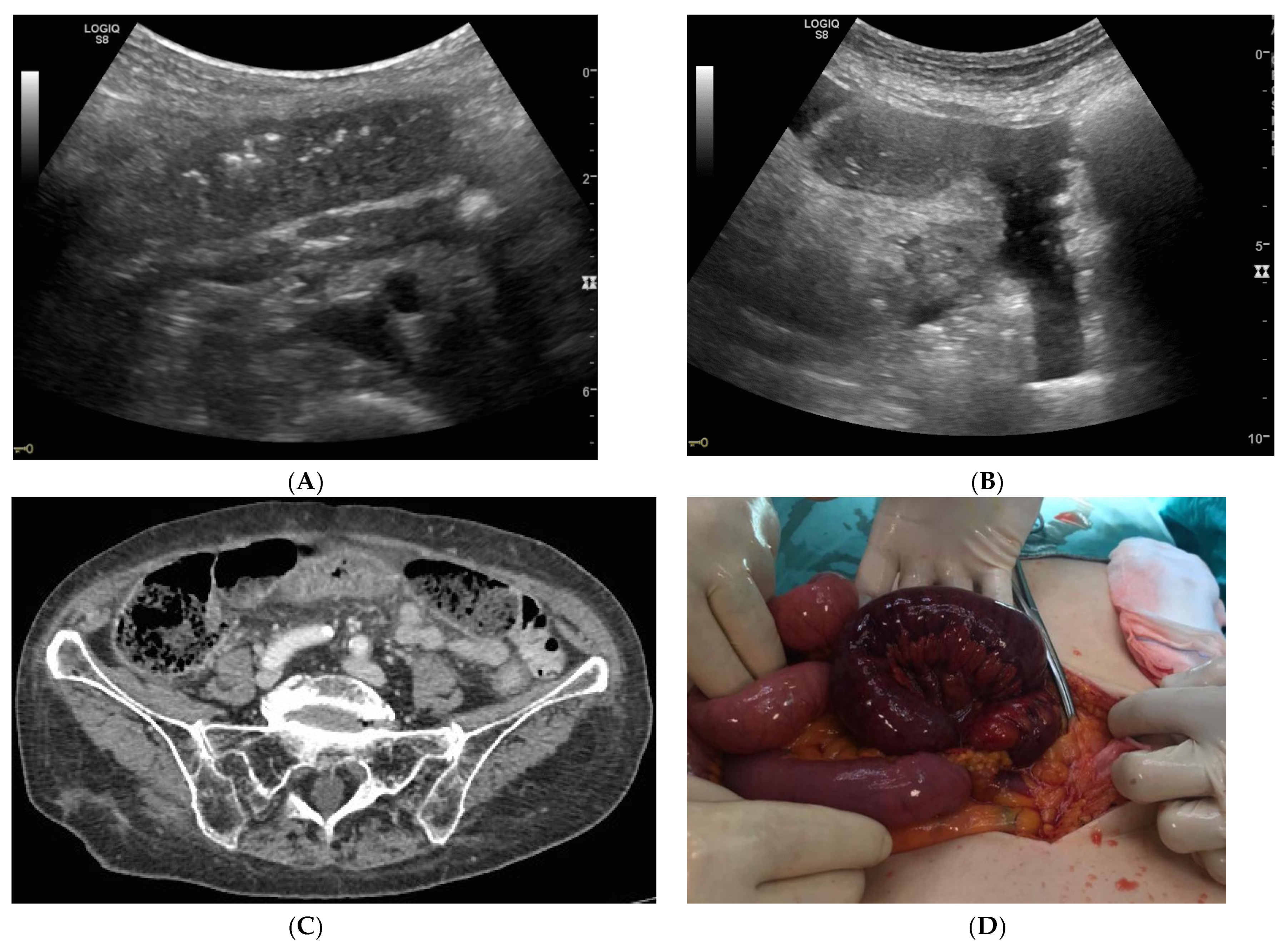
| Parameters | Mean/Percentage |
|---|---|
| Gender | |
| Male | 28.57% (18/63) |
| Female | 71.43% (45/63) |
| Age (years) | 68.69 ± 17.72 |
| Therapy | |
| Conservative | 42.86% (27/63) |
| Surgery | 49.21% (31/63) |
| Conservative + Surgery | 7.94% (5/63) |
| US status | 100% |
| Simple | 26.98% (17/63) |
| Decompensated | 22.22% (14/63) |
| Complicated | 50.79% (32/63) |
| Free fluid (yes) | 74.60% (47/63) |
| Thickened walls (yes) | 82.54% (52/63) |
| Thinned walls (yes) | 44.44% (28/63) |
| Prominence of valvulae conniventes (yes) | 42.86% (27/63) |
| Dilated bowel (>2.5 cm) | 100% |
| Peristalsis | |
| Hyperkinesis | 0% |
| Hypokinesis | 90.48% (57/63) |
| Akinesis | 9.52% (6/63) |
| Bowel jump diameter | |
| Yes | 55.56% (35/63) |
| Not evaluable (NE) | 44.44% (28/63) |
| Bowel jump kinesis | |
| Yes | 36.51% (23/63) |
| NE | 63.49% (40/63) |
| Linear Correlation Analysis | Univariate Analysis R (p-Value) | Multivariate Analysis Rpartial; p-Value |
|---|---|---|
| Multiple linear correlation coefficient = 0.82 | ||
| US staging/dilated bowel # | 0.00 (1.00) | — |
| US staging/peristalsis | 0.03 (0.83) | Rpartial = 0.19; p-value = 0.17 |
| US staging/free fluid | 0.76 (<0.0001) * | Rpartial = 0.75; p-value < 0.0001 * |
| US staging/thickened walls | 0.42 (0.0005) * | Rpartial = 0.11; p-value = 0.40 |
| US staging/thinned walls | 0.09 (0.49) | Rpartial = −0.26; p-value = 0.048 * |
| US staging/prominence of valvulae conniventes | 0.25 (0.0498) * | Rpartial = 0.10; p-value = 0.44 |
| US staging/bowel jump diameter | −0.01 (0.92) | Rpartial = 0.19; p-value = 0.15 |
| US staging/bowel jump kinesis | −0.14 (0.29) | Rpartial = −0.27; p-value = 0.040 * |
| US Sign/Groups | Conservative N. 27 | Surgery N. 31 | Conservative + Surgery N. 5 | Statistical Analysis p-Value (Test Type) |
|---|---|---|---|---|
| Dilated bowel | 100% | 100% | 100% | p = 1.00 (C) |
| Hyperkinesis | 0.0% | 0.0% | 0.0% | p = 1.00 (C) |
| Hypokinesis | 100% | 80.65% (25/31) | 100% | p = 0.0326 * (C), NS |
| Akinesis | 0.0% | 19.4% (6/31) | 0.0% | p = 0.0326 * (C), NS |
| Free fluid | 51.9% (14/27) | 93.6% (29/31) | 80% (4/5) | p = 0.0013 * (C), NS Conservative ***, p = 0.040 (Z) |
| Thickened walls | 70.4% (19/27) | 90.3% (28/31) | 100% | p = 0.077 (C) |
| Thinned walls | 40.7% (11/27) | 45.2% (14/31) | 60% (3/5) | p = 0.72 (C) |
| Prominence of valvulae conniventes | 22.2% (6/27) | 61.3% (19/31) | 40% (2/5) | p = 0.011 * (C), NS |
| Bowel jump diameter | 55.6% (15/27) | 51.5% (16/31) | 80% (4/5) | p =0.50 (C) |
| Bowel jump kinesis | 37.0% (10/27) | 35.5% (11/31) | 40% (2/5) | p = 0.98 (C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburrini, S.; Serra, N.; Lugarà, M.; Mercogliano, G.; Liguori, C.; Toro, G.; Somma, F.; Mandato, Y.; Guerra, M.V.; Sarti, G.; et al. Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction. Diagnostics 2020, 10, 277. https://doi.org/10.3390/diagnostics10050277
Tamburrini S, Serra N, Lugarà M, Mercogliano G, Liguori C, Toro G, Somma F, Mandato Y, Guerra MV, Sarti G, et al. Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction. Diagnostics. 2020; 10(5):277. https://doi.org/10.3390/diagnostics10050277
Chicago/Turabian StyleTamburrini, Stefania, Nicola Serra, Marina Lugarà, Giuseppe Mercogliano, Carlo Liguori, Gabriella Toro, Francesco Somma, Ylenia Mandato, Maria Vittoria Guerra, Giuseppe Sarti, and et al. 2020. "Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction" Diagnostics 10, no. 5: 277. https://doi.org/10.3390/diagnostics10050277
APA StyleTamburrini, S., Serra, N., Lugarà, M., Mercogliano, G., Liguori, C., Toro, G., Somma, F., Mandato, Y., Guerra, M. V., Sarti, G., Carbone, R., Tammaro, P., Ferraro, A., Abete, R., & Marano, I. (2020). Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction. Diagnostics, 10(5), 277. https://doi.org/10.3390/diagnostics10050277






